Mass spectrometry-based proteomics for biomarker discovery in the Drosophila model of Parkinson's disease
Managing Editor: Lili Wang/Ningning Wang
Abstract
Parkinson's disease (PD) is the second most prevalent neurodegenerative disorder worldwide. Despite extensive research, the etiology of both familial and sporadic PD remains unclear. While most PD cases are sporadic, a significant minority are linked to genetic mutations, notably in the synuclein-alpha (SNCA) and leucine-rich repeat kinase 2 (LRRK2) genes. Animal models, such as Drosophila melanogaster (D. melanogaster), enable detailed study of these genetic mutations and their neurotoxic effects. Recent advancements in mass spectrometry-based proteomics have enhanced our understanding of PD by facilitating comprehensive analysis of protein expression and interactions in mutant and wild-type organisms, potentially revealing novel therapeutic targets. This review highlights the pivotal role of mass spectrometry-based proteomics in advancing PD research, emphasizing the contributions of D. melanogaster models in identifying potential biomarkers.
Highlights
Significant findings of the study
-
This review identifies Parkinson's disease (PD)-associated biomarkers from mass-spectrometry proteomics studies utilizing the Drosophila melanogaster model, including proteins related to mitochondrial function, autophagy, and synaptic transmission. These findings reveal the molecular mechanisms of PD and suggest potential therapeutic targets.
What this study adds
-
This study compiles potential PD biomarkers from proteomics studies, and explores links between the various pathways. It reveals unique aspects of disease pathogenesis and therapeutic targets, while further validating Drosophila melanogaster as an effective model for PD.
1 INTRODUCTION
Parkinson's disease (PD) is a progressive neurological disorder marked by the gradual deterioration of motor and nonmotor functions.1 It ranks as the second most prevalent neurodegenerative disorder, following Alzheimer's disease, and significantly affects patients from its onset.2 Although bradykinesia, postural instability, and loss of motor control are the most prominent motor symptoms, PD also presents with nonmotor symptoms, such as depression, memory loss, and sleep difficulties, which may even precede the primary motor manifestations.3
The hallmark of PD is the progressive loss of dopaminergic neurons in the nigrostriatal pathway, a process attributed to multiple well-defined pathological mechanisms.4 In PD, there appears to be a disruption in the equilibrium between free oxygen radicals and their scavengers, leading to significant degeneration of dopaminergic neurons.5
Extensive research has been conducted on the role of fibrillar inclusions, also known as Lewy bodies (LBs), in the brains of patients with PD. Discovered over a century ago, LBs have been extensively studied, revealing that presynaptic protein α-synuclein (α-syn) is an essential constituent.6 While most PD cases are sporadic, a small proportion (50%–10%) are hereditary and linked to mutations in at least 20 known genes.7 The role of genetic mutations in the synuclein-alpha (SNCA) gene, which encodes α-syn, is well established. Notable missense mutations, including A53T, A30P, and E46K missense mutations, have been identified in patients with autosomal dominant PD.8
In addition to the SNCA gene, genetic mutations in the leucine-rich repeat kinase 2 (LRRK2) gene are associated with the cardinal features of PD. These mutations alter kinase and GTPase activities, triggering neuronal death signaling pathways through multiple mechanisms of PD pathogenesis.9
Identifying biomarkers and therapeutic targets for PD is challenging due to its complex pathophysiology and heterogeneity.10, 11 Diagnosing and tracking PD progression are hampered by a lack of reliable biomarkers and objective measures.10 Most treatments focus on managing symptoms rather than modifying the disease, and clinical trials are often complex.12 Furthermore, much remains to be understood about the roles of genetic and environmental factors, neuroinflammation, and immune responses in PD.10, 11 Notably, existing animal models do not completely replicate PD in humans. Although stem cell and organoid models hold potential, further development and consideration of these model systems are required.12
Given that direct examination of brain pathology is only feasible through post-mortem samples from patients with PD,4 animal models are essential for gaining deeper insights into this disorder. Various approaches and techniques have been employed to compare PD models with their wild-type counterparts across different temporal stages. A prominent method involves the use of multidimensional liquid chromatography (LC) combined with mass spectrometry (MS) to analyze neural proteome profiles in PD mutant and wild-type model organisms.13
However, multiple challenges exist in proteomics and the PD research analysis of posttranslational modifications (PTMs). The diversity and variability of brain tissues and biofluid samples create hurdles to achieving extensive protein coverage and reliable measurement.14, 15 Additionally, efficient protein extraction and preparation are technically challenging, often resulting in the loss of proteins or PTMs. Inadequate detection of PTMs, such as phosphorylation and ubiquitination, can lead to an incomplete understanding of protein functions and interactions in PD.16-18
Despite these challenges, the application of MS for identifying and quantifying pathological biomarkers has significantly advanced our understanding of various diseases.19, 20 This review focuses on studies utilizing MS-based proteomics to identify and examine various biomarkers for PD using the Drosophila melanogaster (D. melanogaster) model, as well as their consequential involvement in present-day investigations.
2 OVERVIEW OF PD MODELS AND METHODOLOGIES
2.1 Animal models used in PD
PD research has extensively used animal models to facilitate improved therapeutic interventions. These models are typically categorized as toxin-induced PD models or genetic models.21
The most prominent toxins used in these models are 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-hydroxydopamine (6-OHDA). When administered to rats, these neurotoxins induce neuronal degeneration and trigger PD-like symptoms.22 Rats are often preferred over mice due to their larger size, which facilitates surgical procedures, and their more pronounced expression of characteristic PD phenotypes.23
Regardless of which type of rodent is used, these models have certain limitations. For instance, in 6-OHDA-induced rat models, the nonselective targeting of both dopaminergic and non-dopaminergic transporters results in the involvement of other brain regions.24 Additionally, rats exhibit resistance to MPTP toxicity, while mice often fail to develop the typical symptoms of PD.25 A significant limitation common to both toxin-induced models is the absence of LB formation, a crucial biomarker of PD progression.26 As a result, no single animal model has yet been able to accurately replicate the progression and characteristics features of PD in humans.3
2.2 D. melanogaster as a model for PD studies
D. melanogaster has been extensively used in medical and biological research due to its numerous advantages over vertebrate models, including ease of cultivation and cost-effectiveness. In neurological disorder research, D. melanogaster is particularly favored for its affordability, short lifespan, and ability to exhibit genetic mutation phenotypes.27
As discussed, PD involves the overexpression of several genes, which have been extensively studied in fruit fly models.28 Although D. melanogaster models are not as closely analogous to humans as are rodent models, they facilitate the examination of diverse PD-related effects that are less prominent in mice and rats, such as LB formation and the loss of dopaminergic neurons. Additionally, D. melanogaster contains orthologs for approximately 65% of human disease-causing genes,29-31 reducing the need for inserting foreign genes. This facilitates the direct investigation of mutations and other genetic abnormalities in the gene of interest, allowing for the observation of potential changes similar to those seen in vertebrates.32 Given its efficacy in modeling both toxin-induced and genetic variants of PD and its reproduction, D. melanogaster remains a valuable model organism for PD research.33
2.3 MS-based proteomics
MS has become an indispensable tool in proteomics due to its accuracy and resolving power in quantifying a wide range of biological samples.34 The utility of MS in proteomics studies is well established, primarily because of their effective labeling strategies, ease of comparison between replicates, and precise identification and quantification of peptide fragments.13, 34, 35 Over time, the capabilities of modern MS have significantly advanced, enabling the detailed investigation of protein-protein interactions on both global and organellular scales, detection of PTMs, and identification of potential biomarkers.36 These advances have also paved the way for single-cell proteomics, which offers a more accurate representation of the spatial environment surrounding the cell and the precise proportions of proteins and mRNAs within it.36, 37
MS has been employed in numerous proteomic studies over the years, proving especially useful in detecting local and global proteomic changes caused by mutations or at various stages of diseases. This technology has proven invaluable for studying diseases, such as PD, which typically result from abnormalities in one or more genes. Studies such as those conducted by Xun et al.38, 39 have significantly contributed to the widespread use of the D. melanogaster PD model in MS-based proteomics research,38, 39 making it a cornerstone for investigating proteomic alterations associated with PD due to its robust and reproducible outcomes.
Numerous α-syn mutations associated with PD, such as A53T and A30P, have been extensively studied in D. melanogaster. These mutations typically result in LB formation and dopaminergic neuronal loss, providing valuable insights into the mechanisms underlying synucleinopathies.40 Additionally, studies utilizing D. melanogaster have explored the risk factors that accelerate the onset and progression of PD, offering insights into how these factors increase disease susceptibility.41 Beyond replicating the motor manifestations of PD, D. melanogaster models also mimic nonmotor symptoms observed in human patients, such as sleep disorders, learning difficulties, and visual impairment, thus enhancing their applicability in comprehensive PD research.41
The LRRK2 gene is a prominent protein implicated in the pathogenesis of genetic PD. Its D. melanogaster homolog provides researchers ample opportunity to replicate the effects of LRRK2 mutations in humans. LRRK2 mutant models of PD in fruit flies have deepened our understanding of the effects of these mutations and also clarified the functions of the LRRK2 protein itself. The ultimate goal of improving drug development is to leverage the therapeutic doses of various drugs in these models, outlining their pharmacokinetic and pharmacodynamic characteristics.42 Specific genes of interest, including LRRK2, are discussed in detail below.
While several mutations have been associated with PD, independently or in conjunction with other changes, a select few have been identified as critical in most PD cases. Some of the major PD models based on D. melanogaster are listed in Table 1. These genes are the focus of this review, with the most notable findings summarized in Table 2.
| Human gene | Drosophila gene | Inheritance |
|---|---|---|
| SNCA | Not available | Autosomal dominant |
| LRRK2 | Lrrk | Autosomal dominant |
| DJ-1β | Dj-1α Dj-1β |
Autosomal recessive |
| PRKN | Parkin | Autosomal recessive |
| PINK1 | Pink1 | Autosomal recessive |
| GBA | Gba1a Gba1b |
Major risk factor |
- Abbreviations: DJ-1, protein deglycase DJ-1; GBA, glucocerebrosidase; LRRK2, leucine-rich repeat kinase 2; PINK1, PTEN-induced kinase 1; PRKN, parkin RBR E3 ubiquitin protein ligase; SNCA, synuclein alpha.
| Gene investigated | Mutation investigated | Conclusion | Reference |
|---|---|---|---|
| SNCA | Nil | GTP cyclohydrolase and folate metabolism are potential regulators of α-synuclein neurotoxicity | [44] |
| LRRK2 | R1441C | hLRRK2 interacts with various synaptic vesicle proteins, such as synaptojanin-1, which may undergo heightened phosphorylation | [32] |
| LRRK2 | G2019S | LRRK2 regulates the tyrosine hydroxylase-dopamine pathway, resulting in greater levels of dopamine production at the earlier stages, consequently, increased neurodegeneration | [45] |
| LRRK2 | Nil | Neuroaxonal transport of dense core vesicles is directed by LRRK2 and other Parkinson's disease-associated genes | [46] |
| DJ-1ß | Nil | DJ-1ß mutant flies exhibit reduced SERCA activity, which is restored via CDN1163, a specific SERCA activator | [47] |
| PARK7 | Nil | PARK7 helps regulate reactive intermediates from metabolic pathways, notably those involved with carbohydrate metabolism | [48] |
| PINK1 | Pink1B9, Pclc421Su(z)12c253 | PINK1 mutants showcase accelerated deterioration in the regulation of protein synthesis pathways such as those concerned with mitochondria, neurotransmission, etc. | [49] |
| PINK1 | dPINK1B9 | CAT-tailing-like MISTERMINATE events are noted to occur in respiratory chain proteins in mutant PINK1 Parkinson's disease models | [50] |
| PINK1 | Nil | Wild-type PINK1 nullifies the upregulatory effects of LRRK2 mutants on the tyrosine hydroxylase-dopamine pathway, suggesting a natural effect that opposes that of LRRK2 | [45] |
| PINK1 | Nil | PINK1-associated mitochondrial mutations lead to the activation of Relish targets, thus exhibiting age-dependent intestinal dysfunction due to inflammatory signaling | [51] |
| dGba1b | Nil | An enhanced abundance of extracellular vesicles is observed and validated in Gba1b mutants, which is demonstrated to play critical roles in the accumulation and spread of protein aggregates | [52] |
| dGba1b | Nil | Expression of wild-type dGba1b is shown to be associated with a decrease in the levels of ubiquitinated proteins in the Drosophila brain and muscles | [53] |
- Abbreviations: DJ-1, protein deglycase DJ-1; LRRK2, leucine-rich repeat kinase 2; MISTERMINATE, mitochondrial-stress-induced translational termination impairment and protein carboxyl-terminal extension; PARK7, Parkinsonism associated deglycase; PINK1, PTEN-induced kinase 1; SNCA, synuclein alpha.
3 GENETIC MUTATIONS IMPLICATED IN PD
3.1 SNCA
SNCA was the first gene identified to be associated with monogenic PD,54 characterized by its accumulation in the dopaminergic neurons, directly linking it to PD pathogenesis.55 Despite extensive research over decades, the exact mechanism by which SNCA contributes to PD remains elusive. Given that α-syn aggregation leads to the formation of LBs, a hallmark of PD,56 significant resources have been dedicated to elucidating the employed mechanisms. Over the past 25 years, multiple SNCA mutations57 and gene dosage dysregulations have been identified as integral to various forms of PD.58, 59 Notably, SNCA has also been associated with sporadic cases of PD.55, 60
α-Syn, a protein consisting of 140 amino acid residues, is typically highly soluble and predominantly localized in presynaptic regions, where it interacts with lipids and modulates synaptic vesicle exocytosis. Notably, α-syn has a propensity to self-aggregate, forming oligomeric species and LB-like fibrils similar to the insoluble aggregates observed in patients with PD with mutant α-syn.61, 62 These fibrils and their precursor oligomeric species are implicated in neurotoxicity and contribute to neurodegeneration.63 Furthermore, the prion-like characteristics of α-syn have led to various hypotheses about its role in PD pathogenesis.64
3.2 MS-based D. melanogaster studies on SNCA
A pivotal study employed reverse-phase LC-tandem MS (LC-MS/MS) to analyze mutant α-syn in fly heads, revealing significant dysregulation of protein expression across various developmental stages. These findings highlighted notable defects in F-actin regulatory proteins and mitochondrial function during presymptomatic and early disease stages.38 Specifically, dysregulated F-actin regulatory proteins included troponin T, muscle LIM protein at 60 A, and paramyosin. In contrast, mitochondrial proteins, such as manganese-superoxide dismutase and ATP synthase β subunit were similarly affected. In such studies,38, 39 the authors emphasized the importance of elucidating these molecular changes during presymptomatic stages to determine better causation, which may aid in identifying potential biomarkers.
Similarly, A53T α-syn-mutant HEK293 cells and D. melanogaster fly strains were used to demonstrate the significance of Rab7 in facilitating the clearance of α-syn aggregates65 using postmortem substantia nigra tissues obtained from the Brain Bank Center, Würzburg (BrainNet Germany). Rab7 overexpression increased the percentage of mutant HEK293 cells capable of clearing aggregates and improved locomotor functions in the mutant flies.
Likewise, lipid droplets were observed to be positively associated with α-syn accumulation. Specifically, Girard et al.66 reported that lipid droplets induce α-syn resistance to proteolytic digestion in D. melanogaster models, contributing to further protein proliferation. Shotgun MS was used for D. melanogaster retina samples.
Furthermore, integrated proteomic, Gene Ontology enrichment, and comparative analyses using a highly penetrant α-syn D. melanogaster model identified GTP cyclohydrolase (GCH1) and folate metabolism as potential regulators of α-syn neurotoxicity.44 The significance of GCH1 was further validated by observations that transgenic α-syn D. melanogaster Gch1 knockdown exhibited enhanced locomotor and brain metabolic dysfunction. Similarly, folate supplementation exhibits neuroprotective capabilities while partially normalizing brain bioenergetics.44 For MS, the authors utilized a LC-MS3 data collection strategy on an Orbitrap Lumos mass spectrometer (Thermo Fisher Scientific, Cleveland, OH, USA) equipped with a Proxeon Easy nLC 1000 for online sample handling and peptide separations.
3.3 LRRK2
Mutations in LRRK2 are among the most prevalent causes of late-onset autosomal dominant PD, exhibiting clinical attributes similar to those observed in late-onset sporadic PD.67, 68 LRRK2 encodes a large multifunctional protein (approximately 280 kDa) containing two significant enzymatic domains: kinase and GTPase. The kinase domain phosphorylates Rab family GTPases, essential for autophagy and vesicle trafficking. Typically, pathogenic mutations often lead to increased kinase activity, contributing to PD pathogenesis.69, 70 The GTPase domain is vital for regulating cellular signaling through the hydrolyzation of GTP to GDP. The ROC and COR domains mediate this activity. Balance between these processes is crucial for maintaining cellular homeostasis, and disturbances can lead to neurodegenerative diseases.70-72 The prevalence of specific multiprotein interaction domains, including a LRRK2-specific repeat domain, a leucine-rich domain, and a WD40 repeat, has also been observed.73-78 Mutations within enzymatic domains are associated with the mechanistic pathways of the disease.73, 74 The G2019S mutation in the kinase domain is linked with approximately 1% of sporadic late-onset PD and 5%–6% of familial PD globally and is considered the most prominent.79 In most cases, G2019S LRRK2 mutations exhibit LB formation and incomplete penetrance persisting into older age.80 Conversely, GTPase domain mutations, such as R1441 C/G, display variable LB formation and nearly complete penetrance.67, 68 These differences have led to speculation regarding the unique pathways employed by these mutations. Extensive research has suggested that the reciprocal regulation by LRRK2 GTPase and kinase enzyme activities may advance the cellular mechanistic pathways involved in LRRK2 functions.70, 81 LRRK2 has also been associated with processes such as protein translation, vesicle trafficking, mitochondrial function, lysosomal autophagy, and cytoskeletal dynamics.70, 75, 82-85 Despite extensive research, the exact mechanisms by which LRRK2 mutations lead to neurodegeneration in patients with PD remain unclear.
3.4 MS-based D. melanogaster studies on LRRK2
Proteomic studies of the R1441C LRRK2-mutant D. melanogaster have revealed dysregulation of many cytoskeletal, mitochondrial, and synaptic vesicle proteins, including synaptotagmin-1, syntaxin-1A, and Rab3.32 Additional phosphoproteomic analysis highlighted increased phosphorylation of various synaptic vesicle proteins, such as synaptojanin-1, alongside the microtubule-associated protein Futsch in mutant fly brains. The interaction between LRRK2 and synaptojanin-1 was further established using a protein-protein interaction screen.32 LC-MS/MS analysis was performed on fly heads using an EASY nano-LC 1000 coupled to a nano-spray electroionization source and a quadrupole Orbitrap mass spectrometer (QExactive, Thermo Scientific, Waltham, MA, USA).
Similarly, LRRK2 G2019S-mutant D. melanogaster appears to regulate the tyrosine hydroxylase-dopamine pathway, where dopamine production is enhanced during early disease stages, leading to subsequent degeneration of dopaminergic neurons. This effect was reversed upon treatment with α-methyl-L-tyrosine, a tyrosine hydroxylase inhibitor, during the early disease stage.45
In contrast, the loss of LRRK, the D. melanogaster ortholog of human LRRK2, results in the accumulation of Arl8, a lysosome-related organelle regulator, along with dense core vesicles at the most distal boutons of D. melanogaster neuron terminals. The accumulation of Arl8-positive vesicles is dependent on UNC-104 and is regulated by genes associated with PD, such as Auxilin, VPS35, RME-8, and INPP5F, leading to their consideration as upstream regulators of LRRK. These findings indicate that neuroaxonal transport of dense core vesicles is directed by LRRK2 and other PD-associated genes.46
3.5 DJ-1/PARK7
Protein deglycase DJ-1 (DJ-1), encoded by the PARK7/DJ-1 gene, is an antioxidant protein involved in regulating the oxidative environment of dopaminergic neurons.86 DJ-1 is a crucial protein implicated in the mechanisms underlying PD. DJ-1 mutations are often linked with recessive familial PD. Studies using DJ-1 mouse models suggest that a deficiency in this protein is sufficient to induce dysfunctional locomotor activity, even in the absence of nigral neurodegeneration.87, 88 Subsequent research has elucidated the neuroprotective roles of DJ-1 within the central nervous system.89
3.6 MS-based D. melanogaster studies on DJ-1/PARK7
The pathophysiology of PD has been linked to the impact of oxidative stress on crucial modulators and proteins, leading to their modification and disruption. One of such protein is SERCA, an endoplasmic reticulum (ER) Ca2+ channel protein involved in Ca2+ homeostasis, which undergoes significant alterations in PD model flies. Interestingly, DJ-1β-mutant flies exhibit reduced SERCA activity, which can be restored through activation by the specific SERCA activator, CDN1163.47 This finding highlights the role of SERCA in PD pathophysiology and underscores the therapeutic potential of CDN1163 in PD variants associated with SERCA inactivity. The study initially utilized tandem MS/MS to analyze peptide mixtures, with further LC-MS/MS performed for spots that could not be identified. The MS/MS analysis was conducted using 5800 TOF/TOF MALDI (AB Sciex) in the positive reflection mode (3000 shots per position).
Similarly, mutations in the PARK7 gene disrupt mechanisms that mitigate damage from reactive intermediates in metabolic pathways, such as 1,3-bisphosphoglycerate.48 This disruption results in the accumulation of these reactive compounds, potentially causing cellular damage that contributes to PD pathogenesis.
These results have been corroborated in human cell lines, mouse brain tissues, and D. melanogaster models.48 The novel role of PARK7 in sugar-utilizing cells significantly enhances our understanding of the relationship between carbohydrate metabolism and PD,48 unveiling new possibilities for therapeutic targets and potential biomarkers. Metabolites were identified using LC-MS, while peptides were analyzed using nano-LC-MS.
3.7 PRKN
Mutations in the parkin RBR E3 ubiquitin protein ligase (PRKN) gene are associated with approximately 20% of PD cases with unknown origin and 50% of familial cases. Mutant parkin (encoded by PRKN) is one of the most widespread autosomal recessive mutations noted in early-onset PD. Parkin, a ubiquitin ligase, plays a critical role in proteasomal degradation, and mutations typically result in a loss of function,90 leading to a build-up of substrates with neurotoxic features, contributing to parkin-associated PD. Although several parkin knockout models have been studied, their yields have been minimal, with none expressing the classical phenotypes of PD.91 Despite this, numerous studies have demonstrated the neuroprotective role of parkin, including where its overexpression inhibited the degeneration of dopaminergic neurons, indicating potential therapeutic.92
3.8 MS-based D. melanogaster studies on PRKN
Loss-of-function mutations in PRKN are linked to certain familial forms of PD, with a lack of parkin associated with muscle degeneration. Xun et al.93 utilized reverse-phase LC-MS/MS (Q-Exactive, Thermo Fisher) to analyze fly heads, revealing a further association between D. melanogaster parkin nulls and abnormal energy metabolism and protein transporter activity. Certain parkin mutants have also been shown to disrupt interactions with mitochondrial outer membrane targets, autophagy receptors, and general proteasomes.94 This particular study used MS/MS to analyze diGLY peptides.
In contrast, parkin-null mutants were found to promote mitophagy while impairing mitochondrial respiratory chain (RC) subunit turnover,95 which appears to be a more severe variation of the effects observed in autophagy-deficient Atg7 mutants. This impedance of RC turnover was also observed in PTEN-induced kinase 1 (PINK1) mutants, albeit more selectively. These findings demonstrate the involvement of the PINK1-parkin pathway in stimulating mitophagy in vivo as well as the selective turnover of mitochondrial RC subunits.95 MS analysis was conducted using ultra-performance LC and tandem MS/MS.
Overexpression of parkin leads to increased ubiquitination of various proteins, including many mitochondrial proteins and regulators of endosomal trafficking, such as v-ATPase sub-units, Syx5/STX5, ALiX/PDCD6IP, and Vps4.96 The same study elucidated the link between the retromer component Vps35 and validated its parkin-dependent ubiquitination.
3.9 PINK1
The PINK1 gene encodes PTEN-induced kinase 1 (also PINK1), a regulatory kinase of parkin.94 Mutations in PINK1 are traditionally linked to recessive forms of PD. PINK1, which exhibits neuroprotective properties, is primarily localized in mitochondrial and cytosolic regions and participates in neuronal differentiation.97 Mutations in PINK1 have been linked to neuronal degeneration and motor dysfunction. Studies involving PINK1 null D. melanogaster have underscored the role of mitochondrial dysfunction in phenotype expression and the association between PINK1 and parkin, suggesting their involvement in the same or related pathways.98 The remarkable similarities in phenotypes between PINK1 and parkin mutants, such as abnormal wing positions, enlarged mitochondria in disorganized muscle fibers, impaired flight ability, and reduced climbing rates, further support this association.99
3.10 MS-based D. melanogaster studies on PINK1
D. melanogaster PINK1 mutants exhibit selective impairment of mitochondrial RC subunit turnover, leading to an association of PINK1 with this pathway.95 Loss of PINK1 leads to a lack of serine-250 phosphorylation in the NdufA10 subunit of mitochondrial complex I.100 Since this phosphorylation is essential for ubiquinone reduction by complex I, its absence leads to reduced mitochondrial membrane potential, potentially triggering a cascade of effects that contribute to PD.
PINK1 mutants have demonstrated accelerated deterioration in regulating protein synthesis pathways, particularly those involved in mitochondrial function, neurotransmission, and overall proteome homeostasis. Yang et al.49 suggest that such declines are crucial markers preceding the onset of aging and aging-related disorders such as PD. The study analyzed head, muscle, and testes tissues from flies using reverse-phase high-performance LC, followed by LC-MS/MS.
In a study utilizing LC-MS on an Orbitrap Fusion mass spectrometer (Thermo Scientific) with a Nanoacquity M Class UPLC (Waters Corporation, Milford, USA), CAT-tailing-like MISTERMINATE (mitochondrial-stress-induced translational termination impairment and protein carboxyl-terminal extension) events were observed in RC proteins in PD models, including PINK1-mutant D. melanogaster.50 These unique phenomena are associated with failures in proteostasis. It can be speculated that such disruptions may lead to the dysregulation of various proteins, potentially giving rise to pathogenic cascades for diseases, including PD.
Wild-type PINK1 counteracts the upregulatory effects of LRRK2 mutants on the tyrosine hydroxylase-dopamine pathway, indicating the natural antagonistic role of PINK1 against LRRK2 in this specific pathway.45 This discovery presents new possibilities for therapeutic strategies targeting tyrosine hydroxylase inhibition in patients with PD.
Conversely, mutations in mitochondria associated with PINK1 activate Relish targets, leading to age-dependent intestinal dysfunction due to inflammatory signaling. These disruptions result in metabolic reprogramming, neurotoxicity, and subsequent intestinal cell death. Relish suppression in the intestinal midgut was observed to restore mitochondrial function and offer additional neuroprotective effects.51
3.11 GBA
The GBA1 gene codes for glucocerebrosidase (GCase), a lysosomal enzyme synthesized in the ER and considered one of the most significant genetic risk factors for PD.101 Mutations in GCase are known to cause and accelerate the progression of PD directly.102 The heterozygous mutations L444P (severe) and N370S (mild) are the most common GBA mutations associated with PD. These mutations are linked to ER stress, reduced lysosomal GCase activity, and the accumulation ofα-syn aggregates.103, 104
One of the integral models for studying GBA-based PD is D. melanogaster, which contains two homologs of the human GBA1 gene, CG31148 and CG31414, more commonly known as dGBA1a and dGBA1b, respectively. These genes exhibit differential tissue expression, with dGBA1b being expressed primarily in the adult fly brain and at low concentrations in the adult fat body, while dGBA1a is mainly expressed in the adult fly gut but not in the adult brain.105 Compared to wild-type flies, transgenic flies with the N370S and L444P mutations show 82% and 75% reductions in GCase activity, respectively, despite similar GBA expression levels.106 N370S and L444P flies also exhibit a reduced lifespan, accelerated dysfunctional climbing ability, increased ER stress, and degeneration of dopamine neurons, confirming the validity of the GBA fly model.106-108
3.12 MS-based D. melanogaster studies on GBA
Deleterious mutations in the Gba1b ortholog of D. melanogaster show minimal or no overall deceleration of proteomic turnover, unlike the severe impairment seen in autophagy-deficient Atg7 mutants. No significant mitochondrial protein impairments were identified, unlike PRKN mutants. However, an increased abundance of extracellular vesicles was observed and validated, which was further demonstrated to play a critical role in protein aggregate accumulation and spread.52 In this study, isolated fly heads were analyzed using LC-MS/MS with a NanoACQUITY UPLC system (Waters Corporation) and a Q-Exactive HF mass spectrometer (Thermo Fisher, Waltham, MA, USA).
Another study on D. melanogaster models demonstrated that wild-type GCase expression is associated with a reduction in ubiquitinated proteins in the muscles and brain of D. melanogaster. Divergent loss-of-function mutations observed in GBA1 induce alterations in proteins implicated in extracellular vesicles, which are suggested to contribute to protein aggregation linked to PD.53
4 ADVANCED INSIGHTS INTO PD VIA PROTEOMICS AND GENETICS
4.1 MS-based proteomics studies in other models
While some researchers, such as Heremans et al.,48 validated their findings using multiple model systems, such comprehensive approaches are typically beyond the scope of most experiments. Although D. melanogaster is an excellent model for studying PD, it is only one among many models employed in the research of PD and other neurodegenerative disorders.
A notable study utilizing a rat model of PD was conducted by Belloso-Iguerategui et al.109 In this study, adeno-associated viral vectors encoding A53T-mutated human α-syn were injected into the substantia nigra of rats to investigate the degeneration and distribution of α-syn in the midbrain and hippocampus. By the end of the first week, α-syn was observed in the hippocampus and ventral tegmental area, particularly within dopaminergic, glutamatergic, and GABAergic neurons in the hippocampus. By the end of the study, impaired synaptic plasticity and proteome dysregulation at hippocampal terminals were identified as the initial events leading to cognitive deficits in experimental Parkinsonism. This study emphasized the dysfunction of three neurotransmitter systems—dopamine, glutamate, and gamma-aminobutyric acid—in the ventral tegmental area-hippocampus interaction- as relevant to the early stages of Parkinsonism.109 The synaptosomal fraction from the hippocampal tissue was analyzed using sequential window acquisition of all-theoretical mass spectra-MS.
Similarly, Yadav et al.110 used a rat model of rotenone-induced PD to identify possible biomarkers in the blood, specifically focusing on miRNAs and proteins. They utilized high-throughput profiling to analyze miRNAs and LC-MS/MS to examine the global proteome. At the conclusion of the study, miR-144, miR-96, and miR-29a were identified as prospective miRNA biomarkers, while PLP1, TUBB4A, and TUBA1C were identified as potential protein biomarkers for PD.110
4.2 Functional interaction between different PD genes
The intricate interactions between SNCA, LRRK2, DJ-1, PRKN, PINK1, and GBA genes create a network that plays a crucial role in unraveling the progression of PD, as shown in Figure 1. α-syn interacts with several PD-related proteins, including LRRK2, DJ-1, and parkin. Notably, mutations in SNCA can lead to its misfolding and aggregation, processes influenced by the activities of these interacting proteins.111, 112 Mutations in LRRK2 often result in abnormal kinase activity, contributing to neurodegeneration. LRRK2 interacts with α-syn, contributing to its aggregation. Furthermore, it affects mitochondrial function and autophagy through pathways involving parkin and PINK1.111, 113, 114
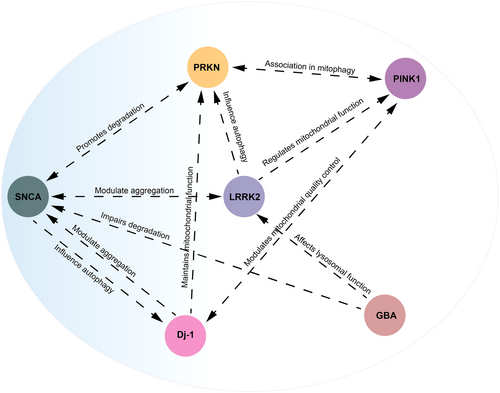
DJ-1 is crucial in maintaining mitochondrial function and responding to oxidative stress through interaction with PINK1 and parkin. Furthermore, it directly interacts with α-syn, influencing its aggregation.115 PINK1 recruits parkin to damaged mitochondria, triggering mitophagy and contributing to the cellular stress response by interacting with DJ-1, indirectly affecting α-syn aggregation.115 GBA mutations impair α-syn degradation, thereby affecting autophagy and lysosomal function via pathways involving LRRK2 and DJ-1.116, 117 These interactions highlight the multifactorial nature of PD, where disruptions in protein degradation, mitochondrial function, and cellular stress responses converge to drive neurodegeneration. Understanding these interactions is crucial for developing precise and focused therapeutics for PD.
5 CONCLUSION
MS-based proteomics in D. melanogaster models has significantly advanced our understanding of PD. This approach allows for the efficient and precise analysis of intricate biological samples, enabling the identification and measurement of proteins, peptides, metabolites, and other biomolecules involved in PD. Proteomics underscores its utility in PD research by identifying critical dysregulated pathways, protein interactions, and potential biomarkers. Despite technical challenges, advancements in MS technology and the continued use of D. melanogaster as a model organism hold promise for discovering novel therapeutic targets. This review highlighted the crucial contributions of MS-based proteomics in identifying potential biomarkers and elucidating the mechanisms of PD.
Proteomic studies in D. melanogaster have identified key dysregulated pathways and proteins involved in PD. For example, LRRK2 mutations enhance dopamine production in the early stages of the disease, leading to subsequent neurodegeneration.45 This insight enhances our understanding of genetic contributions to PD and suggests new therapeutic targets. DJ-1, which is crucial for mitigating oxidative damage and maintaining mitochondrial function, becomes dysfunctional with mutations, leading to decreased SERCA activity—reversible by targeted activators, indicating its therapeutic potential.47 Additionally, α-syn interactions with lipid droplets, leading to resistance to proteolytic digestion, offer insights into protein aggregation and neurotoxicity.66 Understanding the functional interactions between essential PD-related genes such as SNCA, LRRK2, DJ-1, PRKN, PINK1, and GBA is crucial. These genes form a complex network where disruptions in protein degradation, mitochondrial function, and cellular stress responses converge to drive neurodegeneration. Thus, comprehensive knowledge of these interactions is essential for developing targeted therapies to address the molecular dysfunctions of PD. These studies have paved the way for developing more effective and precise treatments for this debilitating neurodegenerative disorder. Although progress has been made, obstacles remain in validating and applying these biomarkers in clinical settings. Further research and collaboration within the scientific community are required.
Despite significant advancements, challenges remain in MS-based proteomics. Technical limitations, such as sample purity and proteolytic cleavage specificity, can affect the accuracy of proteomic analyses. Methodological variability across studies can also complicate result interpretation. Future research should aim to standardize proteomic techniques and enhance MS analysis resolution to overcome these challenges.
AUTHOR CONTRIBUTIONS
Mohammad Radid Khan: Data curation, investigation, visualization, writing—original draft preparation, writing—review & editing. Ramisha Anan Rahman: Writing—original draft preparation, writing—review & editing. Md Harunur Rashid: Funding acquisition, writing—original draft preparation, writing—review & editing. Md Shariful Islam: Conceptualization, funding acquisition, project administration, supervision, visualization, writing—original draft preparation, writing—review & editing.
ACKNOWLEDGMENTS
This work is fully supported by NSU CTRG Research Grant (2021-2022) (CTRG-21-SEPS-11) and NSU CTRG Research Grant (2022-2023) (CTRG-23-SEPS-14).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




