Changes in the specific absorption rate (SAR) of radiofrequency energy in patients with retained cardiac leads during MRI at 1.5T and 3T
Corresponding Author
Laleh Golestanirad
Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, Massachusetts
Department of Radiology, Northwestern University, Feinberg School of Medicine, Chicago, Illinois
Correspondence
Laleh Golestani Rad, AA Martinos Center, Massachusetts General Hospital, Harvard Medical School, Building 75, Third Ave, Charlestown, MA 02129 Email: [email protected] or Department of Radiology, Feinberg School of Medicine, 737 N Michigan Ave. Suite 1600, Chicago IL 60611.
Email: [email protected]
Search for more papers by this authorAmir Ali Rahsepar
Department of Radiology, Northwestern University, Feinberg School of Medicine, Chicago, Illinois
Search for more papers by this authorJohn E Kirsch
Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, Massachusetts
Search for more papers by this authorKenichiro Suwa
Department of Radiology, Northwestern University, Feinberg School of Medicine, Chicago, Illinois
Search for more papers by this authorJeremy C. Collins
Department of Radiology, Northwestern University, Feinberg School of Medicine, Chicago, Illinois
Search for more papers by this authorLeonardo M. Angelone
Division of Biomedical Physics, Office of Science and Engineering Laboratories, Center for Devices and Radiological Health, U.S. Food and Drug Administration, Silver Spring, Maryland
Search for more papers by this authorBoris Keil
Department of Life Science Engineering, Institute of Medical Physics and Radiation Protection, Giessen, Germany
Search for more papers by this authorRod S. Passman
Division of Cardiology, Department of Medicine, Northwestern University, Feinberg School of Medicine, Chicago, Illinois
Search for more papers by this authorGiorgio Bonmassar
Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, Massachusetts
Search for more papers by this authorPeter Serano
Division of Biomedical Physics, Office of Science and Engineering Laboratories, Center for Devices and Radiological Health, U.S. Food and Drug Administration, Silver Spring, Maryland
Search for more papers by this authorJames C. Carr
Department of Radiology, Northwestern University, Feinberg School of Medicine, Chicago, Illinois
Search for more papers by this authorLawrence L. Wald
Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, Massachusetts
Search for more papers by this authorCorresponding Author
Laleh Golestanirad
Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, Massachusetts
Department of Radiology, Northwestern University, Feinberg School of Medicine, Chicago, Illinois
Correspondence
Laleh Golestani Rad, AA Martinos Center, Massachusetts General Hospital, Harvard Medical School, Building 75, Third Ave, Charlestown, MA 02129 Email: [email protected] or Department of Radiology, Feinberg School of Medicine, 737 N Michigan Ave. Suite 1600, Chicago IL 60611.
Email: [email protected]
Search for more papers by this authorAmir Ali Rahsepar
Department of Radiology, Northwestern University, Feinberg School of Medicine, Chicago, Illinois
Search for more papers by this authorJohn E Kirsch
Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, Massachusetts
Search for more papers by this authorKenichiro Suwa
Department of Radiology, Northwestern University, Feinberg School of Medicine, Chicago, Illinois
Search for more papers by this authorJeremy C. Collins
Department of Radiology, Northwestern University, Feinberg School of Medicine, Chicago, Illinois
Search for more papers by this authorLeonardo M. Angelone
Division of Biomedical Physics, Office of Science and Engineering Laboratories, Center for Devices and Radiological Health, U.S. Food and Drug Administration, Silver Spring, Maryland
Search for more papers by this authorBoris Keil
Department of Life Science Engineering, Institute of Medical Physics and Radiation Protection, Giessen, Germany
Search for more papers by this authorRod S. Passman
Division of Cardiology, Department of Medicine, Northwestern University, Feinberg School of Medicine, Chicago, Illinois
Search for more papers by this authorGiorgio Bonmassar
Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, Massachusetts
Search for more papers by this authorPeter Serano
Division of Biomedical Physics, Office of Science and Engineering Laboratories, Center for Devices and Radiological Health, U.S. Food and Drug Administration, Silver Spring, Maryland
Search for more papers by this authorJames C. Carr
Department of Radiology, Northwestern University, Feinberg School of Medicine, Chicago, Illinois
Search for more papers by this authorLawrence L. Wald
Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, Massachusetts
Search for more papers by this authorFunding information: National Institutes of Health, Grant/Award Numbers: K99EB021320, R01EB00684, R01MH111875, and R03 EB024705
Abstract
Purpose
To evaluate the local specific absorption rate (SAR) and heating around retained cardiac leads during MRI at 64 MHz (1.5T) and 127 MHz (3T) as a function of RF coil type and imaging landmark.
Methods
Numerical models of retained cardiac leads were built from CT and X-ray images of 6 patients with retained cardiac leads. Electromagnetic simulations and bio-heat modeling were performed with MRI RF body and head coils tuned to 64 MHz and 127 MHz and positioned at 9 different imaging landmarks covering an area from the head to the lower limbs.
Results
For all patients and at both 1.5T and 3T, local transmit head coils produced negligible temperature rise (
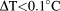 ) for
) for
 . For body imaging with quadrature-driven coils at 1.5T,
. For body imaging with quadrature-driven coils at 1.5T,
 during a 10-min scan remained < 3°C at all imaging landmarks for
during a 10-min scan remained < 3°C at all imaging landmarks for
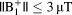 and <6°C for
and <6°C for
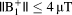 . For body imaging at 3T,
. For body imaging at 3T,
 during a 10-min scan remained < 6°C at all imaging landmarks for
during a 10-min scan remained < 6°C at all imaging landmarks for
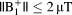 . For shorter pulse sequences up to 2 min,
. For shorter pulse sequences up to 2 min,
 remained < 6°C for
remained < 6°C for
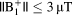 .
.
Conclusion
For the models based on 6 patients studied, simulations suggest that MRI could be performed safely using a local head coil at both 1.5T and 3T, and with a body coil at 1.5T with pulses that produced
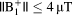 . MRI at 3T could be performed safely in these patients using pulses with
. MRI at 3T could be performed safely in these patients using pulses with
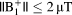 .
.
REFERENCES
- 1Greenspon AJ, Patel JD, Lau E, et al. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol. 2012; 60: 1540-1545.
- 2Sommer T, Naehle CP, Yang A, et al. Strategy for safe performance of extrathoracic magnetic resonance imaging at 1.5 Tesla in the presence of cardiac pacemakers in non–pacemaker-dependent patients a prospective study with 115 examinations. Circulation. 2006; 114: 1285-1292.
- 3Higgins JV, Gard JJ, Sheldon SH, et al. Safety and outcomes of magnetic resonance imaging in patients with abandoned pacemaker and defibrillator leads. Pacing Clin Electrophysiol. 2014; 37: 1284-1290.
- 4Roguin A. Magnetic resonance imaging in patients with implantable cardioverter-defibrillators and pacemakers. J Am Coll Cardiol. 2009; 54: 556-557.
- 5Kalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol. 2005; 28: 326-328.
- 6Nazarian S, Hansford R, Roguin A, et al. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. Ann Intern Med. 2011; 155: 415-424.
- 7Pavlicek W, Geisinger M, Castle L, et al. The effects of nuclear magnetic resonance on patients with cardiac pacemakers. Radiology. 1983; 147: 149-153.
- 8Shellock FG, Fischer L, Fieno DS. Cardiac pacemakers and implantable cardioverter defibrillators: in vitro magnetic resonance imaging evaluation at 1.5-tesla. J Cardiovasc Magn Reson. 2007; 9: 21-31.
- 9Wahlstrand CD, Hoegh TB, Hrdlicka GA, Cross TE Jr, Olsen JM. Lead electrode for use in an MRI-safe implantable medical device. Google Patent US20050222647A1, 2007.
- 10Serano P, Angelone LM, Katnani H, Eskandar E, Bonmassar G. A novel brain stimulation technology provides compatibility with MRI. Sci Rep. 2015; 5: 9805.
- 11 American Section of the International Association for Testing Materials. Standard test method for measurement of radio frequency induced heating on or near passive implants during magnetic resonance imaging, in F2182-11. West Conshohocken: ASTM; 2011.
- 12Gallik DM, Ben-Zur UM, Gross JN, Furman S. Lead fracture in cephalic versus subclavian approach with transvenous implantable cardioverter defibrillator systems. Pacing Clin Electrophysiol. 1996; 19: 1089-1094.
- 13Henrikson CA, Brinker JA. How to prevent, recognize, and manage complications of lead extraction. Part III: procedural factors. Heart Rhythm. 2008; 5: 1352-1354.
- 14Friedman RA, Zandt H, Collins E, LeGras M, Perry J. Lead extraction in young patients with and without congenital heart disease using the subclavian approach. Pacing Clin Electrophysiol. 1996; 19: 778-783.
- 15Silvetti MS, Drago F. Upgrading of VVIR pacemakers with nonfunctional endocardial ventricular leads to VDD pacemakers in adolescents. Pacing Clin Electrophysiol. 2006; 29: 691-696.
- 16Love CJ, Wilkoff BL, Byrd CL, et al. Recommendations for extraction of chronically implanted transvenous pacing and defibrillator leads: indications, facilities, training. Pacing Clin Electrophysiol. 2000; 23: 544-551.
- 17Koshy A, Nanayakkara S, McGiffin D, Martin J, Bergin P, Mariani J. Retained defibrillator leads following orthotopic heart transplantation. Int J Cardiol. 2016; 215: 87-89.
- 18Kim J, Hwang J, Choi JH, et al. Frequency and clinical impact of retained implantable cardioverter defibrillator lead materials in heart transplant recipients. PLoS One. 2017; 12: e0176925.
- 19Lund LH, Edwards LB, Dipchand AI, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-third adult heart transplantation report—2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant. 2016; 35: 1158-1169.
- 20Rezai AR, Baker KB, Tkach JA, et al. Is magnetic resonance imaging safe for patients with neurostimulation systems used for deep brain stimulation? Neurosurgery. 2005; 57: 1056-1062.
- 21Rezai AR, Phillips M, Baker KB, et al. Neurostimulation system used for deep brain stimulation (DBS): MR safety issues and implications of failing to follow safety recommendations. Invest Radiol. 2004; 39: 300-303.
- 22Nitz WR,
Oppelt A,
Renz W,
Manke C,
Lenhart M,
Link J. On the heating of linear conductive structures as guide wires and catheters in interventional MRI. J Magn Reson Imaging. 2001; 13: 105-114.
10.1002/1522-2586(200101)13:1<105::AID-JMRI1016>3.0.CO;2-0 CAS PubMed Web of Science® Google Scholar
- 23Nordbeck P, Weiss I, Ehses P, et al. Measuring RF-induced currents inside implants: impact of device configuration on MRI safety of cardiac pacemaker leads. Magn Reson Med. 2009; 61: 570-578.
- 24Mattei E, Triventi M, Calcagnini G, et al. Complexity of MRI induced heating on metallic leads: experimental measurements of 374 configurations. Biomed Eng Online. 2008; 7: 11.
- 25Wilkoff BL, Albert T, Lazebnik M, et al. Safe magnetic resonance imaging scanning of patients with cardiac rhythm devices: a role for computer modeling. Heart Rhythm. 2013; 10: 1815-1821.
- 26 U.S. Food and Drug Administration. Electromagnetic Modeling. http://www.fda.gov/MedicalDevices/ScienceandResearch/ResearchPrograms/ucm477379.htm. Published 2017. Updated February, 2, 2018. Accessed May, 7, 2018.
- 27Nordbeck P, Fidler F, Weiss I, et al. Spatial distribution of RF-induced E-fields and implant heating in MRI. Magn Reson Med. 2008; 60: 312-319.
- 28Nordbeck P, Ritter O, Weiss I, et al. Impact of imaging landmark on the risk of MRI-related heating near implanted medical devices like cardiac pacemaker leads. Magn Reson Med. 2011; 65: 44-50.
- 29Bottomley PA, Karmarkar PV, Allen JM, Edelstein WA. MRI and RF compatible leads and related methods of operating and fabricating leads. Google Patents, 2016.
- 30Marshall M, Seifert K. Medical electrical lead having improved inductance. Google Patents, 2006.
- 31Horner M. Human body modeling with ANSYS software. https://support.ansys.com/staticassets/ANSYS/Conference/Minnesota/downloads/Human%20Body%20Modeling%20ANSYS%20Software.pdf. ANSYS Inc. Published October, 24, 2011. Accessed May, 7, 2018.
- 32 American Society for Testing and Materials. Standard test method for measurement of radio frequency induced heating near passive implants during magnetic resonance imaging (F2182–02a). West Conshohocken: ASTM International; 2004.
- 33Mattei E, Triventi M, Calcagnini G, et al. Temperature and SAR measurement errors in the evaluation of metallic linear structures heating during MRI using fluoroptic® probes. Phys Med Biol. 2007; 52: 1633-1646.
- 34Murbach M, Neufeld E, Kainz W, Pruessmann KP, Kuster N. Whole-body and local RF absorption in human models as a function of anatomy and position within 1.5 T MR body coil. Magn Reson Med. 2014; 71: 839-845
- 35 IEEE P1528.4™/D1.0, Recommended Practice for Determining the Peak Spatial Average Specific Absorption Rate (SAR) in the Human Body from Wireless Communications Devices, 30 MHz - 6 GHz: Requirements for Using the Finite-Element Method for SAR Calculations, specifically involving Vehicle Mounted Antennas and Personal Wireless Devices. 2014.
- 36Cheng HLM, Plewes DB. Tissue thermal conductivity by magnetic resonance thermometry and focused ultrasound heating. J Magn Reson Imaging. 2002; 16: 598-609.
- 37Langman DA, Goldberg IB, Finn JP, Ennis DB. Pacemaker lead tip heating in abandoned and pacemaker-attached leads at 1.5 tesla MRI. J Magn Reson Imaging. 2011; 33: 426-431.
- 38Mattei E, Gentili G, Censi F, Triventi M, Calcagnini G. Impact of capped and uncapped abandoned leads on the heating of an MR-conditional pacemaker implant. Magn Reson Med. 2015; 73: 390-400.
- 39Luechinger R, Zeijlemaker VA, Pedersen EM, et al. In vivo heating of pacemaker leads during magnetic resonance imaging. Eur Heart J. 2005; 26: 376-383.
- 40Amjad A, Kamondetdacha R, Kildishev A, Park S, Nyenhuis J. Power deposition inside a phantom for testing of MRI heating. IEEE Trans Magn. 2005; 41: 4185-4187.
- 41Ibrahim T, Lee R, Baertlein B, Robitaille PL. B1 field homogeneity and SAR calculations for the birdcage coil. Phys Med Biol. 2001; 46: 609-619.
- 42Ibrahim TS, Mitchell C, Abraham R, Schmalbrock P. In-depth study of the electromagnetics of ultrahigh-field MRI. NMR Biomed. 2007; 20: 58-68.
- 43Collins CM, Liu W, Wang J, et al. Temperature and SAR calculations for a human head within volume and surface coils at 64 and 300 MHz. J Magn Reson Imaging. 2004; 19: 650-666.
- 44Chen J, Feng Z, Jin J-M. Numerical simulation of SAR and B1 field homogeneity of shielded RF coils loaded with the human head. IEEE Trans Biomed. Eng. 1998; 45: 650-659.
- 45McElcheran CE, Yang B, Anderson KJ, Golenstani-Rad L, Graham SJ. Investigation of parallel radiofrequency transmission for the reduction of heating in long conductive leads in 3 Tesla magnetic resonance imaging. PLoS One. 2015; 10: e0134379.
- 46McElcheran CE, Yang B, Anderson KJ, Golenstanirad L, Graham SJ. Parallel radiofrequency transmission at 3 tesla to improve safety in bilateral implanted wires in a heterogeneous model. Magn Reson Med. 2017; 78: 2406-2415.
- 47Golestanirad L, Iacono MI, Keil B, et al. Construction and modeling of a reconfigurable MRI coil for lowering SAR in patients with deep brain stimulation implants. Neuroimage, 2017; 147: 577-588.
- 48Golestanirad L, Keil B, Angelone LM, Bonmassar G, Mareyam A, Wald LL. Feasibility of using linearly polarized rotating birdcage transmitters and close-fitting receive arrays in MRI to reduce SAR in the vicinity of deep brain simulation implants. Magn Reson Med. 2017; 77: 1701-1712.
- 49Lucano E, Liberti M, Mendoza G, et al. Assessing the electromagnetic fields generated by a radiofrequency MRI body coil at 64 MHz: defeaturing vs. accuracy. IEEE Trans Biomed Eng. 2016; 63: 1591-1601.
- 50Yarmolenko PS, Moon EJ, Landon C, et al. Thresholds for thermal damage to normal tissues: an update. Int J Hyperthermia. 2011; 27: 320-343.
- 51Jones S, Carley S, Harrison M. An introduction to power and sample size estimation. Emerg Med J. 2003; 20: 453–458.
- 52Liu Z, Ahmed M, Weinstein Y, Yi M, Mahajan RL, Goldberg SN. Characterization of the RF ablation-induced ‘oven effect’: the importance of background tissue thermal conductivity on tissue heating. Int J Hyperthermia. 2006; 22: 327-342.




