Estimating Resting Energy Expenditure in Obesity
Abstract
FOSTER, GARY D. AND BRIAN G. MCGUCKIN. Estimating resting energy expenditure in obesity. Obes Res. 2001;9:367S–372S.
In the treatment of obesity, assessment of resting energy expenditure (REE) can provide the basis for prescribing an individualized energy intake to attain a desired level of energy deficit. Indirect calorimetry is the most frequently used method to measure REE, but the great expense of equipment precludes its widespread use. As a result, REE is often estimated by predictive formulas based on weight, height, age, and gender. This paper examines the accuracy of these formulas in estimating REE among obese patients, discusses the assumptions underlying their use, and reviews the need for technological advances that will make the assessment of REE accurate, portable, and inexpensive.
Introduction
The management of obesity is fundamentally a manipulation of the energy (im)balance equation. Despite the plethora of gimmicks that promise easy weight loss, the treatment of obesity is rooted in the balance between energy consumed and energy expended. The factors that affect this balance (e.g., genes, environment, and their interaction) are numerous and complex, but the basics of weight management could not be more straightforward. Thus, virtually all obesity treatments seek to identify and modify the amount of energy consumed and expended ((1),(2)). In a companion paper in this supplement, Dr. Patrick O'Neil ((3)) describes the various methods to monitor energy intake and their importance in the management of obesity. This paper will focus on the energy expenditure side of the equation and review three main areas: 1) the importance of resting energy expenditure (REE) in the treatment of obesity, 2) the accuracy of clinical estimates of REE, and 3) the clinical implications of estimating and measuring REE.
Energy Expenditure and Obesity
Components
There are three principal components of total daily energy expenditure: REE, the energy cost of physical activity, and the thermic effect of food. REE, often called resting metabolic rate or RMR, is the energy required to sustain the body's functions at rest and accounts for ~65% of total daily energy expenditure in sedentary individuals ((4)). The energy cost of physical activity (often referred to as the thermic effect of activity) accounts for 25% of total daily energy expenditure in sedentary individuals. The thermic effect of food (often referred to as diet-induced thermogenesis) is the energy associated with digestion and metabolism of nutrients and accounts for ~10% of total daily energy expenditure. Because of its large contribution to daily energy expenditure, REE has been frequently examined in the development and treatment of obesity. The following section briefly summarizes the major points about REE and obesity. Two excellent comprehensive reviews of this topic were published recently ((5),(6)).
REE
In general, REE is higher among the obese than the non-obese because it takes more energy to support more mass. In samples of varying body weights. REE is strongly related to the amount of fat-free mass ((4)). Men, however, have higher REEs than women, even after controlling for weight and fat-free mass ((7)). In addition, increasing age is associated with decreasing REE, partially due to diminished fat-free mass ((8),(9)). However, even among those of similar weight, age, and gender, REE can vary markedly ((6)). In addition, several studies have suggested that ethnicity may independently affect REE ((10-14)).
Although some studies have shown that REE and total energy expenditure predict the development of obesity in infants and children ((15)), most have not ((5),(16-18)). Ravussin et al. ((19)) showed that in adult Pima Indians, lower initial REEs predicted greater weight gain. Regardless of whether REE influences the development of obesity, it is clearly important in weight management for those who are already obese. Initial REE has been a significant predictor of weight loss in several clinical trials ((20),(21)). In a metabolic ward setting where intake was tightly controlled, Garrow et al. ((22)) found that 64% of the variance in weight loss over 3 weeks was accounted for by initial REE. Whereas it is a matter of some controversy whether the decrement in REE after weight loss is greater than or the same as that expected for a new lower body weight, it is unequivocal that REE is significantly reduced after weight loss ((23-26)). This lower REE establishes a new energy requirement for weight maintenance or for further weight loss. This decrement persists even after patients return to energy balance after weight loss.
Measurement
The clinical rationale for assessing REE is straightforward. Knowing REE can provide the basis for estimating total daily energy expenditure (1.3 × REE) ((27),(28)). Once total energy expenditure is known, an energy intake can be prescribed to attain a desired energy deficit (e.g., 500 kcal/d). Prescribing the same energy level (e.g., 1400 kcal/d) to all patients will necessarily result in different energy deficits and weight losses because their REEs (and total daily energy expenditure) vary considerably. Because REE is a large component of daily energy expenditure and it changes with weight loss, patient reassessment would help tailor energy intakes over time. Unfortunately, REE is rarely measured in the clinical setting. Indirect calorimetry is the most frequently used method, but the great expense of equipment precludes its widespread use. As a result, REE is often estimated by predictive formula as described in the next section.
Clinical Estimates of REE
Over the course of the last century, a number of equations to estimate REE have been proposed and put into practice (Table ). The first such equation was published in 1919 by Harris and Benedict of the Carnegie Institution of Washington and uses weight, height, age, and gender to estimate REE ((29)). The equation was based on a study of 239 men and women. The 136 male participants had a mean age of 27 ± 9 years (range, 16 to 63 years) and a mean body mass index (BMI) of 21.4 ± 2.8 kg/m2 (range, 15.2 to 32.5 kg/m2). The mean age for the 103 female participants was 31 ± 14 years (range, 15 to 74 years) and the mean BMI was 21.5 ± 4.1 kg/m2 (range, 12.3 to 34.6 kg/m2). Whereas more recent equations have been shown to be more accurate than the Harris-Benedict in estimating REE ((30)), any equation that relies heavily on body weight is likely to overestimate the REE for obese patients. This is due to the fact that obese patients’ excess weight is mostly fat tissue which is less metabolically active ((31)). Numerous studies have been conducted to assess the accuracy of these equations, and the following section will review the literature estimating REE in obese populations.
| Equation | ||
|---|---|---|
| Men | Women | |
| Harris and Benedict ((29)) | 66 + {13.7 × weight (kg)} + {5 × height (cm)} − {6.8 X age (year)} | 665 + {9.6 × weight (kg)} + {1.8 × height (cm)} − {4.7 × age (year)} |
| Owen et al. (40,41) | 879 + {10.20 × weight (kg)} | 795 + {7.18 × weight (kg)} |
| Mifflin et al, (30) | {9.99 × weight (kg)} + {6.25 × height (cm)} − 14.92 × age (year)} + 5 | {9.99 × weight (kg)} + {6.25 × height (cm)} − {4.92 × age (year)} − 161 |
| Bernstein et al. (37) | {11.0 × weight (kg)} + {10.2 × height (cm)} − 15.8 × age (year)} − 1032 | {7.48 × weight (kg)} + {0.42 × height (cm)} − (3.0 × age (year)} + 844 |
| James (39) | Age 18 to 30: 692 + {15.1 × weight (kg)} Age 31 to 60: 873 + {11.6 × weight (kg)} | Age 18 to 30: 487 + {14.8 × weight (kg)} Age 31 to 60: 845 + {8.17 × weight (kg)} |
| World Health Organization ((47)) | Age 18 to 30: [{64.4 × weight (kg)} − {113 × height (m)} + 3000]/4.184 | Age 18 to 30: [{55.6 × weight (kg)} + {1397.4 × height (m)} + 146]/4.184 |
| Age 31 to 60: [{19.2 × weight (kg)} + {66.9 × height (m)} + 3769]/4.184 | Age 31 to 60: [{36.4 × weight (kg)} − {104.6 × height (m)} + 3619]/4.184 | |
| Cunningham (38) | 501.6 + 21.6 [79.5 − {0.24 × weight (kg)} − {0.15 × age (year)} × weight (kg)]/73.2 | 501.6 + 21.6 [69.8 − {0.26 × weight (kg)} − (0.12 × age (year)} × weight (kg)]/73.2 |
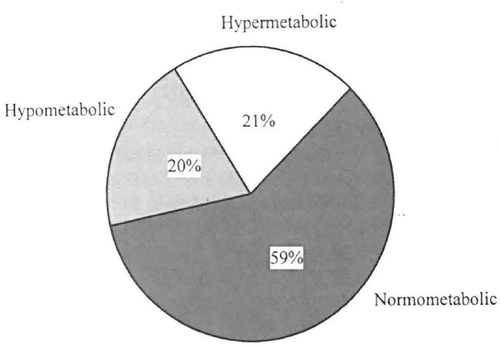
Percentage of patients in each of these categories based on their measured and predicted REEs (Harris-Benedict). Hypometabolic, measured REE < 90% of predicted REE; normometabolic, measured REE between 90% and 100% of predicted REE: hypermetabolic, measured REE > 110% of predicted REE. Chart adapted from Foster et al. ((33)).
Pavlou et al. ((32)) compared the measured REEs of 31 moderately obese (107.3 ± 17.1 kg and an approximate BMI of 34 kg/m2) men with their predicted REEs. REE was measured using indirect calorimetry and predicted by the Harris-Benedict equation. They found that the measured REE was 92 ± 10% of predicted REE. Individual variation, however, ranged from 65% to 109%. Only 64% of patients had measured REEs that were within 10% of predicted values.
Foster et al. ((33)) conducted a similar comparison in 80 moderately obese women (mean BMI, 38.9 ± 7 kg/m2). REE was measured using indirect calorimetry and predicted using the following three formulas: ((1)) Harris-Benedict equation ((29)), ((2)) tables of Boothby ((34)), and ((3)) the Kleiber equation ((35)). For mean values, measured REE was 99.3 ± 12.2% of Harris-Benedict predictions, 98.8 12.1% of Boothby's predictions, and 97.9 ± 12.2% of Kleiber's predictions. Despite these accurate predictions for mean REE, only 59% of patients’ measured REE values were within 10% of Harris-Benedict predictions (Figure 1). Similar values were observed for the Boothby predictions, and only 55% of measured values fell within 10% of Kleiber's predictions. Attempts to predict the subjects’ degree of hypometabolism or hypermetabolism (i.e., measured values that were below or above predicted) using a variety of factors were unsuccessful. No combination of independent variables (including weight, height, age, fat, fat-free mass, fat-cell size, and fat-cell number) could account for more than 6% of the variance in the degree of over or underestimation.
Among this group of 80 women, 5 were of similar height, weight, and age; therefore, they had virtually the same predicted REEs based on the Harris-Benedict formula (-1740 kcal/d). By contrast, the measured REEs for these subjects were markedly different, ranging from 1263 to 2152 kcal/d (Table ). Assuming that total daily energy expenditure is a minimum of 130% of REE ((27),(28)), prescribing the same caloric intake of 1200 kcal/d for these five individuals would result in energy deficits ranging from 442 to 1598 kcal/d. Furthermore, assuming that the energy equivalent of 1 kg of fat is 7700 kcal and that all of the weight lost is fat, 1 month of treatment using a 1200 kcal/d diet would result in losses of 1.7 to 6.2 kg (Table ). On the basis of their estimated REEs, all five subjects would be expected to lose 4 kg. These individual cases reveal the clinically significant inaccuracies of applying the Harris-Benedict formula to obese patients.
| Predicted REE (kcal/d) | Measured REE (kcal/d) | REE × 1.3 (kcal/d) | 1-Month weight loss1 (kg) | |
|---|---|---|---|---|
| Subject A | 1714 | 1263 | 1642 | 1.7 |
| Subject B | 1740 | 1523 | 1980 | 3.0 |
| Subject C | 1743 | 1778 | 2311 | 4.3 |
| Subject D | 1744 | 1979 | 2573 | 5.3 |
| Subject E | 1743 | 2152 | 2798 | 6.2 |
- * Assuming a 1200 kcal/d diet and that 1 kg of fat = 7700 kcal. Table adapted from Foster et al. ((33)).
Heshka et al. ((36)) examined 12 methods of predicting REE in a sample of 126 obese men (BMI, 41.5 ± 8.5 kg/m2) and women (BMI, 35.2 ± 7.2 kg/m2). Indirect calorimetry was used to measure REE while the following prediction equations were employed: Bernstein et al. ((37)), Cunningham ((38)), Harris-Benedict ((29)), James ((39)), Mifflin ((30)), Owen et al. ((40),(41)), Pavlou et al. ((32)), Aub and Dubois ((42)), Boothby et al. ((34)), Fleisch ((43)), and Robertson and Reid ((44)). The Robertson and Reid ((44)) equation was the only one that produced estimated REE values that were not statistically different from measured REE values for both the men and women. The equations proved slightly more accurate overall for the women than for the men; a finding the authors attributed to the greater obesity of the male sample.
Feurer et al. ((45)) measured REE in 112 morbidly obese men and women (BMI, 48 kg/m2) awaiting gastric bypass surgery. REE was measured using indirect calorimetry and predicted using the Harris-Benedict formula. Measured REE was significantly different from predicted values for both men and women (88.4 ± 15.0% of predicted for men and 89.5 ± 16.9% for women). Individual variation ranged from 57% to 135% of predicted, and only 39% of the measured REEs were within 10% of predicted values.
Faulty Assumptions
These studies reveal several faulty assumptions of applying predictive formula to individual obese patients. The first is that similar mean values for measured and predicted REE in a sample of patients leads to accuracy for an individual. Similar mean values in the studies above reflected underestimation in some patients and overestimation in others. Thus, on average the prediction appears accurate, but that is due to significant error in both directions.
A second faulty assumption is that formulas developed in one population can be extrapolated to others with significantly different body composition. Applying formulas developed in lean samples to those who are obese is likely to lead to significant errors. For example, in the studies described above, the percentage of participants whose measured REE values were within 10% of predicted values ranged from 58% in those with a BMI of 34 kg/m2 ((32)) to only 39% in the morbidly obese (BMI, 48 kg/m2) ((45)). Studies of hospitalized patients receiving total parenteral nutrition show a similarly small number of patients (48%) within 10% of predicted values ((46)). This should not be surprising because the Harris-Benedict formulas were based on samples of healthy men and women with a BMI of ~21 kg/m2. It is unreasonable to assume that they can accurately predict REE in unhealthy or obese samples.
A third faulty assumption of using predictive formulas is that REE is independent of energy balance status. It is common practice for predicted REE to be recalculated based on a new lowered body weight. If a patient is still in a negative energy balance, REE will be lower than if at the same weight and not in an energy deficit. Similarly, REE will be higher than expected based on weight alone if patients are gaining weight (as they often are when presenting for treatment).
Clinical Implications
If we accept an estimate within 10% as accurate, obtaining a prediction of REE from the Harris-Benedict equation for obese patients is roughly equivalent to the likelihood of getting heads or tails on a coin toss (50%). Despite this, predictive formulas continue to be widely used in the clinical setting. The danger in using these formulas among the obese is that both patients and practitioners are given a false sense of surety because they have “a number” that seems to be scientifically derived. Unfortunately, this number is the basis of determining energy requirements. Given this set-up, it is reasonable for patients to think that if they adhere to the prescribed energy regimen, they will lose a specified amount of weight. Based on the data reviewed above, however, ~50% will not lose what is expected because the original estimate has a >10% error. From our perspective, the process of estimating REE invites unrealistic expectations. The dilemma is that most settings do not afford the opportunity to measure REE by indirect calorimetry. In such settings, we recommend that REE not be estimated based on the well-documented inaccuracies of current formulas. There are enough uncertainties and unrealistic expectations in the weight loss process without adding another that can be easily avoided.
Potential Benefits of Measuring REE
Clearly, there is a need for technological advances that will make the assessment of REE both portable and inexpensive. Such methods, of course, will need to be evaluated carefully to assess their reliability and validity. If such technology were available, how might it impact the research and treatment of obesity? On the research side, making the measurement of REE less costly and more accessible will allow researchers to obtain more frequent assessments without undue patient burden. Portable technology may also allow for the assessment of energy expenditure associated with specific bouts of physical activity in more free-living environments. Clinically, the ability to measure REE will permit tailored energy goals (e.g., 500 kcal/d deficit). Whereas prescribing an intake that is less than most person's energy requirements (e.g., 1200 kcal/d) will produce weight loss in the majority of patients, such prescriptions may be overly restrictive for some. Thus, measuring REE may minimize the likelihood of excessive restriction and weight loss. On the other hand, if a measured REE indicates that little if any weight loss is likely on a 1200 kcal/d diet, such information can be used to help patients focus on aspects of their health (physical activity, stress management) that have positive effects independent of weight change. Revealing, before treatment, that a REE is unusually low can prevent patients from multiple unsuccessful attempts at weight loss. An intuitive but untested idea is that providing patients with more feedback (about intake and expenditure) may improve outcome. For example, knowing the actual energy expenditure associated with 30 minutes of walking may prevent patients from overcompensating with increased intake. Rather than using the scale alone as the arbiter of success, more interim assessments of intake and expenditure can be used to make adjustments. In addition, serial measurements of REE will help patients adjust their intake downward as they lose weight. In short, the accurate assessment of REE (and other components of energy expenditure) coupled with complete assessments of energy intake will demystify the weight loss process and allow patients, practitioners and researchers to focus on the issues most relevant to weight control: energy in and energy out.
Acknowledgments
Preparation of this manuscript was partially supported by HealtheTech. This paper was based on a presentation at a HealtheTech-sponsored symposium at the annual NAASO meeting in Long Beach, California, in November 2000.





