A novel perspective in stroke neuroprotection: Leveraging hypoxic pocket insights for studying preconditioning and postconditioning
Managing Editor: Ningning Wang
Abstract
This perspective article delineates the significant role of hypoxic pockets—localized, transient reductions in cerebral oxygenation—and their implications for stroke neuroprotection strategies. It posits that preconditioning and postconditioning, through interventions like isoflurane, exercise, remote limb ischemic conditioning, can mitigate these hypoxic pockets, potentially protecting the brain against ischemic events. These strategies exploit the brain's intrinsic adaptive capabilities to resist ischemic damage, underscoring a novel avenue for enhancing recovery and prevention efforts. The study emphasizes the need for further exploration into optimizing these interventions to harness their full potential in combating stroke's debilitating effects, marking a pivotal shift towards targeted neuroprotective measures focused on cerebral microenvironmental optimization.
Highlights
-
Explores the critical role of localized reductions in brain oxygenation, challenging traditional views and enabling targeted stroke interventions.
-
Highlights exercise's role in improving cerebral blood flow and oxygenation, reducing hypoxic pockets, and aiding stroke recovery.
-
Demonstrates isoflurane's effectiveness in enhancing blood flow and oxygen delivery, mitigating hypoxic pockets.
-
Shows how sensory-induced increases in blood flow and oxygenation, like whisker stimulation, can reduce hypoxic pockets, offering a nonpharmacological neuroprotective approach.
-
Discusses how strategies like exercise and isoflurane exposure and remote limb ischemic conditioning enhance the brain's resilience to ischemic damage.
1 INTRODUCTION
Stroke, a leading cause of disability, drastically impacts lives and healthcare systems. Preconditioning and postconditioning are innovative strategies to protect the brain from ischemic damage by applying mild stressors before or immediately after a stroke, triggering protective mechanisms.1-4 In this review article, we highlight hypoxic pockets, which are defined as localized, transient zones of reduced oxygenation within the brain, which are not uniformly distributed but instead occur sporadically in certain microenvironments. These pockets are typically short-lived, lasting only a few seconds to minutes, and are characterized by sharp reductions in oxygen levels that can lead to temporary neuronal stress. The formation of hypoxic pockets is influenced by microcirculatory changes and can be mitigated by interventions that enhance blood flow and oxygen delivery.5 We provide novel perspective that addresses these hypoxic pockets through preconditioning and postconditioning could significantly advance stroke recovery and prevention efforts, representing a new direction in neuroprotection research.
2 A NEW FRONTIER IN STROKE RESEARCH: DEFINE HYPOXIC POCKETS AND THEIR SIGNIFICANCE IN THE CEREBRAL MICROENVIRONMENT
Hypoxic pockets represent a critical and newly recognized aspect of stroke research, shedding light on the intricate and dynamic nature of oxygen distribution within the brain's microenvironment. These pockets are defined as localized, transient areas of reduced oxygen levels within the brain tissue, distinct from the broader cerebral oxygen landscape.5 Their discovery is pivotal, as they challenge the conventional understanding of cerebral oxygenation, which has traditionally been viewed as relatively homogenous across the brain's cortex.
The significance of hypoxic pockets lies in their potential impact on neuronal health and function. In the delicate balance of the brain's oxygen supply and demand, even brief periods of reduced oxygenation can have profound effects on cellular processes.6 Neurons, in particular, are highly sensitive to changes in their microenvironment, and inadequate oxygen supply can lead to cellular stress, dysfunction, or even death.7 This sensitivity makes the study of hypoxic pockets especially relevant in the context of stroke, where disruptions to cerebral blood flow can exacerbate the formation and impact of these oxygen-deprived zones.
Understanding the mechanisms underlying the formation of hypoxic pockets, as well as their spatial and temporal characteristics, is crucial for developing targeted interventions aimed at mitigating their occurrence and minimizing their detrimental effects on brain tissue. Moreover, elucidating the role of hypoxic pockets in the pathophysiology of stroke and their potential as therapeutic targets can pave the way for novel approaches to stroke prevention, treatment, and recovery.
2.1 Major findings about hypoxic pockets
This recent study marks a significant advancement in our comprehension of oxygen dynamics within the cerebral cortex, focusing on a newly identified phenomenon termed “hypoxic pockets.”5 These localized, transient reductions in cerebral oxygenation were visualized using a cutting-edge method involving a genetically encoded bioluminescent oxygen indicator, Green enhanced Nano-lantern (GeNL).8 This novel approach allowed for real-time observation of oxygen dynamics in the cortex of awake, behaving mice, revealing the spontaneous formation of hypoxic pockets characterized by sharply defined borders and rapid onset and resolution.
These hypoxic pockets are defined by their distinctive negative amplitude in pO2, suggesting areas within the cortex that temporarily experience reduced oxygen levels. Throughout a 20-min observation period in anesthetized mice, an average of 200 ± 22 hypoxic pockets were detected, covering a small but significant portion of the field of view. The findings suggest that these pockets are not random but occur in specific areas, often reappearing in the same locations and displaying a near-circular shape.
Crucially, the study posits that these hypoxic pockets result from changes in the microcirculation, particularly interruptions in capillary blood flow. This hypothesis is supported by intrinsic optical spectroscopy imaging (IOSI) data, which indicate that fluctuations in total hemoglobin concentration within the cortex mirror the dynamics of hypoxic pockets, thereby linking them directly to cerebral blood volume and flow. Additionally, the study finds that behavioral states such as exercise and arousal significantly mitigate the presence and impact of these hypoxic pockets, suggesting a dynamic interplay between physiological state and cortical oxygenation.
By uncovering the existence and characteristics of hypoxic pockets, this research opens new avenues for exploring brain physiology under various conditions and investigating the significance of oxygen tension in both normal brain function and neurological diseases. This breakthrough contributes substantially to our understanding of how transient disruptions in oxygen supply can affect the brain, providing a novel perspective on cerebral oxygen dynamics and their implications for health and disease.
2.2 Isoflurane attenuates the formation of hypoxic pockets through induced vasodilation and improved oxygen delivery
The study reveals that isoflurane, a commonly used anesthetic, has the capability to attenuate the formation of hypoxic pockets within the brain's cortex.5 This effect is largely attributed to isoflurane's property of inducing vasodilation, thereby enhancing cerebral blood flow and oxygen delivery to brain tissues.9, 10 Vasodilation, the widening of blood vessels, increases the blood volume that can flow through the vessels, ensuring that areas of the brain receive adequate oxygen to meet their metabolic needs. This mechanism of action is critical in preventing or reducing the occurrence of hypoxic pockets, areas within the brain that temporarily experience reduced oxygen levels.
By improving oxygen delivery and distribution across the brain, isoflurane helps maintain a more uniform oxygenation status within the cerebral cortex, mitigating the conditions that lead to the formation of hypoxic pockets.5 The presence of these pockets is associated with transient reductions in cerebral oxygenation, which could potentially impact neuronal function and overall brain health. Therefore, the use of isoflurane in a preconditioning context, or even during surgery, could play a crucial role in preserving cerebral oxygenation and protecting the brain against the formation of hypoxic areas.
2.3 The role of exercise in enhancing cerebral blood flow and oxygenation, reducing the prevalence of hypoxic pockets
The study illustrates the impact of exercise on the dynamics of hypoxic pockets within the brain, emphasizing its role in enhancing cerebral oxygenation.5 Specifically, the research findings indicate that increased physical activity, exemplified by mice voluntarily running on a Styrofoam sphere, leads to a notable reduction in the occurrence and size of hypoxic pockets. These hypoxic pockets are transient areas of reduced oxygenation that could potentially impair neuronal function and overall brain health.
The reduction in hypoxic pockets through exercise is attributed to improved cerebral blood flow and oxygenation, mechanisms essential for maintaining the brain's metabolic demands.11-13 This outcome suggests a protective effect of regular physical activity against the development of conditions associated with cerebral hypoxia, including cognitive impairments and other neurodegenerative diseases.
The exercise regimen in our study, specifically voluntary running on a Styrofoam sphere, effectively enhanced cerebral blood flow and reduced the formation of hypoxic pockets. Aerobic exercises, such as treadmill running or swimming, are likely to be particularly beneficial in this context. It is important to clarify that the hypoxic pockets described are localized and transient, with subtle effects on oxygenation that do not lead to significant functional impairments, such as limb paralysis. Therefore, these pockets are unlikely to interfere with the ability to perform the exercises mentioned.
2.4 Sensory-induced increases in local blood flow and oxygenation can diminish hypoxic pockets, providing insights into nonpharmacological interventions for neuroprotection
The paper details an intriguing aspect of cerebral physiology, showcasing how sensory stimulation, exemplified by whisker stimulation in mice, significantly impacts the formation and resolution of hypoxic pockets within the brain.5 This form of sensory-induced activation is associated with increases in local blood flow and oxygenation in the stimulated regions, directly contributing to the diminishment of hypoxic pockets. These findings are particularly notable as they provide a mechanistic insight into how sensory stimulation could serve as a nonpharmacological intervention for neuroprotection.
The sensory stimulation, in this case through whisker activation, is believed to enhance neurovascular coupling – a crucial mechanism where increased neuronal activity leads to a rise in local blood flow to meet the elevated metabolic demands.14, 15 This improved blood flow not only ensures adequate oxygen delivery to active brain regions but also potentially mitigates the formation or severity of hypoxic pockets by ensuring a more uniform distribution of oxygen across the brain tissue.
The study's findings on whisker stimulation suggest that other sensory regions, such as the visual or auditory cortex, could similarly influence hypoxic pocket formation due to the general principles of neurovascular coupling. Neurovascular coupling, which enhances blood flow in response to neural activity, is a common mechanism across sensory modalities. While whisker stimulation has demonstrated efficacy, the potential for visual or auditory stimulation to reduce hypoxic pockets warrants further investigation. Future research should explore these possibilities to expand nonpharmacological approaches for neuroprotection.
3 FROM INSIGHTS TO INTERVENTIONS: PRECONDITIONING AND POSTCONDITIONING AGAINST STROKE
We speculate that the rationale for employing preconditioning and postconditioning strategies against stroke is deeply rooted in their potential to influence the formation and resolution of hypoxic pockets within the brain. This innovative approach is based on the understanding that modulating these transient zones of reduced oxygenation could significantly impact cerebral health and resilience to ischemic events.
3.1 The role of isoflurane conditioning and other anesthetics in modulating hypoxic pockets and stroke outcomes
Isoflurane, a commonly used anesthetic, has been shown to induce vasodilation, thereby enhancing cerebral blood flow and oxygen delivery.9 Further exploration into the protective mechanisms of isoflurane reveals its role in not only mitigating the direct effects of stroke but also in influencing downstream cellular and molecular pathways that contribute to stroke outcomes.16-20 These include the regulation of inflammatory responses, modulation of apoptosis, and the preservation of blood-brain barrier integrity, each contributing to the compound's overall neuroprotective profile.
As isoflurane has been recognized for its potential in preconditioning the brain against ischemic stroke through mechanisms that enhance cerebral blood flow and oxygen delivery. Its capacity to induce vasodilation stands at the core of its neuroprotective effects, contributing to a reduction in the formation or severity of hypoxic pockets within the brain's microenvironment. These hypoxic pockets, areas of transient reduced oxygenation, are critical targets in the context of ischemic stroke, where their presence can exacerbate neuronal damage and functional outcomes.5
In the pursuit of effective neuroprotective strategies against ischemic stroke, isoflurane's ability to attenuate the formation and impact of hypoxic pockets,5 if it is true, offers a compelling avenue for research and clinical application. By leveraging its vasodilatory effects to enhance cerebral oxygenation preemptively, isoflurane preconditioning represents a promising approach to reducing the incidence and severity of ischemic damage, underscoring the importance of further investigations to fully harness its potential in stroke neuroprotection.
In addition to isoflurane, other anesthetics may also significantly impact cerebral physiology in the context of hypoxic pockets and stroke. Sevoflurane induces vasodilation and enhances cerebral blood flow, reducing infarct size and improving neurological outcomes through antiapoptotic, antioxidant, and anti-inflammatory mechanisms.21 Propofol, with its antioxidant properties and GABAergic enhancement, mitigates oxidative damage and excitotoxicity, supporting brain tissue protection.22 Dexmedetomidine, an α2-adrenergic agonist, enhances cerebral blood flow autoregulation and reduces inflammatory cytokine production, decreasing neuronal apoptosis and infarct volume.23, 24
In essence, in addition to isoflurane, as other anesthetics such as sevoflurane, propofol, and dexmedetomidine, also enhance cerebral blood flow, reduce oxidative stress, modulate inflammatory responses, and inhibit excitotoxicity, they may contribute to the attenuation of hypoxic pockets and improved outcomes in ischemic stroke. Incorporating these anesthetics into preconditioning and postconditioning strategies offers a comprehensive approach to stroke neuroprotection, highlighting the importance of a multifaceted therapeutic regimen.
3.2 Exercise conditioning
Similarly, exercise has been recognized for its role in promoting cerebral oxygenation and vascular function.5, 11-13 Regular physical activity can lead to improvements in neurovascular coupling and blood-brain barrier integrity, crucial factors in maintaining optimal cerebral blood flow and oxygen delivery. As such, exercise serves as a natural preconditioning method that prepares the brain to better handle the challenges of ischemic conditions, likely through its ability to mitigate the formation of hypoxic pockets.
The study highlights the significant role of exercise in promoting cerebral blood flow and oxygenation, which in turn contributes to the reduction in the prevalence of hypoxic pockets within the brain.5 The mechanism through which exercise reduces the prevalence of hypoxic pockets is multifaceted. Primarily, it involves the upregulation of various factors that contribute to vasodilation and the formation of new blood vessels (angiogenesis) in the brain.25, 26 This increase in vascularization ensures a more consistent and effective distribution of oxygen throughout the brain, mitigating the conditions that lead to the formation of hypoxic pockets.
Furthermore, regular exercise has been shown to improve the brain's ability to regulate blood flow in response to changes in metabolic demand—a process known as neurovascular coupling.27 By enhancing this critical function, exercise helps the brain more effectively match oxygen supply with its metabolic needs, further reducing the risk of developing hypoxic pockets. Exercise also contributes to overall cardiovascular health, which is intrinsically linked to brain health.28-31 A healthier cardiovascular system can pump blood more efficiently, supporting the brain's need for continuous oxygen supply and nutrient delivery. By highlighting the link between exercise, enhanced cerebral blood flow, and the reduction of hypoxic pockets, we argue for the role of exercise in mitigating risks associated with cognitive impairments and promoting overall brain resilience in stroke.
3.3 Remote limb ischemic conditioning (RLIC)
RLIC is a noninvasive procedure that involves inducing brief, controlled periods of ischemia in a limb, followed by reperfusion.32 This is typically achieved by inflating and deflating a blood pressure cuff placed around the upper arm or thigh. The standard protocol often includes cycles of 5 minutes of ischemia (cuff inflation) followed by 5 minutes of reperfusion (cuff deflation), repeated three to four times. This procedure can be performed before (preconditioning), during (perconditioning), or after (postconditioning) a stroke event.
RLIC is believed to confer neuroprotection by triggering systemic protective responses that enhance cerebral blood flow, reduce inflammation, and improve oxygen delivery to the brain. Implementing RLIC in clinical and experimental settings has shown promise in reducing the severity of ischemic brain injury and improving outcomes, making it a valuable adjunctive strategy in stroke management.33, 34
The exact mechanisms are still under investigation, but they likely involve both humoral and neural mechanisms. The humoral mechanisms involve the release of protective factors such as adenosine, bradykinin, and opioids into the bloodstream.35 These factors can cross the blood-brain barrier and trigger protective pathways in the brain, reducing inflammation, oxidative stress, and apoptosis. Additionally, RLIC promotes the release of endothelial nitric oxide, which enhances cerebral blood flow and oxygen delivery.
The neural mechanisms involve the activation of afferent nerves in the limb during ischemia, which sends signals to the central nervous system.36 This neural activation triggers a cascade of protective responses, including the release of neuroprotective mediators and modulation of the autonomic nervous system. These responses help to stabilize cerebral blood flow, reduce excitotoxicity, and enhance the brain's resilience to ischemic injury.
We speculate that RLIC also contribute to neuroprotection by mitigating formation of hypoxic pockets, as its similarity compared to whisker stimulation. The study indicates that whisker stimulation, as a form of sensory-induced cerebral activation, highlights the capacity of nonpharmacological interventions to modulate cerebral blood flow and oxygenation.5 The increase in local blood flow and oxygenation associated with whisker stimulation suggests that targeted sensory activation could similarly serve as a preconditioning or postconditioning strategy against stroke. This approach underscores the potential of harnessing the brain's natural responses to sensory stimuli to enhance its resilience against ischemic damage and reduce the occurrence of hypoxic pockets. Similarly, RLIC, by inducing transient ischemia in a peripheral limb, can stimulate systemic protective responses that may enhance cerebral blood flow and oxygenation, offering another avenue for neuroprotection.37-42 This transition from local sensory stimulation to systemic intervention like limb ischemia underscores a broadened understanding of protective mechanisms against cerebral ischemia and hypoxic pockets.
Together, these interventions illuminate a path forward in the development of preconditioning and postconditioning strategies against stroke. By targeting the physiological underpinnings associated with hypoxic pockets, these approaches offer a novel means to bolster the brain's defenses, enhance neuroprotection, and ultimately improve outcomes following stroke. Further exploration into the mechanisms by which these strategies exert their effects is essential for optimizing their application and realizing their full potential in stroke prevention and recovery.
In conclusion, the identification and characterization of hypoxic pockets within the brain offer a novel paradigm for understanding and intervening in the pathophysiology of stroke. By developing preconditioning and postconditioning protocols that specifically target these microenvironmental zones of reduced oxygenation, it is possible to advance the field of stroke neuroprotection, offering hope for significantly improved outcomes for individuals at risk of or recovering from stroke. Further research into the mechanisms, optimal interventions, and clinical applicability of these findings is essential to realize their full potential in stroke care.
4 FUTURE DIRECTIONS IN STROKE NEUROPROTECTION RESEARCH
The identification of hypoxic pockets within the brain's microenvironment may significantly propel the field of stroke pathological and neuroprotective mechanism research, highlighting these areas as pivotal in cerebral ischemia. Based on previous studies, we propose a novel hypothesis that conditioning attenuates brain injury post-stroke by inhibiting the formation of hypoxic pockets (Figure 1). Future research endeavors should be aimed at elucidating the mechanisms through which preconditioning and postconditioning interventions impact these localized oxygen-deprived zones (Figure 1). Such studies hold the promise of not only deepening our understanding of the dynamics surrounding hypoxic pockets but also unveiling new molecular targets and pathways for neuroprotection.43, 44
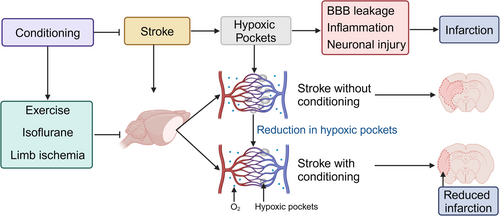
To advance neuroprotection strategies against stroke, future investigations must focus on several key areas. A detailed examination of how various interventions—ranging from pharmacological approaches like isoflurane administration to nonpharmacological strategies including exercise and sensory stimulation—can modulate hypoxic pockets is essential.45 Understanding the cellular and molecular basis behind these interventions will be crucial for enhancing their protective efficacy.46 Moreover, exploring novel molecular targets and signaling pathways triggered by hypoxia in the brain will open up new avenues for therapeutic intervention.
Furthermore, translating these laboratory findings into clinical applications remains a paramount challenge. Bridging this gap will require conducting comprehensive clinical trials to validate the effectiveness of these neuroprotective strategies in human patients, potentially paving the way for personalized treatment plans based on individual risk factors and physiological conditions. Additionally, technological advancements in imaging and monitoring will be instrumental in better understanding hypoxic pockets, offering new diagnostic tools and biomarkers for identifying at-risk patients and evaluating the success of neuroprotective interventions.
By focusing on these critical areas, the scientific community can build upon the foundational knowledge of hypoxic pockets, exploring innovative approaches for stroke prevention and treatment.3 The ultimate aim is to translate these scientific insights into effective, evidence-based strategies that significantly improve patient outcomes following stroke, heralding a new era in the quest for effective stroke neuroprotection.
5 GUIDING CLINICAL PRACTICE: REDUCING THE FORMATION OF HYPOXIC POCKETS AND ACHIEVING CLINICAL TRANSLATION
The clinical application of strategies to reduce the formation of hypoxic pockets in stroke patients can be guided by focusing on three strategies: the use of specific anesthetics, the implementation of RLIC, and the promotion of regular exercise.
Certain anesthetics, such as isoflurane, sevoflurane, and propofol, have been shown to enhance cerebral blood flow and reduce hypoxic pockets through vasodilation and improved oxygen delivery. Clinicians can incorporate these anesthetics into preconditioning and intraoperative protocols to leverage their neuroprotective effects. For example, using sevoflurane during surgeries that pose a risk of ischemic events can help maintain optimal cerebral oxygenation and minimize ischemic injury.47
RLIC involves inducing brief, controlled periods of ischemia in a limb, followed by reperfusion. To translate RLIC into clinical practice, protocols can be developed and standardized for patients at risk of stroke or undergoing procedures with high ischemic potential, and for patients having stroke. Training healthcare providers on the proper technique and timing of RLIC is crucial for its effective implementation.
Regular physical activity has been shown to improve cerebral blood flow and may reduce the occurrence of hypoxic pockets. Clinicians should encourage patients, particularly those at risk of stroke, to engage in regular aerobic exercise. Exercise programs can be tailored to individual patient needs and capabilities, emphasizing activities that enhance cardiovascular health and cerebral oxygenation. Structured exercise programs and rehabilitation protocols can be integrated into clinical practice to support patients in maintaining regular physical activity.
To achieve clinical translations of these strategies, multidisciplinary collaboration and continuous education are essential. Developing clinical guidelines and protocols based on the latest research can help standardize practices. Additionally, integrating these strategies into patient care pathways and rehabilitation programs can facilitate their adoption. Monitoring and evaluating patient outcomes through clinical trials and real-world studies will provide further evidence of efficacy and guide refinements in practice.
6 CONCLUSION
The recent advancements in understanding hypoxic pockets within the brain's microenvironment offer a promising avenue for understanding stroke neuroprotection through innovative preconditioning and postconditioning strategies. These interventions, grounded in modulating cerebral oxygenation and potentially mitigating the formation of hypoxic pockets, hold significant potential for enhancing patient outcomes following stroke.
AUTHOR CONTRIBUTIONS
Heng Zhao: Conceptualization (lead); writing—original draft (lead). Yan Meng: Writing—original draft (supporting). Sijie Li: Writing—original draft (supporting). Guiyou Liu: Writing—original draft (supporting); writing—review and editing (supporting). Xunming Ji: Writing—review and editing (lead).
ACKNOWLEDGMENTS
Images were created using BioRender. The authors have no funding to report.
CONFLICT OF INTEREST STATEMENT
Professor Xunming Ji and Guiyou Liu are members of Neuroprotection editorial board. They are therefore excluded from the peer-review process and all editorial decisions related to the publication of this manuscript.
ETHICS STATEMENT
Not available.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in this article.




