Macrophage-Mediated Myelin Recycling Promotes Malignant Development of Glioblastoma
A recent study published in the journal Cell [1] revealed the critical role of macrophages in the malignant development of brain cancers, particularly glioblastoma. Through a series of in-vivo and in-vitro experiments, the study researched how macrophages provide essential lipids and nutrients to brain cancer cells by mediating the recycling and reuse of myelin, the insulating layer in the nervous system, which in turn promotes growth and invasion of tumor. They explored the heterogeneity of tumor-associated macrophages (TAM) in glioblastoma tumor microenvironment using single-cell and multi-omics analyses and revealed their specific interactions with different glioblastoma subtypes. The study reported the dynamic contexture of the glioblastoma tumor microenvironment at single-cell levels during primary or recurrent tumor progression, revealed the colocated diversity of niche-specific interactions between TAMs and glioblastoma subtypes at spatial transcriptomic levels, evaluated the chromatin landscape changes and immuno-suppressive features associated with the lipid-laden phenotype using multi-omics sequences, addressed the transfer route of lipid flux from myelin to macrophages at last to mesenchymal-like (MES-like) glioblastoma cells, demonstrated the intrinsic lipid traffic in macrophages and the altered metabolic manner in glioblastoma cells using lipidomics analysis and experiments, presented the protumorigenic functions of lipid-laden macrophages (LLMs) in glioblastoma and their relevance to clinical survival or immunotherapeutic response.
The study found that in the brain tumor microenvironment, macrophages are able to take up and accumulate myelin debris in large quantities. These myelin fragments are converted by macrophages into cholesterol and other lipids, which are then delivered to brain cancer cells to support their growth and malignant transformation. They also found that specific types of macrophages, such as TAMs with high glycoprotein nonmetastatic melanoma protein B (GPNMB) expression, are closely associated with areas of high myelin debris accumulation and exhibit unique patterns of lipid metabolism and inflammatory activity. The study further demonstrated that macrophage-mediated lipid delivery not only provides an energy source for brain cancer cells, but also promotes the invasion and metastasis ability of cancer cells. By interfering with the lipid metabolism pathway of macrophages, the progress of brain cancer can be significantly inhibited. When macrophages take in myelin fragments, their inflammatory activity is suppressed and they shift to an “anti-inflammatory” state. This anti-inflammatory state may help maintain the stability of the tumor microenvironment, thus providing favorable conditions for tumor cell growth. The study was also verified using patient sample data and found similar patterns of macrophage activity in the tumor microenvironment of glioma patients, which suggests that macrophage-mediated myelin recycling may be an important target in brain cancer therapy.
Researchers used two mouse models to study the heterogeneity of tumor cells and macrophages and subtype transitions [2]. They found that primary tumors changed from astrocyte-like to oligodendrocyte-like upon recurrence, while oligodendrocyte-progenitor became MES-like. Macrophages in the tumor microenvironment were divided into four subsets, with GPNMB-high TAM increasing in recurrent tumors, which showed the characteristics of lipid-laden macrophages [3, 4]. These macrophages showed lipid accumulation, suggesting extracellular cholesterol origin. Previous work has shown that phagocytic action of cholesterol-rich myelin fragments can lead to cholesterol accumulation and cholesterol biosynthesis shutdown [5] and in this work myelin was identified as a lipid source in these LLMs, confirmed by electron microscopy. In vitro studies showed that myelin phagocytosis enhanced lipid accumulation and altered gene expression in macrophages. LLMs also had increased lipid transporters and contributed to cholesterol outflow from tumors. Coculture experiments revealed a symbiotic relationship between LLMs and tumor cells, with LLMs promoting tumor cell proliferation through lipid efflux (Figure 1).
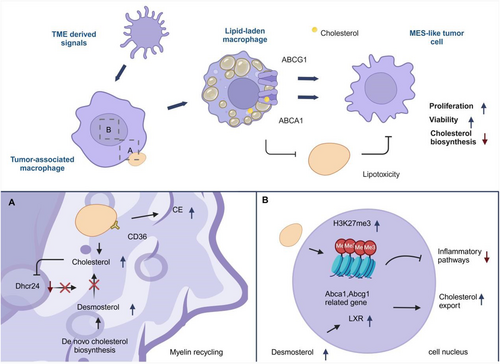
In summary, this work demonstrates that MES-like glioblastoma cells promote their own survival and proliferation through metabolic crosstalk with macrophages, that is, promoting the uptake of myelin by macrophages and processing into lipids that can be utilized by tumor cells, and the discovery of this mechanism is crucial for updating antitumor therapeutic strategies. For the first time, a TAM subpopulation was found to acquire a LLM by recycling cholesterol-rich myelin fragments. This metabolic remodeling enables LLM cells to exhibit immunosuppressive properties, providing a new perspective for understanding the role of TAM subpopulations in tumor progression. It was demonstrated that LLM cells can transfer myelin-derived lipids to glioblastoma cells, thereby providing nutrients for them and promoting tumor progression and recurrence. This reveals the driving effect of immunometabolic interaction in tumor microenvironment on tumor malignant process, and provides a new idea for potential therapeutic strategies targeting metabolic remodeling of TAM. LLM cells were found to be associated with aggressive MES-like glioblastoma subtypes and were associated with poor patient outcomes. This study provides a basis for further exploring the dynamic changes of TAM subsets among different tumor subtypes and its clinical significance.
However, the article also has some limitations: The molecular mechanisms of how LLM cells regulate immunosuppressive function and its direct or indirect effects on lymphocyte function have not been thoroughly explored. Associations between LLM cells and tumor progression have only been observed in animal models and clinical samples, and direct proof of causality that LLM cells drive tumor progression is lacking. Maybe there can have further elucidation of the molecular regulation mechanism of LLM cell immunosuppressive function and its influence on tumor immune microenvironment and explore potential therapeutic strategies for the remodeling of LLM cell metabolism and provide a new therapeutic target for improving the prognosis of glioma patients. This might involve: (1) Conducting an in-depth analysis of the molecular mechanism of myelin recycling, identifying of specific carriers of lipids delivered by TAM to tumor cells, and screening of key genes regulating myelin phagocytosis and metabolism. (2) Exploring clinical translational potential, developing drugs that target myelin recycling, evaluating their synergies with existing therapies, and validating preclinical efficacy of targeting lipid metabolism. (3) Focus on the immune regulation of myelin recycling, studying whether myelin metabolites indirectly promote immune escape by regulating TAM polarization or inhibiting T cell function, and exploring whether myelin fragments trigger inflammation by activating pattern recognition receptors, forming a positive feedback loop that promotes cancer. (4) Expanding to other nervous system diseases and cancers to study whether abnormal myelin accumulation has a common mechanism with other nervous system tumors or neurodegenerative diseases, and exploring whether peripheral nerve injury promotes remote tumor metastasis through a similar mechanism.
Author Contributions
Huanhuan Wang wrote the manuscript and prepared the figure. Feng Xie provided valuable discussion. Feng Xie and Long Zhang approved the final version of the manuscript. All authors have read and approved the final manuscript.
Acknowledgments
This work was supported by the Chinese National Natural Science Funds (31925013, W2411011 and 82473119). We want to express our gratitude for the drawing materials provided by BioRender.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
Author Long Zhang is an Editorial board member of MedComm - Oncology. Author Long Zhang was not involved in the journal's review of or decisions related to this manuscript. The remaining authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




