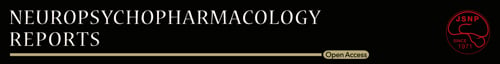Antidepressant amitriptyline-induced matrix metalloproteinase-9 activation is mediated by Src family tyrosine kinase, which leads to glial cell line-derived neurotrophic factor mRNA expression in rat astroglial cells
Abstract
Background
Astrocytes have been implicated in the pathophysiology of mood disorders and in the mechanism of the pharmacological effects of antidepressant drugs by the production of neurotrophic/growth factors. Previous studies have identified astrocyte-expressed Gαi/o-coupled lysophosphatidic acid receptor 1 (LPAR1), as being involved in antidepressant-induced production of glial cell line-derived neurotrophic factor (GDNF) and matrix metalloproteinase-9 (MMP-9) activation, an important step in the production of GNDF. However, the precise mechanism of MMP-9 activation by antidepressants has yet to be identified, in particular the intracellular signaling pathway between LPAR1/Gαi/o and MMP-9.
Methods and Results
Treatment of rat C6 astroglial cells (C6 cells) with amitriptyline increased Src family tyrosine kinase phosphorylation in a time and concentration-dependent manner. Amitriptyline-induced GDNF mRNA expression was blocked by Src family tyrosine kinase inhibitors. In addition, inhibiting Src family tyrosine kinase blocked amitriptyline-induced zymographic MMP-9 activation in C6 cells. The amitriptyline-induced zymographic MMP-9 activity was completely blocked by selective inhibition of Gαi/o protein and LPAR1. Furthermore, the amitriptyline-induced Src family tyrosine kinase phosphorylation was blocked by LPAR1, but not MMP-9 inhibition, indicating that Src family tyrosine kinase involvement is downstream of LPAR1.
Conclusions
The current findings suggest that the pharmacological effect of antidepressant such as amitriptyline is mediated through an intracellular signaling pathway via the LPAR1/Gαi/o/Src family tyrosine kinase, which leads to MMP-9 activation and GDNF production.
1 INTRODUCTION
Neurotrophic/growth factors, such as brain-derived neurotrophic factor (BDNF), fibroblast growth factor 2 (FGF2), and glial cell line-derived neurotrophic factor (GDNF), support neurogenesis, gliogenesis, neural plasticity, and survival are mainly produced by astrocytes. In addition, both preclinical and clinical studies have demonstrated that such factors play important roles in the pharmacological effects of antidepressants.1-3 Glial cell line-derived neurotrophic factor, a member of the transforming growth factor β superfamily, is decreased in the peripheral blood of patients with major depressive disorder (MDD),4, 5 whereas antidepressant treatment of MDD patients increases blood levels of GDNF.6 As an alternative (or in addition) to the presumed mechanism of action of antidepressants, neurotransmitter reuptake inhibition, modulating GDNF production could underlie the therapeutic effect of antidepressants. Therefore, understanding the mechanism of antidepressant-induced production of GDNF by astrocytes could provide novel insights into the pathophysiology of MDD, an alternative mechanism of action of antidepressants and possible treatments for MDD based on a novel mechanism.
Distinct classes of antidepressants, such as tricyclic, tetracyclic antidepressants, and selective 5-HT reuptake inhibitor (SSRI), increase GDNF production in rat C6 glial cells (C6 cells) and primary cultured rat astrocytes (primary astrocytes), but not in primary neurons.7, 8 In addition, the antidepressant amitriptyline increased GDNF production via Gαi/o-coupled lysophosphatidic acid receptor 1 (LPAR1)/matrix metalloproteinase-9 (MMP-9)/fibroblast growth factor (FGFR)/ERK cascade.7, 9-12 However, the precise intracellular signaling pathway between LPAR1/Gαi/o and MMP-9 is yet unknown. It has been reported that G proteins regulate MMPs via intracellular signaling molecules, such as Src family tyrosine kinase, calcium (Ca2+) ions, and protein kinase C (PKC).13 A previous report showed that ERK activation, an important downstream step in astrocytic GDNF production, induced by amitriptyline was not inhibited by Ca2+ chelators and PKC inhibitors.7 The Src family tyrosine kinase belongs to nonreceptor protein tyrosine kinases and is widely expressed in mammalian cells, including astrocytes.14, 15 Furthermore, Src family tyrosine kinase and MMP-9 modulate FGFR functioning via LPAR1/Gαi/o activation, a FGFR transactivational mechanism. In this mechanism, Src family tyrosine kinase and MMP-9 are thought to mediate “crosstalk” between LPAR1, a G protein-coupled receptor (GPCR), and FGFR, a receptor tyrosine kinase (RTK) by the shedding of RTK ligands, such as FGF2, and following activation of RTK downstream signaling, such as ERK cascade.13, 16 Therefore, the Src family tyrosine kinase could be a crucial link between LPAR1/Gαi/o and MMP-9. The current study attempted to elaborate the involvement of Src family tyrosine kinase in MMP-9 activation, which could be key in the upregulation of GDNF mRNA expression in rat astroglial cells following antidepressant treatment.
2 METHODS
2.1 Reagents
Drugs were obtained from the following sources: amitriptyline (Wako Pure Chemical Industries, Ltd., Osaka, Japan); PP1 (Sigma-Aldrich Co., St. Louis, MO); pertussis toxin (PTX), NF449, PP2, and PP3 (Calbiochem, San Diego, CA); Ki16425 (Cayman, Ann Arbor, MI); AM966 (Medchem Express, Monmouth Junction, NJ); H2L5186303 (Tocris Bioscience, Minneapolis, MN); and MMP-9 inhibitor (Abcam Biochemicals., Cambridge, UK). YM-254890 was a gift from Taiho Pharmaceutical Co., Ltd. (Tokyo, Japan).
2.2 Cell culture
Preparation of C6 cells, a rat astrocytic cell line, has been described previously.12 For drug treatment, the medium was replaced with serum-free Opti-MEM (Invitrogen, Carlsbad, CA) containing 0.5% bovine serum albumin (Sigma-Aldrich Co.), and cells were then treated with various drugs of interest. In the current study, C6 cells were incubated in 25 μmol/L of amitriptyline as previous reports indicated that 25 μmol/L amitriptyline is not toxic to C6 cells when increased for 24 hours8 and to primary astrocytes increased for 48 hours.7 Since antidepressants accumulate in the brain at concentrations approximately 30-fold higher than that in blood (0.36-0.9 μmol/L) because of their highly lipophilic properties,17, 18 the in vitro concentration of amitriptyline in the current study mirrored the clinical concentration. In vitro concentrations of drugs and incubation times were based on previous reports.9-11, 19
2.3 Western blotting
Western blotting was performed as previously described19 using the following antibodies: phospho-Src family (Tyr416) (D49G4) rabbit mAb (for phospho-Src family tyrosine kinase: p-Src) (Cell Signaling Technology, Beverly, MA) and anti-Src antibody (clone GD11; Merck KGaA, Darmstadt, Germany). C6 cells were collected in ice-cold phosphate-buffered saline (PBS) and solubilized in sample buffer (100 mmol/L Tris-HCl (pH 6.8), 20% glycerol, 4% SDS). The total amount of protein in each sample was normalized. After the addition of 1,4-dithiothreitol, the samples were boiled for 5 minutes. The proteins were separated by SDS-polyacrylamide gel electrophoresis and transblotted to polyvinylidene difluoride membranes. The membranes were blocked with 10% (w/v) skim milk for 6 hours at 4°C and incubated with respective antibodies overnight at 4°C. After washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature. Chemiluminescent detection was performed using Immun-Star WesternCTM kit (Bio-Rad), and the net intensities of each band were quantified using ChemiDocTM XRS® (Bio-Rad).
2.4 Gelatin zymography
The current study used a previously described method of gelatin zymography.11 After drug treatment, the culture supernatant was collected and centrifuged at 3300 g for 5 minutes. Total protein in each sample was normalized, and samples were mixed with an equivalent amount of sample buffer (0.125 mol/L Tris-HCl (pH 6.8), 10% glycerol, 2% SDS, 0.01% bromophenol blue) and subjected to electrophoresis in 8% SDS-polyacrylamide gels containing 0.1% gelatin under nonreducing conditions. Gels were washed for 1 hour in wash buffer (2.5% Triton X-100) to remove SDS and further incubated for 16-24 hours in incubation buffer (50 mmol/L Tris-HCl (pH 7.4), 10 mmol/L CaCl2, 0.02% NaN3) at 37°C. Gels were then stained for 3 hours in gel staining solution (0.125% Coomassie Brilliant Blue R-250, 30% methanol, and 10% acetic acid) and destained in gel destaining solution (40% methanol/7% acetic acid) until clear bands of gelatinolysis appeared on a dark background. Band intensity was analyzed with ChemiDocTM XRS® (Bio-Rad). Strictly speaking, MMP-9 band intensity does not provide an absolute measure of total MMP-9 activity because of the absence of their endogenous inhibitors, such as tissue inhibitors of metalloproteinases that are expressed in astrocytes.20 Although 92 kDa MMP-9 is actually the pro-peptide, it has been reported that pro-MMPs, like that MMPs themselves, have catalytic activity.21 Therefore, the current study regarded MMP-9 band intensity as “zymographic MMP-9 activity.”
2.5 RNA extraction
For the collection of total RNA, C6 cells were cultured at a density of 1.6 × 105/cm2 on a 6-well plate with 3 mL of growth medium. After drug treatment, total RNA was isolated using a RNeasy Mini kit (Qiagen, Valencia, CA) following the manufacturer's protocols. RNA quantity and purity were determined with the MultiSpectrophotometer (Dainippon Sumitomo Pharma Co. Ltd., Osaka, Japan).
2.6 Real-time RT-PCR assay
First strand cDNA was synthesized from 500 ng of total RNA using the PrimeScript® RT Master Mix (Takara Bio., Shiga, Japan). The cDNA was used as a template for real-time RT-PCR assay that was performed with a Thermal Cycler Dice II Real Time System (Takara Bio.), using TaqMan probes and primers for rat GDNF (TaqMan® Gene Expression Assays: Rn00569510_m1) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Rn99999916_s1) (Applied Biosystems). The reaction conditions and analysis have been described elsewhere.12
2.7 Data analysis
Statistical analyses were performed using SPSS (SPSS, Chicago, IL). The results are expressed as the mean ± SEM. One-way analysis of variance was used to compare the effects of treatments. Differences between the groups were analyzed by Tukey's honest significant difference and Dunnett's test. The significance level was set at P < 0.05.
3 RESULTS
3.1 Amitriptyline increases Src family tyrosine kinase phosphorylation in C6 cells
Treatment of C6 cells with amitriptyline increased phosphorylation levels of Src family tyrosine kinase (between 50 and 60 kDa) over time, beginning at 5 minutes, with statistically significant increases at 10 and 30 minutes (Figure 1A). Beyond 30 minutes, however, no further increase in Src family tyrosine kinase phosphorylation was observed. In addition, amitriptyline increased Src family tyrosine kinase phosphorylation in a concentration-dependent manner, with statistically significant increases at 25, 50, and 100 μmol/L (Figure 1B).
3.2 Src family tyrosine kinase inhibition blocked amitriptyline-induced GDNF mRNA expression in C6 cells
Pretreatment with either PP1 or PP2, selective Src family tyrosine kinase inhibitors, blocked amitriptyline-induced GDNF mRNA expression (Figure 1C). By contrast, PP3, a PP2 negative control, had no effect on amitriptyline-induced GDNF mRNA expression. In vitro concentrations of Src family tyrosine kinase inhibitors and incubation times were based on a previous report, which showed block of Src family tyrosine kinase in C6 cells.19
3.3 Src family tyrosine kinase inhibition blocked amitriptyline-induced zymographic MMP-9 activation
Pretreatment of C6 cells with either PP1 or PP2 blocked amitriptyline-induced zymographic MMP-9 activation (Figure 2A). By contrast, PP3 had no effect on amitriptyline-induced zymographic MMP-9 activation.

3.4 G protein αi/o subunit inhibition and LPAR1 inhibition blocked amitriptyline-induced zymographic MMP-9 activation
C6 cells were incubated with G protein α-subunit inhibitors and LPAR inhibitors. Amitriptyline-induced zymographic MMP-9 activation was completely inhibited by pertussis toxin (PTX), a Gαi/o inhibitor. By contrast, neither NF449, a Gs inhibitor, nor YM-254890, a Gq inhibitor, had any effect on zymographic MMP-9 activity in C6 cells (Figure 2B). Furthermore, amitriptyline-induced zymographic MMP-9 activation was completely inhibited by Ki16425, a LPAR1/3 inhibitor, and AM966, a selective LPAR1 inhibitor. By contrast, H2L5186303, a selective LPAR2 inhibitor, had no effect on zymographic MMP-9 activity in C6 cells (Figure 2C).
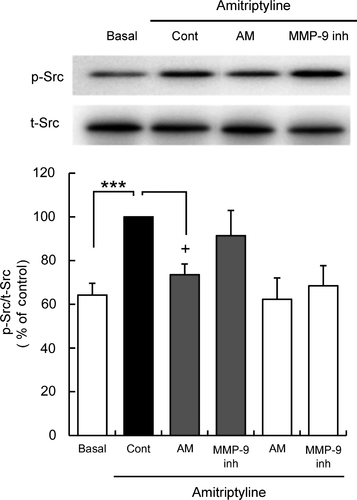
3.5 LPAR1 inhibition but not MMP-9 inhibition blocked amitriptyline-induced Src family tyrosine kinase phosphorylation in C6 cells
Amitriptyline-induced Src family tyrosine kinase phosphorylation was blocked by a selective LPAR1 inhibitor AM966, but not MMP-9 inhibitor (Figure 3).
4 DISCUSSION
It has been proposed that the pharmacological effect of antidepressants such as amitriptyline is, in part, mediated by astrocytic expression of trophic factors such as GDNF. The current study further elaborated a cellular cascade by which amitriptyline increases GDNF mRNA expression, from activation of astroglial cell surface receptor LPAR1 through MMP-9 activation. Amitriptyline, through Gαi/o-dependent LPAR1, activates Src family tyrosine kinase which, in turn, activates zymographic MMP-9 activity, which, in turn, leads to the upregulation of GDNF expression. Thus, the current findings indicate that amitriptyline activates a LPAR1/Gαi/o/Src family tyrosine kinase/MMP-9 cascade in astroglial cells, which could be a novel intracellular mechanism that underlies of the effects of antidepressants.
Beyond its other well-characterized physiological roles, the LPAR1 could also have a key role in the pathophysiology of MDD22 and the pharmacological effect of antidepressants. The endogenous ligand for LPAR1 induces GDNF expression. Blocking LPAR1 expressed in C6 cells with LPAR1 antagonists or knockdown of LPAR1 gene expression in prevented amitriptyline-evoked GDNF expression.10 Amitriptyline, and other classes of antidepressants, significantly and selectively increased zymographic MMP-9 activity, a key step in the upregulation of GDNF.11 In the current study, the nonselective LPAR1/3 antagonist Ki16425 and selective LPAR1 antagonist AM966 blocked amitriptyline-induced activation of MMP-9. The effect of amitriptyline on MMP-9 is most likely mediated by LPAR1, as siRNA knockdown of LPAR3 did not affect GDNF expression.10 Thus, the current findings support previous findings of a role of LPAR1 in amitriptyline-induced expression of GDNF.
While MMP-9 activation is a crucial step in the upregulation of GDNF, the cellular signaling pathway between LPAR1 activation and MMP-9 activation has yet to be characterized. G proteins are associated with LPAR1.23 A previous study showed that antidepressants rapidly increased G protein activity in C6 cells, which was PTX-sensitive to and intensive to other G protein inhibitors.9, 10 The present study showed the involvement of a PTX-sensitive G protein, the Gαi/o subunit in amitriptyline-induced MMP-9 activation, which confirms previous findings of Gαi/o subunit involvement in MMP-9 activation.
Activated G protein α-subunits are involved in the phosphorylation of tyrosine kinases, including the Src family tyrosine kinase.24, 25 The Src family tyrosine kinase, a family of nonreceptor-type tyrosine kinases, includes at least nine members with significant amino acid sequence homology (Src, Frk, Yes, Fyn, Fgr, Hck, Lck, Blk, and Lyn), and these kinases share a common domain structure that includes the catalytic domain.26 Five of these tyrosine kinases, Src, Yes, Fyn, Lck, and Lyn, are expressed in the mammalian CNS, including within astrocytes, and are known to play important roles in regulating cell proliferation and differentiation.25 Furthermore, Fyn (59 kDa) and Lyn (53/56 kDa) are found in C6 cells27 and Lyn is found in human astrocytes and rat primary astrocytes.28, 29 The current study confirms that phosphorylation of Src family tyrosine kinase is a crucial intracellular step that leads to amitriptyline-induced GDNF expression in C6 cells and it is possible that Lyn contributes to the intracellular signaling pathway of GDNF expression. The particular Src family tyrosine kinase subtype, however, has yet to be identified. In the current study, the amitriptyline-sensitive phospho-Src family tyrosine kinase band was shown merged with the total Src family tyrosine kinase band (between 50 and 60 kDa). The anti-phospho-Src antibody and anti-Src antibody appear to be cross-reactive with other Src family tyrosine kinase members, including Hck (59/61 kDa), Lck (56 kDa), Yes (60 kDa), Fyn, and Lyn. Further studies will be needed to identify the specific Src family tyrosine kinase subtype that is phosphorylated to antidepressants with anti-Lyn antibodies or selective Lyn inhibitors.
A previous study demonstrated that amitriptyline treatment did not change MMP-9 mRNA expression even though MMP-9 activity was significantly increased,11 indicating that amitriptyline increased the release of MMPs already expressed in C6 cells rather than increasing MMP gene expression. Matrix metalloproteinase-9 is intracellularly synthesized and secreted to the extracellular matrix.30 The intracellular distribution and transport of MMP-9 in astrocytes are vesicularly mediated, co-localizing with motor proteins along the cytoskeleton, and secreted upon stimulation.31 For example, Lyn, a member of Src family tyrosine kinase, rapidly translocates to the plasma membrane following LPAR stimulation.32 In addition, concurring with the present findings, it has reported that Src family tyrosine kinase is involved in MMP-9 release.33 Thus, it is possible that amitriptyline affects LPAR1/Gαi/o and increases Src family tyrosine kinase, such as Lyn, phosphorylation near the membrane, which might promote the release of MMP-9 in astroglial cells.
The current in vitro study showed that amitriptyline induced rapid Src family tyrosine kinase activation, within 30 minutes, and GDNF mRNA expression, within 3 hours. A long duration of GDNF release, over a period of at least 48 hours, has been observed with amitriptyline treatment.7, 34 The early in vitro effects on Src family tyrosine kinase observed following amitriptyline treatment suggests the early acute intracellular signaling mechanism necessary to initiate GDNF expression. Thus, repeated treatment of amitriptyline would be needed in order to sustain steady-state level of GDNF. Neurotrophic/growth factors, such as GDNF, support neurogenesis, gliogenesis, neural plasticity and survival in the brain.1, 2 Long-term expression of GDNF with long treatment with amitriptyline could promote neuronal survival and protect neurons from the damaging effects of stress, thereby reversing the effects of depression on brain structure. Chronic unpredictable stress (CUS)-induced depression-like behavior and decreased GDNF expression in rat hippocampus, which were reversed with 3 weeks of clomipramine, a tricyclic antidepressant.35 Clinical studies have shown decreased GDNF levels in the peripheral blood of patients with MDD,4 and GDNF levels were significantly increased after a few weeks of antidepressant treatment6 and electroconvulsive therapy (ECT).36 It appears that a course of antidepressant therapy in general increases GDNF expression. While GDNF levels appear to be increased in vivo with long-term antidepressant therapy, the acute time course of GDNF expression is not known and whether early expression of GDNF is associated with a therapeutic effect is also not known. If GDNF expression is a key mediator of the therapeutic effects of amitriptyline and other treatments, then this could in part explain the need for long-term treatment courses, rather than acute courses.
The present study demonstrated that Src family tyrosine kinase phosphorylation, via Gαi/o-dependent LPAR1 activation, mediates amitriptyline-induced MMP-9 activation in astroglial cells. Activation of MMP-9, in turn, leads to the upregulation of GDNF, the trophic factor that, in part, underlies the therapeutic effect of antidepressants. The LPAR1/Gαi/o/Src family tyrosine kinase/MMP-9 cascade could be a mechanism of action underlying other antidepressants as many of the cellular components described in the current study have been observed in other antidepressants.9-11 The currently elaborated signaling pathway could be utilized to understand the pathophysiology of MDD from an alternate perspective as well as to develop antidepressants with a novel mechanism of action.
ACKNOWLEDGMENTS
This work was supported in part by JSPS KAKENHI grant numbers JP18H02756, JP18K07620, and JP18K15534, AMED grant number 18059511, and a grant from the Takeda Science Foundation. We wish to thank Dr. Aldric Hama for careful editorial assistance.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DATA REPOSITORY
Raw data are shown in Supporting Information (Data S1).
AUTHOR CONTRIBUTION
HA, NK, MOT, and MT conceived and designed the experiments. HA, NK, and MOT performed the experiments and analyzed the data. NK, MOT, WO, MY, and MT revised the paper critically for important intellectual content. HA, NK, MOT, WO, MY, and MT wrote the paper.