Hyperpolarized 129Xe gas transfer MRI: the transition from 1.5T to 3T
Funding information: National Institutes of Health, Grant/Award Numbers: R01HL126771, R01HL105643, HHSN268201700001C
Abstract
Purpose
Hyperpolarized 129Xe MRI depicting 3D ventilation, interstitial barrier uptake, and transfer to red blood cells (RBCs) has emerged as a powerful new means of detecting pulmonary disease. However, given the challenging susceptibility environment of the lung, such gas transfer imaging has, thus far, only been implemented at 1.5T. Here, we seek to demonstrate the feasibility of Dixon-based 129Xe gas transfer MRI at 3T.
Methods
Seven healthy subjects and six patients with pulmonary disorders were recruited to characterize 129Xe spectral structure, optimize acquisition parameters, and acquire representative images. Imaging used randomized, gradient-spoiled 3D-radial encoding of 1000 gas (0.5° flip) and dissolved (20° flip) views, reconstructed into 3-mm isotropic voxels. The center of k-space was sampled when barrier and RBC compartments were 90° out of phase (TE90). A single dissolved phase spectrum was appended to the sequence to measure the global RBC–barrier ratio for Dixon-based decomposition.
Results
A 0.69 ms sinc was found to generate minimal off-resonance gas-phase excitation (3.0 ± 0.3% of the dissolved-phase), yielding a TE90 = 0.47 ± 0.02 ms. The RBC and barrier resonance frequencies were shifted by 217.6 ± 0.6 ppm and 197.8 ± 0.2 ppm. The RBC 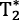 was estimated to be ∼1.1 ms, and therefore each read-out was limited to 1.3 ms. 129Xe gas and dissolved-phase images have sufficient SNR to produce gas transfer maps of similar quality and sensitivity to pathology, as previously obtained at 1.5T.
was estimated to be ∼1.1 ms, and therefore each read-out was limited to 1.3 ms. 129Xe gas and dissolved-phase images have sufficient SNR to produce gas transfer maps of similar quality and sensitivity to pathology, as previously obtained at 1.5T.
Conclusions
Despite short dissolved-phase 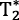 , 129Xe gas transfer MRI is feasible at 3T.
, 129Xe gas transfer MRI is feasible at 3T.