Tumor Metastasis: Mechanistic Insights and Therapeutic Intervention
Lin Tang, Shao-Cong Peng, Xiao-Wan Zhuang, Yan He, and Yu-Xiang Song contributed equally to this work and share first authorship.
ABSTRACT
Metastasis remains a leading cause of cancer-related deaths, defined by a complex, multi-step process in which tumor cells spread and form secondary growths in distant tissues. Despite substantial progress in understanding metastasis, the molecular mechanisms driving this process and the development of effective therapies remain incompletely understood. Elucidating the molecular pathways governing metastasis is essential for the discovery of innovative therapeutic targets. The rapid advancements in sequencing technologies and the expansion of biological databases have significantly deepened our understanding of the molecular drivers of metastasis and associated drug resistance. This review focuses on the molecular drivers of metastasis, particularly the roles of genetic mutations, epigenetic changes, and post-translational modifications in metastasis progression. We also examine how the tumor microenvironment influences metastatic behavior and explore emerging therapeutic strategies, including targeted therapies and immunotherapies. Finally, we discuss future research directions, stressing the importance of novel treatment approaches and personalized strategies to overcome metastasis and improve patient outcomes. By integrating contemporary insights into the molecular basis of metastasis and therapeutic innovation, this review provides a comprehensive framework to guide future research and clinical advancements in metastatic cancer.
Graphical Abstract
This graphical abstract illustrates the complex mechanisms of metastasis, emphasizing the contributions of genetic variation, post-transcriptional regulation, tumor metabolism, and the tumor microenvironment. It further explores the prospects of innovative therapeutic strategies, highlighting the demand for an integrative approach to address metastatic cancer effectively.
1 Introduction
Metastasis represents a highly intricate, multi-step process wherein tumor cells migrate from the primary site to distant organs. The “seed and soil” theory, introduced by Paget in 1889, highlights the crucial interaction between tumor cells and their microenvironment, which ultimately determines the metastatic potential of cancer cells [1]. The “pre-metastatic niche” theory, based on this concept, asserts that primary tumors establish a conducive “soil” at distant sites through a cascade of events, preparing the microenvironment and creating conditions for subsequent metastatic dissemination. Genetic and epigenetic alterations further modulate an individual's susceptibility to metastasis, with tumor cells acquiring novel traits through the continuous accumulation of mutations, enabling them to detach and disseminate from the primary tumor. Each phase of metastasis is marked by dynamic and adaptive changes, such as metabolic reprogramming, angiogenesis, epithelial–mesenchymal transition (EMT), and extracellular matrix remodeling. These adaptive responses, driven by interactions among multiple signaling pathways, culminate in the successful colonization of distant organs and the establishment of secondary tumors.
Despite significant advances in the treatment of metastatic cancers, the 5-year survival rate for patients remains dismally low. Metastasis prevention and treatment require a more systematic approach than primary tumor management, as the clinical presentation and treatment of metastases differ markedly across cancers such as metastatic prostate, colorectal, breast, and non-small cell lung cancer. The precision and sensitivity of biomarker screening for metastases will benefit greatly from advancements in diagnostic technologies. Prognostic and predictive markers now play a pivotal role in shaping clinical treatment decisions for metastatic patients, becoming integral to personalized oncology care. Traditional drug development, which is resource-intensive and relies on target screening in tumor cell lines, further emphasizes the need for a deep understanding of metastatic mechanisms to develop effective strategies against metastasis.
This review focuses on the genetic, epigenetic, and molecular mechanisms that drive metastatic progression. We also discuss the significant role of the tumor microenvironment (TME) and its influence on metastatic behavior, integrating insights from various disciplines such as molecular biology, genetics, and immunology. By incorporating a multidisciplinary approach, this review aims to provide a holistic view of the metastatic cascade and identify key areas for future investigation, with the ultimate goal of advancing therapeutic strategies to target metastatic disease more effectively.
2 Metastatic Process: Phases and Mechanisms
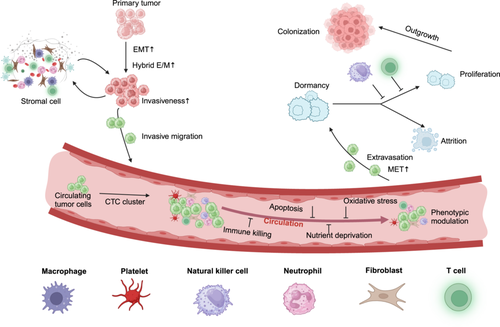
Metastasis involves a sequence of biological events in which primary tumor cells progressively acquire the capacity to invade deeper tissues, survive dissemination via the bloodstream, lymphatic system, or direct infiltration of adjacent structures, seed distant organs, and eventually resume proliferation at remote sites. This sequence, known as the metastatic cascade [2], is essential for tumor spread (Figure 1).
2.1 Intrusion-Related Reprogramming
The initial step in metastasis is the local invasion of surrounding tissues, requiring tumor cells to undergo changes that enhance motility, invasiveness, and their ability to penetrate the vascular system, either directly or via the lymphatic system, to enter the bloodstream. Cancer stem cells (CSCs) are typically characterized by aggressive proliferation. They possess the capacity for self-renewal, the ability to generate phenotypically diverse tumor cell subpopulations, and tumor-initiating potential, positioning them at the apex of the tumor cell hierarchy [3]. Two key factors enable CSCs to escape from their primary site: epithelial–mesenchymal plasticity (EMP) and alterations within the TME [4] (Figure 1). Biomechanical changes in the extracellular matrix (ECM) also play a significant role in promoting cellular invasiveness [5]. Degradation of the ECM by tumor and stromal cells reduces its structural integrity, creating additional space for tumor expansion. Alterations in ECM composition and increased mechanical stiffness, common in most solid tumors, contribute to resistance against apoptosis and enhance tumor cell invasiveness [6]. EMT is recognized as a pivotal cellular mechanism in malignant tumor progression and metastasis [7]. Under abnormal activation of EMT, tumor cells are rich in a variety of dynamic changes, including decreased adhesion, loss of polarity, and tight junctions, resulting in an enhanced ability to migrate and invade adjacent tissues. EMT is implicated in tumorigenesis, metastasis, and therapeutic relapse across various cancer types, including breast cancer [8] prostate cancer [9], colorectal cancer [10], esophageal cancer [11], gastric cancer [12], and lung cancer [13]. It facilitates the acquisition of tumor heterogeneity and cancer stem cell-like properties, serving as a critical driver of tumor invasion and metastasis [14, 15].
The EMT and mesenchymal-epithelial transformation (MET) programs have traditionally been viewed as binary switches between two distinct static states. However, recent research has revealed that EMT is not an all-or-none process. Instead, it often occurs in an incomplete manner, with individual cancer cells activating EMT to varying extents, resulting in hybrid or partial E/M states. Such tumor cells simultaneously express epithelial and mesenchymal markers at different levels, exhibiting mixed phenotypic, transcriptional, and epigenetic traits characteristic of both epithelial and mesenchymal cells [16]. Emerging evidence indicates that the hybrid E/M state confers enhanced stem cell-like properties, tumor-initiating potential, and increased invasiveness [17]. Moreover, prostate cancer cells in a hybrid E/M state express elevated levels of stem cell markers and form faster-growing, larger tumors in vivo [18]. In triple-negative breast cancer, Bierie et al. identified an E/M hybrid subpopulation expressing integrin β4 (ITGB4), which exhibited greater stem-like traits, tumor-initiating capacity, and poorer prognosis [19]. Collectively, these findings suggest that stem-like properties and tumor-initiating capacity are predominantly associated with the acquisition of a hybrid E/M state rather than a fully epithelial or mesenchymal phenotype.
2.2 Metastatic Circular Transmission-Dissemination
After undergoing invasive transformations, malignant tumor cells can detach from the primary tissue and invade the bloodstream or lymphatic vessels, becoming circulating tumor cells (CTCs). CTCs hold immense clinical value, with current research on liquid biopsy of CTCs primarily focusing on three areas: capturing CTCs to determine their quantity, identifying CTC phenotypes to evaluate tumor status, and analyzing genetic variations in CTCs to reveal tumor heterogeneity [20]. Studies on CTCs provide insights into predicting distant metastases, assessing prognosis, and monitoring therapeutic responses [21-23].
These CTCs typically circulate in microclusters that acquire mesenchymal or stem cell-like properties. They are often encapsulated by platelets, immune cells, or stromal cells of tumor origin, which enhance their stem cell characteristics, proliferative potential, and ability to evade immune detection [24] (Figure 1). CTC clusters, in particular, exhibit greater viability than individual CTCs, clustered structures provide CTCs with a unique hypoxic microenvironment that promotes their survival [25], significantly contributing to their dissemination and subsequent metastasis formation [26]. However, while in circulation, CTCs face numerous environmental stressors, including immune killing, apoptosis, oxidative stress, blood shear forces, and nutrient deprivation. As a result, the majority of CTCs undergo apoptosis, with only a small fraction surviving and successfully initiating metastasis [27]. To navigate these challenges, CTCs must integrate intrinsic cellular modifications with external microenvironmental signals, adapting to the dynamic conditions. This leads to substantial temporal and spatial heterogeneity in the molecular phenotype, transcriptome, translatome, and cytological characteristics of CTCs, even within the same parental tumor population [28]. Interactions between CTCs and circulating microenvironment components further modify the microenvironment, facilitating the migration and long-distance spread of CTCs [29-31]. For instance, Lin et al. demonstrated that in brest cancer (BCa) cells, transforming growth factor-beta (TGF-β) signaling can induce tumor-associated fibroblasts (CAFs) to transform into a (platelet-derived growth factor receptor alpha (PDGFRα+) integrin subunit alpha 11 (ITGA11+) subpopulation. This subpopulation promotes lymphangiogenesis and ECM remodeling, creating pathways that enhance the invasion of lymphatic vessels by BCa cells and augment their overall invasive potential [32]. Liu et al. discovered that CTCs can bind to platelet-derived regulator of G protein signaling 18 (RGS18), leading to the upregulation of the immune checkpoint molecule major histocompatibility complex, class I, E (HLA-E), thereby enabling CTCs to evade immune surveillance by natural killer (NK) cells [33]. During extravasation, CTCs are restored to an epithelial phenotype by MET and regain their adhesion capacity and stem cell properties, which are important for the metastatic regeneration of CTCs in distant organs [34, 35].
Currently, various therapeutic strategies targeting the TME or CTCs themselves have been developed to limit CTC survival, thereby reducing metastasis or preventing disease progression into more aggressive phenotypes. These approaches include inhibiting the EMT, reversing the MET, and clearing CTCs from circulation. For example, in breast cancer, Rg3 liposome loading with docetaxel has been used to capture circulating CTCs and disrupt the lung microenvironment, effectively suppressing metastasis [36]. Additionally, eribulin treatment in breast cancer patients has been shown to improve survival by modulating the epithelial–mesenchymal state of CTCs [37]. However, early clinical trials have been terminated due to low recruitment rates or challenges in detecting CTCs, highlighting the critical need for improved CTC isolation and enrichment technologies for future clinical studies.
CTCs serve as a critical bridge linking primary tumors and metastatic sites. They not only represent certain biological characteristics of the primary tumor but also reflect some behaviors of metastatic lesions. Therefore, in-depth investigations into the unique traits of CTCs—such as stemness, epithelial–mesenchymal state, immune evasion, and drug resistance—are essential. Identifying key molecules on or within CTCs and exploring their roles in mediating organ-specific metastasis will be pivotal in uncovering the mechanisms underlying distant organ-specific tumor dissemination [28]. Furthermore, developing targeted therapies against CTC-specific antigens holds great promise for clinically inhibiting metastasis.
2.3 Organ Infiltration and Metastatic Colonization
Disseminated tumor cells (DTCs) have the capacity to spread to nearly all organs, yet distinct cancer types exhibit a preference for specific organs, a phenomenon referred to as metastatic organ tropism. This tendency is shaped by the characteristics of the targeted organs and the organ-specific microenvironment that facilitates DTC seeding and colonization [38]. Upon arrival in distant tissues, the majority of DTCs are swiftly eliminated—either by innate or adaptive immune responses or due to their failure to adapt to the new environment, resulting in cell death [39, 40]. Surviving DTCs may enter a dormant phase, wherein they remain viable but cannot sustain proliferation, as their growth is suppressed by immune mechanisms or constrained by the stromal components of the TME, resulting in minimal net metastatic expansion [2, 41] (Figure 1). In this dormant state, DTCs can persist for extended periods. However, due to their low numbers and minimal activity, detecting micrometastatic clusters clinically remains challenging, making it difficult for healthcare providers to accurately assess patient status at this stage [42, 43].
The TME comprises a variety of immune and stromal cells that co-evolve and interact with tumor cells. Initially, the TME tends to exert anti-tumor effects, inhibiting tumor cell growth during the early phases of colonization. Over time, however, tumor cells adapt and co-opt the TME, evading immune surveillance while promoting colonization through mechanisms such as regeneration, angiogenesis, and the activation of immunosuppressive pathways [44]. For instance, breast cancer cells can alter fibroblast phenotypes by secreting IL-1α and IL-1β, which induces lung fibroblasts to produce C-X-C motif chemokine ligand 9 (CXCL9) and CXCL10 via nuclear factor kappa-B (NF-κB) signaling, thereby promoting the development of lung metastases [45]. Additionally, fibroblasts activated by insulin-like growth factor 2 secreted from inhibitor of DNA binding 1 (ID1)-overexpressing cancer cells can secrete vascular endothelial growth factor (VEGF), which primes the TME and facilitates the involvement of bone marrow cells in supporting tumor growth and distant metastasis [46]. Similarly, (transforming growth factor-beta 1 (TGF-β1) secreted by colorectal cancer (CRC) cells induces anterior gradient 2 release from recruited neutrophils, creating a positive feedback loop that accelerates CRC metastasis [47]. A deeper understanding of how cancer cells hijack host environments is crucial to curbing metastatic colonization. By advancing this knowledge, preventing the establishment of metastatic colonization may remain the most effective strategy against the progression of secondary tumors.
The metastatic cascade is multifaceted, with the same molecules and pathways often playing distinct roles depending on the context—particularly during the primary tumor's proliferation and its dissemination. Phenotypic plasticity, defined as the dynamic, non-genetic adaptation of tumor cells to the metastatic cascade and their ability to respond to shifts in the TME, is now recognized as a key hallmark of metastasis and a critical area of research [19]. Currently, studies have found that circadian rhythms as well as the microbiome play key roles in the immune response against cancer metastasis. The latest study explored the impact of the biological clock on the TME and tumor metastasis and identified an important role for the biological clock-regulated gut microbiota and its metabolites in the evolution of the TME and tumor metastasis [48]. Metastasis represents an ongoing process of cellular and microenvironmental reprogramming, where the selective evolution of tumor cells is able to withstand microenvironmental stresses [49]. As metastasis progresses, unchecked tumor growth leads to systemic organ dysfunction and deterioration, ultimately culminating in the collapse of vital organ function and death.
3 Genetic and Epigenetic Regulation
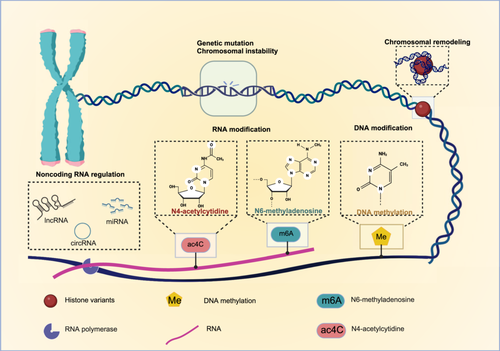
In the process of tumor metastasis, gene inheritance and expression regulation are critical factors that influence the metastatic potential of tumor cells. Both genetic alterations and epigenetic modifications significantly impact cell migration, invasiveness, and adaptability to the TME, ultimately promoting metastatic progression. Therefore, the effects of genetic regulation (gene mutation and chromosome instability) and epigenetic modification (chromosome remodeling, DNA methylation, RNA modification, and non-coding RNA modification) on tumor metastasis will be described. These processes collectively shape the metastatic behavior of tumor cells (Figure 2).
3.1 Genetic Inheritance of Tumor Metastases
Understanding the relationship between gene mutations and metastasis is essential for advancing knowledge of tumor biology and developing targeted therapies to inhibit metastasis. Chromosomal instability (CIN) is another critical factor, as it leads to abnormal chromosomal alterations that enhance the adaptability of tumor cells to various environments, thus promoting invasion and metastasis [50].
3.1.1 Genetic Mutations in Tumor Metastasis
Each metastatic tumor cell constitutes an evolutionary offshoot of its parental primary tumor, inheriting its genomic makeup and key oncogenic alterations. While primary cancer cells do not actively select for metastatic capabilities, they can acquire such potential through the accumulation of specific adaptations and molecular markers [51].
Among the numerous genetic alterations found in metastatic tumors, only a subset is classified as “driver alterations.” These changes equip primary cancer cells with the adaptive features necessary for metastasis. The concept of cancer stemness is considered fundamental to tumor progression. Specific mutations, such as those in tumor protein p53 (TP53) and kirsten rats arcomaviral oncogene homolog (KRAS), drive malignant transformation and metastasis, while structural variants like gene amplifications and deletions are common in CSCs, influencing gene expression and enhancing metastatic potential [52]. Genomic analyses comparing metastatic and primary tumor samples reveal that metastatic lesions may exhibit either enrichment or loss of specific genes relative to primary tumors, indicating the presence of metastasis-specific genes [53, 54]. These observations contribute to understanding the temporal dynamics of metastasis and the origins of metastasis-related genes.
The linear progression model posits that genomic instability escalates early in cancer cell proliferation, with certain cells gradually accumulating genetic alterations through successive cycles of mutation and selection, thereby acquiring metastatic traits. According to this model, metastasis is a late event, with both primary and metastatic cells sharing a substantial number of genomic alterations [55]. In contrast, the parallel progression model suggests that tumor cells disseminate early in the disease course and develop distinct genetic profiles at metastatic sites in response to local microenvironmental conditions. This model asserts that metastasis is an early event, with metastatic cells exhibiting unique genomic features based on their location [56, 57]. In the TRACERx renal study, a digital approach was used to analyze tumor models, suggesting that during tumor progression, a group of highly invasive and metastatic cell clones might form in the early stages, although it is also possible that they emerge in the later stages [58]. This volumetric growth model helps to explain that the heterogeneity observed in tumor growth may arise both in the early and late stages.
3.1.2 Chromosomal Instability
CIN, a key hallmark of human cancers, is linked to poor prognosis, metastasis, and treatment resistance. CIN arises from mitotic errors during chromosome segregation, resulting in both structural and numerical chromosomal abnormalities [59]. Persistent mis-segregation of chromosomes maintains a cytoplasmic DNA pool, essential for sustaining a pro-metastatic state [60].
The resulting diversity of somatic copy number alterations (SCNAs) is a key variation driving tumor metastatic evolution [50]. Studies have established a positive correlation between CIN and metastatic burden across 11 tumor types, with evidence suggesting that SCNAs, which are closely associated with metastasis, may be initiated early in tumor development [61]. CIN and cyclic GMP-AMP synthase (cGAS) activation in micronuclei drive endoplasmic reticulum (ER) stress, immune suppression, and metastasis in triple-negative breast cancer, highlighting a potential therapeutic target [59]. Thus, CIN-induced somatic copy number changes likely represent specific events in tumor evolution.
Chromosome segregation errors can also lead to the formation of micronuclei in cancer cells, which frequently rupture, exposing genomic double-stranded DNA (dsDNA) to the cytoplasm. This cytoplasmic dsDNA, a hallmark of metastatic cancer cells [62], is detected by cGAS, triggering the cyclic GMP-AMP synthase stimulator of interferon genes (cGAS-STING) innate immune pathway. Activation of this pathway promotes inflammatory signaling through antiviral responses and stimulates the atypical NF-κB signaling pathway, thereby enhancing tumor metastasis [60, 62].
Interestingly, while the cGAS-STING pathway in normal cells is activated upon pathogen recognition, inducing type I interferon responses and initiating an immune reaction, its activation in tumor cells does not result in downstream interferon production. This inhibition prevents immune clearance of tumor cells, contributing to metastasis [59]. These findings offer new insights into the immune evasion mechanisms mediated by cGAS-STING activation in cancer cells.
3.2 Epigenetic Regulation of Tumor Metastasis
For primary tumors to metastasize, a cascade of adaptive changes driven by both genetic and epigenetic modifications is required. Epigenetics encompasses regulatory mechanisms that influence gene expression without altering the underlying DNA sequence [63]. This dynamic regulation plays a pivotal role in metastasis [64], as aberrant activation of epigenetic elements is a hallmark of tumor cells, contributing to both drug resistance and metastatic potential [65]. Therefore, epigenetic regulation is fundamental to tumor progression. Mechanisms such as chromatin remodeling, DNA modification, RNA modification, and non-coding RNA play significant roles in tumor metastasis and merit further exploration [66].
3.2.1 Chromatin Remodeling
Mutations in chromatin remodeling machinery are prevalent across solid tumors [67], and the adenosine triphosphate (ATP)-dependent chromatin remodeling complexes involved in metastasis promote nucleosome sliding, histone assembly or disassembly, and the exchange of histone variants [68]. Notably, components of the SWItch/sucrose non-fermentable (SWI/SNF) complex are frequent targets of mutational inactivation [69], with these specific subunit mutations being commonly observed in various tumors, establishing the SWI/SNF complex as a bona fide tumor suppressor [70].
Histone variants are essential in remodeling chromatin by replacing promoters and enhancers [67], thereby increasing chromatin accessibility and activating transcriptional programs that drive tumor progression and metastasis [71, 72]. In CRC liver metastases, the DPP4 promoter exhibits elevated levels of the acetylation of lysine 27 on histone H3 (H3K27ac) histone activation marker, indicating active chromatin remodeling and upregulated promoter expression [73]. Additionally, histone chaperones are key regulators of chromatin structure, facilitating the interaction between classical histones and chaperones to mediate epigenetic regulation in tumors [74].
3.2.2 DNA Modification
DNA modification is a key epigenetic mechanism in tumor metastasis, with DNA methylation being the most prevalent form, often silencing gene expression [75]. Certain DNA methylation target genes are recognized as cancer drivers, and the accumulation of clonal patches with abnormal methylation can establish a pre-cancerous field, increasing susceptibility to oncogenic mutations prior to driver mutations [76]. Methylation changes in cytosine-phosphate-guanine (CpG) islands at specific loci have been implicated in tumorigenesis and cancer progression [77].
Aberrant methylation can manifest as localized hypermethylation of CpG island promoter regions in specific genes or genome-wide hypomethylation in repetitive genomic regions, both of which are linked to malignancies. Specific hypomethylation events, such as the hypomethylation of the signal-induced proliferation-associated protein 1 (SIPA1) gene, further illustrate its role in cancer [78]. In small-cell lung cancer (SCLC), hypomethylation of enhancer of zeste homolog 2 (EZH2) leads to its overexpression, which subsequently downregulates the TGF-β–SMAD pathway, promoting metastasis [79].
Interestingly, different metastatic sites of the same primary tumor exhibit distinct DNA methylation profiles. For instance, CAFs isolated from liver metastases show higher levels of NAD(P)H:quinone oxidoreductase 1 (NQO-1) methylation compared to normal liver fibroblasts, while CAFs from lung metastases retain baseline methylation levels [80]. Additionally, methylation patterns in glioblastoma vary among its differentiated forms [81]. Over the past few years, DNA methylation's regulatory role in tumor immunity has been extensively studied, providing further insights into its impact on cancer progression [82]. Research has found that ten-eleven translocation 2 (TET2) condensation regulates DNA demethylation. Disruption of TET2 condensation promotes aberrant DNA demethylation and inhibits leukemia cell growth [83]. Moreover, this finding is similar to the effects of hypomethylating treatments in leukemia patients, suggesting potential clinical applications.
3.2.3 RNA Modification
RNA modification is a significant epigenetic mechanism in tumor metastasis, playing a pivotal role in various biological processes, with its dysregulation frequently linked to cancer progression [84]. Four primary RNA modifications—N6-methyladenine (m6A), N1-methyladenine (m1A), alternative polyadenylation (APA), and adenosine-to-inosine (A-to-I) RNA editing—have been implicated in tumorigenesis and metastasis.
Both m6A and N4-acetylcytosine (ac4C) modifications influence RNA stability and translation efficiency [85]. Extensive research has highlighted the role of m6A modification in cancer biology [86, 87], particularly its involvement in tumor angiogenesis [88]. For instance, m6A modifications regulate the production of non-coding RNAs such as miR-17-92 and let-7e-5p, which in turn affect the expression of thrombospondin (Tsp), a key factor in angiogenesis [89].
Ac4C modification, a newly identified and highly conserved RNA modification, is the sole acetylation event in eukaryotic RNA, involving the addition of an acetyl group (CH3CO) to cytosine (C) in RNA. This modification enhances the stability and translation efficiency of mRNA, promoting tumor development [90]. N-acetyltransferase 10 (NAT10), the catalytic enzyme responsible for RNA acetylation [85], has been shown to stabilize mRNA through ac4C modification [91]. This research reveals a novel NAT10-ac4C/eEF2-HMGB2 (eukaryotic elongation factor 2–high mobility group box 2) axis that regulates the growth and metastasis of hepatocellular carcinoma (HCC) [92]. The recent discovery of lysine 2-hydroxyisobutyrylation (Lys2-HIB) modification of NAT10 has deepened the understanding of ac4C modification, as Lys2-HIB significantly enhances NAT10's acetyltransferase activity, facilitating ac4C modification of mRNA and promoting cancer cell metastasis [93]. Although research on ac4C modification is still in its early stages, its potential implications for tumor metastasis offer promising avenues for future investigations and therapeutic development.
3.2.4 Noncoding RNA Regulation
Noncoding RNAs (ncRNAs) have significant potential to regulate cellular processes [71, 94]. Numerous studies have demonstrated that ncRNAs play critical roles in tumor metastasis, with long noncoding RNAs (lncRNAs), circular RNAs (circRNAs), and microRNAs (miRNAs) being particularly important in this context [95].
LncRNAs are precisely regulated in various biological processes, including tumor cell proliferation and migration [96]. For example, metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is pivotal in tumor initiation and metastasis reactivation across multiple cancer types [97]. In highly metastatic lung cancer cells, lnc-MLETA1 has been identified as upregulated, particularly in metastatic cells and their secreted exosomes [98]. Additionally, urothelial cancer-associated 1 (UCA1) promotes tumor invasion and migration by regulating EMT and interacting with diverse signaling pathways [99].
CircRNAs, generated through back-splicing of introns or exons [100], have been found to exhibit abnormal expression in numerous tumor types [101]. They can neutralize the inhibitory effects of miRNAs on target gene mRNAs by competitively binding to them [102]. Furthermore, circRNAs are implicated in tumor angiogenesis, particularly in hepatocellular carcinoma [103].
MiRNAs are key regulators of metastasis, controlling the expression of pro-metastatic genes across various tumor types and influencing critical stages of the metastatic cascade, such as tumor cell detachment, EMT, migration, and invasion [104]. Overexpression of certain miRNAs has been linked to enhanced metastasis through their regulation of tumor suppressor genes and associated pathways [105]. For instance, circNOX4 promotes tumor growth and metastasis by activating the fibroblast niche via the miR-329-5p/FAP/IL-6 axis [106].
4 Post-Translational Modifications of Protein (PTMs)
PTMs play a central role in tumorigenesis [107], primarily through the addition of functional groups to specific amino acids in proteins [108]. In addition to common modifications such as phosphorylation, acetylation, and ubiquitination, emerging modifications exhibit complex regulatory roles. These modifications are mediated by various enzymes and serve distinct functions as writers, erasers, or readers. The intricate regulation of PTMs presents promising opportunities for the development of novel therapeutic strategies [109] (Figure 3).

4.1 Phosphorylation
Phosphorylation is one of the most evolutionarily conserved PTMs, notable for its dynamic and reversible characteristics, leading to diverse functional outcomes. This process involves the addition of phosphate groups to amino acids such as serine, threonine, or tyrosine, regulated by ATP, protein kinases, and protein phosphatases [110, 111]. The reversible nature of phosphorylation is mediated by phosphatases, including specific serine and threonine phosphatases [108]. Phosphorylation is integral to numerous cellular functions, including protein–protein interactions, stability, signal transduction, transcriptional regulation, intracellular localization, and apoptosis [112]. Aberrant phosphorylation has been recognized as a key factor in disease development and progression, particularly in cancer. For instance, mitogen-activated protein kinase 1 (MAPK) signaling-mediated phosphorylation and the subsequent subcellular translocation of pyruvate dehydrogenase E1 component subunit alpha (PDHE1α) have been linked to tumor immune evasion. Furthermore, low PDHE1α phosphorylation levels are associated with poor prognosis in patients with lung cancer [113]. Research indicates that mutations in kinases or phosphatases can alter phosphorylation patterns, disrupting the intricate balance of cancer-related proteins and acting as both contributors to and consequences of tumorigenesis [114]. These insights emphasize the importance of unraveling the complex interplay between phosphorylation and disease pathogenesis, particularly in cancer.
4.2 Acetylation
Acetylation is a fundamental mechanism for gene expression regulation, primarily governed by lysine acetyltransferases (KATs) and lysine deacetylases [115, 116]. KATs, also known as histone acetyltransferases (HATs), consist of a catalytic subunit and accessory proteins that transfer acetyl groups to lysine residues [117, 118]. Histone deacetylases (HDACs), classified into four groups—Class I, Class IIA, Class IIB, Class III, and Class IV—remove acetyl groups, thereby modulating acetylation [119]. Acetylation plays a vital role in chromatin remodeling and transcriptional activation, influencing essential biological processes such as tumor cell proliferation, apoptosis, metastasis, and stem cell behavior. Research underscores the significance of histone acetylation regulators in managing HCC and improving patient outcomes, offering promising potential for clinical application [120]. NAT10 has also been implicated in promoting colon cancer progression by inhibiting ferroptosis through N4-acetylation and stabilization of ferroptosis suppressor protein 1 (FSP1) mRNA [121]. Recent findings highlight histone H2B N-terminal multisite lysine acetylation (H2BNTac) as an active enhancer, regulated by CBP/p300 and HDACs 1 and 2, marking active enhancers and serving as a predictor for CBP/p300 target genes [122]. Acetylation modifications also influence immune function and tumor immunity [123], suggesting important implications for developing novel cancer therapies.
4.3 Ubiquitination
Ubiquitination is a key post-translational modification involved in regulating numerous cellular processes, including proteasomal degradation, DNA damage repair, and cell cycle progression [108]. The attachment of ubiquitin to target proteins determines their fate by modulating interactions with proteins containing various ubiquitin-binding domains. The role of ubiquitination in cancer is multifaceted. For instance, SMAD-specific E3 ubiquitin protein ligase 2 (SMURF2) has been shown to act as a tumor suppressor by regulating genomic stability through ubiquitin-dependent modification of ring finger protein 20 (Rnf20). Additionally, SMURF2-mediated ubiquitination and degradation of NAD-dependent deacetylase sirtuin 1 (SIRT1) inhibit CRC cell proliferation and tumor growth [124]. Linear ubiquitination also plays a significant role in modulating NF-κB-dependent inflammatory signaling and immune responses by mediating epidermal growth factor receptor (EGFR)-induced NF-κB activation, which contributes to tumorigenesis [125]. Recent studies have also highlighted the importance of non-lysine ubiquitination in various biological processes, such as endoplasmic reticulum-associated degradation, immune signaling, and neuronal functions. This expands the scope of ubiquitination beyond protein substrates to non-protein targets, revealing a novel ubiquitination cascade that regulates the integrated stress response (ISR). Activation of ISR has emerged as a potential therapeutic strategy [126]. In addition to ubiquitination, studies on the deubiquitinase family can offer new insights into the understanding of cancer pathology [127]. These insights are expected to propel significant advances in ubiquitin research, offering new opportunities for therapeutic intervention [128].
4.4 Ubiquitin-Like (UbL)
The PTM of UbL proteins constitutes a complex signaling language that governs nearly all cellular processes [129]. The UbL pathway has become a promising therapeutic target due to its central role in cancer, and research into UbL modifications has been abundant in several areas.
4.4.1 ISGylation
Iinterferon-stimulated gene 15 (ISG15), the first discovered UbL protein, was identified in studies of type I interferon-treated cells. It consists of two UbL domains connected by a short hinge region, sharing approximately 30% amino acid sequence homology with ubiquitin. Unlike ubiquitination, ISGylation has limited target specificity, with only a few host proteins non-covalently binding to ISG15, the best-characterized being ubiquitin-specific peptidase 18 (Usp18) [130]. ISG15 exists in both intracellular and extracellular forms, although the function of free ISG15 remains poorly understood and warrants further investigation [131]. Under physiological conditions, ISG15 expression is low in normal cells and tissues, but elevated in various cancers, including BCa, colon adenocarcinoma (COAD), pancreatic ductal adenocarcinoma, nasopharyngeal cancer, and oral cancer [132]. The process of ISGylation is tightly regulated, and the constitutive overexpression of ISG15 and its conjugates is an intrinsic feature of malignancies, promoting tumorigenesis and metastasis [133]. Recent research has shown that ISG15 acts as a post-translational modifier of programmed cell death ligand 1 (PD-L1), reducing PD-L1 stability, thus highlighting ISG15 as a potential target for cancer immunotherapy [134].
4.4.2 Sumoylation
Sumoylation, similar to ubiquitination, is a tightly regulated enzymatic process involving both conjugating and deconjugating enzymes. It begins with the synthesis of small ubiquitin-like modifier (SUMO) as an inactive precursor, which is then cleaved by Sentrin-specific proteases to generate mature SUMO molecules. Activation of these molecules is driven by the SUMO-activating enzyme (SAE1/SAE2 heterodimer) in an ATP-dependent process. Subsequently, SUMO is transferred to the catalytic cysteine of ubiquitin-conjugating enzyme E2 I (Ubc9), the sole SUMO-specific conjugating enzyme, which facilitates its attachment to the designated substrate [135]. Recent studies have revealed that SUMOylation regulates extracellular vesicles, promoting lymphatic metastasis in non-small cell lung cancer [136]. Additionally, proteins from both RNA and DNA virus families have been shown to undergo SUMO coupling, enhancing viral replication, thus highlighting the potential of SUMOylation as a therapeutic target in virus-induced diseases [137].
4.4.3 Neddylation
Neddylation is a reversible process in which the eural precursor cell expressed developmentally down-regulated 8 (NEDD8) moiety is covalently linked to the lysine (K) residue of target proteins through a three-step enzymatic cascade. This cascade activates NEDD8 and facilitates its attachment to the lysine (K) residue, playing a pivotal role in regulating ubiquitination. While the roles of E1 activating enzymes (NAE) and E2 conjugating enzymes (Ubc12) and ubiquitin-conjugating enzyme E2 F (UBE2F) are well-established, the specific functions of E3 ligases remain less defined. Neddylation is essential for maintaining cellular function and controlling various biological processes [138]. Studies have shown that enzymes in the NEDD8 pathway are frequently over-activated in lung adenocarcinoma and squamous cell carcinoma. Additionally, high NEDD8 expression has been identified as a negative prognostic factor in nasopharyngeal carcinoma development [139].
4.4.4 UFMylation
Ubiquitin fold modifier 1 (UFM1) is a compact Ubl modifier composed of 85 amino acids with a molecular weight of 9.9 kDa. It is conjugated to the lysine residue of target proteins through a process catalyzed by three enzymes. To date, only a limited number of UFM1 substrates have been identified, including UFM1 binding protein 1 (UFBP1), cyclin-dependent kinase 5 regulatory subunit associated protein 3 (CDK5RAP3), and activating signal cointegrator 1 (ASC1) [138]. The UFM1 system plays a role in various cellular pathways, particularly in scaffolding large protein complexes to maintain endoplasmic reticulum homeostasis, coordinating DNA damage responses, and supporting tumor suppressor functions. Dysregulation of UFMylation has been implicated in the pathogenesis of several diseases, including cancer, diabetes, and inflammatory disorders [129].
4.4.5 FAT10ylation
Ubiquitin-like modifier human leukocyte antigen F-associated transcript 10 (FAT10), also known as ubiquitin D (UBD), is a ubiquitin-like modifier that contains two ubiquitin-like domains and has been directly linked to tumorigenesis [138]. Its overexpression in tumor tissues significantly enhances its role in promoting malignancy. Research indicates that FAT10 interacts with key proteins such as mitotic arrest deficient 2 (Mad2), p53, and β-catenin, facilitating the survival, proliferation, invasion, and metastasis of both cancerous and non-cancerous cells. FAT10 expression is markedly elevated in several cancers, including COAD, HCC, gastrointestinal, pancreatic, and glioma cancers, with a strong correlation to disease progression and poor prognosis, including increased metastatic potential [140]. For instance, FAT10 is highly expressed in CRC, where it promotes cell proliferation by accelerating p53 degradation [141].
5 Metabolic Adaptations
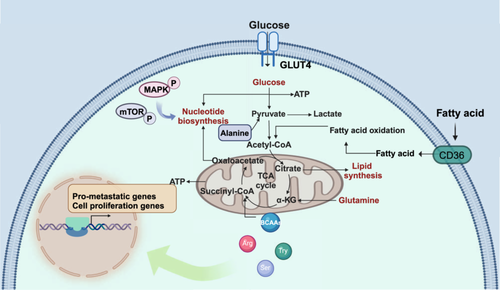
Tumor metastasis encompasses not only changes in cellular properties but also profound shifts in the microenvironment. A hallmark of cancer is metabolic reprogramming, which drives tumor progression by optimizing the regulatory pathways of various metabolic substances to foster conditions conducive to metastasis (Figure 4). Recent research on tumor metabolism identifies two key traits of metastatic cells: metabolic plasticity and flexibility. Metabolic plasticity enables cancer cells to modify their metabolic pathways in response to environmental fluctuations, while metabolic flexibility allows them to alternate between metabolic processes to fulfill the energy and biosynthetic requirements for metastasis. These attributes are essential for cancer cell survival and dissemination under diverse microenvironmental conditions [142].
5.1 Glucose
Glucose is a critical energy source for the survival and proliferation of tumor cells. Due to mitochondrial dysfunction and hypoxic conditions, cancer cells primarily rely on aerobic glycolysis to extract glucose from their surroundings [143]. Additionally, tumor cells can absorb glycolytic byproducts such as lactate and pyruvate, which further promote invasion and migration. Lactate activates the NF-κB signaling pathway, thereby enhancing metastasis, and also regulates DNA repair mechanisms, contributing to both tumor invasion and chemotherapy resistance [144, 145]. Elevated plasma pyruvate levels stimulate the hypoxic response via hypoxia-inducible factor 1α (HIF1α), which sustains circulating tumor cells and influences angiogenesis, as well as EMT processes [144]. Pyruvate also inhibits HDAC3, facilitating tumor cell migration and promoting metastasis [146, 147]. Tumor cells display markedly increased expression of pyruvate kinase 2, which activates EMT and signal transducer and activator of transcription 3 (STAT3) signaling pathways [148] and enhances the activity of fructose-2,6-bisphosphatase (PFKFB2), sustaining glycolysis and driving cancer progression [149, 150]. Further investigation into the metabolic regulation of the microenvironment reveals that glucose uptake and metabolism profoundly influence tumor-associated macrophages (TAMs), which in turn promotes metastasis and heightens resistance to chemotherapy [142, 151]. Elevated fructose levels also contribute to cancer progression through increased glycosylation [152]. Moreover, oxidative phosphorylation plays a pivotal role in cancer metabolism [153]. Understanding these metabolic adaptations and their effects on the TME sheds light on the mechanisms underlying cancer progression and treatment resistance.
5.2 Lipid
Excessive lipid uptake, coupled with abnormal expression or hyperactivation of key enzymes involved in de novo lipid synthesis and catabolism, supports tumor growth, invasion, and metastasis [154]. For instance, the transmembrane protein cluster of differentiation 36 (CD36), responsible for fatty acid transport, is upregulated during metastasis, leading to substantial fatty acid absorption. This accumulation drives the EMT and accelerates cancer progression [155]. The lipid synthesis pathway, particularly mediated by fatty acid synthase, has been identified as a vital driver of metastasis [156]. Fatty acid-binding proteins (FABPs) are pivotal in regulating lipid metabolism and energy balance, with several FABPs overexpressed in tumors and implicated in tumorigenesis. Notably, FABP4 facilitates metabolic crosstalk between ovarian cancer cells and adipocytes during omental metastasis [157]. FABP5 promotes lymph node metastasis in cholangiocarcinoma by activating lipolysis and fatty acid synthesis, increasing intracellular fatty acids and triggering NF-κB signaling [158]. Moreover, fatty acid oxidation generates mitochondrial reactive oxygen species, which promote EMT in tumor cells [159]. Recent studies have also highlighted the significant role of cholesterol metabolism in tumor development [160], and tissue-specific lipid metabolism has been linked to the organ selectivity of metastasis [161]. These findings on lipid metabolism adaptations offer novel insights into the mechanisms driving cancer progression.
5.3 Amino Acid
Reprogramming of amino acid metabolism similarly orchestrates energy production, redox homeostasis, and other metabolic pathways critical to cancer metastasis. Arginine metabolism, for example, is intertwined with fatty acid metabolism and regulates ovarian tumor progression [162]. Additionally, arginine acts as a feedback regulator of tumor metabolism, functioning similarly to a second messenger, thereby furthering tumor progression [163]. Variations in branched-chain amino acids (BCAAs) significantly affect tumor cell behavior. The enzyme branched-chain amino acid transferase 1, which catalyzes BCAA metabolism, is highly expressed in many malignant tumors and is linked to increased tumor proliferation, invasion, and metastasis [164]. Elevated amino acid metabolism is especially evident in tumor cells, particularly for glutamine. Glutamine metabolism produces α-ketoglutarate, tricarboxylic acid cycle intermediates, and ATP, which metastatic tumors rely on to modify cell adhesion and activate invasion signaling [144]. Additionally, tryptophan metabolism is frequently upregulated in tumors, enhancing the invasion-metastasis cascade [165]. This increase in tryptophan metabolism fosters tumor stemness, mediated by BicC family RNA binding protein 1, and contributes to drug resistance [166]. Glycine metabolism, regulated by glycine decarboxylase and serine hydroxymethyltransferase 2, plays a significant role in tumor progression [167, 168]. Furthermore, serine metabolism promotes perineural invasion and metastasis by modulating S-adenosylmethionine levels [169, 170]. These insights into the reprogramming of amino acid metabolism deepen our understanding of the metabolic adaptations that underlie cancer metastasis.
5.4 Nucleotide
The invasive behavior of cancer cells, regardless of type or genetic background, consistently depends on metabolic processes, particularly the aberrant activation of nucleotide synthesis and utilization [171]. Nucleotide metabolism plays a multifaceted role in cancer metastasis. Dysregulation of key signaling pathways, such as MAPK/ERK (extracellular regulated protein kinases) and mammalian target of rapamycin, in tumor cells enhances nucleotide synthase activity, which in turn supports rapid cell proliferation and increases invasive potential [172]. Elevated nucleotide synthesis also aids in repairing DNA damage caused by the oxidative and stressful conditions within cancer cells, thereby improving the survival of migrating cells and contributing to treatment resistance [173]. Moreover, nucleotide metabolism influences the TME by regulating immune evasion. For example, increased adenosine synthesis by tumor cells inhibits T-cell cytotoxicity [174], while the secretion of uracil nucleotides facilitates the recruitment of TAMs and further promotes immune escape [175]. Targeting these metabolic pathways offers a promising therapeutic approach to inhibit tumor metastasis.
5.5 Other Metabolites
Beyond the three major metabolites—glucose, lipids, and amino acids—tumor metastasis is intricately regulated by other metabolites. Acetic acid, for instance, upregulates c-MYC expression, thereby reshaping metabolic pathways and promoting immune evasion [176]. Succinic acid has been shown to drive metastasis both directly and indirectly [177, 178]. Similarly, d-2-hydroxyglutarate (D-2-HG) induces epithelial–mesenchymal transition and is linked to distant metastasis in colorectal cancer [179]. Interestingly, the precursor α-ketoglutarate (α-KG) can act synergistically with SLC25A (mitochondrial carrier subfamily of solute carrier protein 25) inhibitors to reprogram metabolism and enhance tumor sensitivity to radiotherapy [180]. These metabolites in the TME often exhibit dual functions, reflecting the complexity and diversity of their roles. Understanding their precise mechanisms remains a significant challenge in unraveling the intricacies of tumor metastasis.
6 The Tumor Microenvironment (TME)
6.1 Overview of the TME
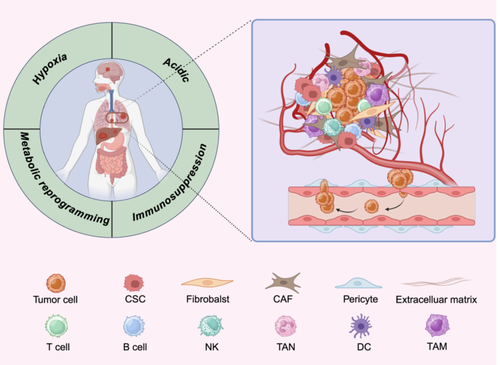
The TME is an intricate and dynamic system that plays a pivotal role in promoting tumor development, progression, metastasis, and resistance to treatment [181]. It comprises diverse components, including tumor cells, non-tumor cells, ECM, and exosomes. Key non-tumor cells include endothelial cells, pericytes, CAFs, immune-inflammatory cells [182], and lipids [183] (Figure 5). The TME is defined by several hallmark features: acidity, hypoxia, angiogenesis, chronic inflammation, and immunosuppression. These conditions create a complex interplay of processes that drive tumor progression [184, 185].
6.1.1 Hypoxia and Acidity
Hypoxia within the TME is particularly critical in cancer progression, as it triggers responses through hypoxia-inducible factors (HIFs) in both cancer cells and surrounding stromal cells. Recent studies utilizing single-cell and spatial transcriptomics have shed light on the mechanisms underlying hypoxia and acidity in the TME, revealing the complex interplay between tumor cells and their microenvironment [186, 187]. Hypoxia often leads to the conversion of fibroblasts into CAFs, which restructure the ECM to facilitate metastasis [188]. It also stimulates the production of VEGF-A by cancer cells, promoting angiogenesis by binding to VEGFR2 on adjacent endothelial cells. In parallel, hypoxia suppresses immune responses, allowing cancer cells to evade immune detection [185, 189]. Notably, HIF-1 primarily governs acute hypoxic responses, while HIF-2 regulates chronic hypoxia, offering potential therapeutic targets for modulating these pathways.
Additionally, tumor cells exhibit pronounced acidification of the extracellular environment due to altered energy metabolism, uncontrolled proliferation, and insufficient perfusion. The reliance on glycolysis in tumor cells results in lactic acid and acidic metabolite production, contributing to an acidic microenvironment. This acidity not only correlates with aggressive tumor behavior and treatment resistance but also fosters genetic instability, chromosomal abnormalities, and epigenetic modifications, accelerating the transition from a precancerous to a malignant state. Moreover, the acidic TME significantly impairs immune cell function, hindering immune cell infiltration and reducing antigen presentation and cytotoxic activity. The resulting immunosuppressive environment promotes tumor immune escape and contributes to resistance to immunotherapy [190].
6.1.2 Chronic Inflammation and Immune Evasion
Chronic inflammation plays a critical role in shaping the TME [191]. Tumors initiate inflammatory responses by secreting cytokines, chemokines, and growth factors that recruit various immune cells into the TME, thereby fostering tumor growth and metastatic progression [192]. Inflammation in tumorigenic tissues promotes cancer development by driving Th2-type immune responses and polarizing bone marrow cells toward immunosuppression. Moreover, chronic inflammation disrupts communication between adaptive immune cells in developing cancers, an irreversible process that undermines effective immune responses [193]. A defining feature of the TME is immune evasion, a major target in anti-cancer research. Tumors deploy multiple strategies to evade immune detection: (1) expressing immune checkpoint molecules like PD-L1; (2) losing antigen presentation capabilities; (3) accumulating immunosuppressive metabolites such as lactate and succinate; (4) recruiting immunosuppressive cells and factors, including regulatory T (Treg) cells, myeloid-derived suppressor cells (MDSCs), polarizing TAMs toward the M2 phenotype, and secreting immunosuppressive cytokines such as IL-10 and TGF-β; and (5) impaired immune cell transport. These mechanisms collectively create an immunosuppressive milieu that enables tumors to evade immune surveillance and resist immunotherapy, highlighting a promising avenue for developing therapies that target immune evasion mechanisms [194].
6.2 Implication of Cellular Components of TME on Metastasis
The TME consists of a variety of cell types, including stromal and immune cells, which interact to create a dynamic environment that can either promote or inhibit tumor development and metastasis.
6.2.1 Cancer Stem Cells
CSCs, characterized by their clonogenicity, self-renewal, and multilineage differentiation, are critical components of this microenvironment [195, 196]. The TME supports CSCs by facilitating interactions through cytokines and signaling molecules, thereby fostering conditions conducive to tumor metastasis and therapy resistance. One of the key properties of CSCs is their plasticity, allowing them to transition between differentiated and stem cell-like states [52]. This plasticity leads to the formation of CSC subpopulations that exhibit low immunogenicity or the ability to eliminate immune cells through immunogenic peptides [197]. Furthermore, CSCs contribute to immune evasion by mimicking other cell phenotypes, thus enhancing metastasis and immunotherapy resistance [198]. Recent research has also uncovered a strong link between circadian regulation and the cooperative promotion of metastasis by CSCs and the TME [199], underscoring the complexity and dynamic role of CSCs in cancer progression.
6.2.2 Stromal Cells
CAFs are the predominant stromal cells in the TME and play a pivotal role in promoting cancer progression [200]. CAFs contribute to tumor growth by secreting growth factors that stimulate the EMT and releasing protein hydrolases that remodel the extracellular matrix, thereby facilitating metastasis [201]. In addition to aiding cancer cell clusters in entering the bloodstream and colonizing distant sites, CAFs also promote lymphangiogenesis, which leads to early-stage lymphatic metastasis [201, 202]. Other key stromal cells include vascular endothelial cells and pericytes. Vascular endothelial cells form a critical barrier to tumor cell extravasation. To combat hypoxia and nutrient deprivation, tumor cells activate endothelial cells through VEGF and neurogenic locus notch homolog (NOTCH) signaling pathways, triggering vascular sprouting. The high-pressure conditions of the TME create an incomplete endothelial barrier, making it easier for tumor cells to invade blood vessels and metastasize [203]. Endothelial cells also undergo endothelial-to-mesenchymal transition, which, through paracrine signaling, further modulates the TME to support tumor progression [204, 205]. Pericytes, mesenchymal cells associated with smooth muscle, support endothelial cells and contribute to tumor angiogenesis. They can directly interact with cancer cells; for example, β3 integrin-deficient pericytes have a paracrine effect that promotes tumor growth [206]. Furthermore, pericytes interact with non-tumor cells in the TME [207] and play a pivotal role in remodeling the perivascular stromal microenvironment, enhancing cancer metastasis [208].
6.2.3 Immune Cells
The immune-infiltrating cells within the TME exhibit dual roles, both hindering and advancing tumor development [209, 210]. TAMs are one of the key immune cell types involved. The polarization from M1-like to M2-like, driven by tumor cells, is a dynamic process closely linked to poor clinical outcomes in patients with cancers [211]. TAMs influence metastasis by interacting with various components of the TME [212], including promoting angiogenesis, activating IL-4 receptors, and facilitating lymphovascular invasion [213-215]. Exosomal signals from tumors can further polarize macrophages and enhance their recruitment, creating a positive feedback loop that accelerates tumor metastasis. Additionally, macrophages produce chemokines like CXCL1 and CXCL5, which stimulate cancer cell migration through the CXCR2/STAT3 feedforward loop [216].
Other immune cells, such as T cells, B cells, NK cells, dendritic cells (DCs), and tumor-associated neutrophils (TANs), are also present within the TME, forming a complex immune microenvironment that promotes cancer progression through intricate signaling networks [209, 217]. Neutrophils can convert CTCs into DTCs, promoting their adhesion to the endothelium and enhancing invasiveness. Additionally, neutrophils release extracellular traps (NETs) that inhibit lymphocytes cytotoxicity and facilitate tumor cell extravasation [218]. Paradoxically, they frequently contribute to cancer progression [219]. Meanwhile, the cytotoxic activity of NK cells and CD8+ T cells in the TME is suppressed, with other immune cells further dampening their tumor-killing capacity. Recent advancements in single-cell transcriptome sequencing technology have enabled researchers to more precisely delineate the heterogeneity of T cells within the TME. This advancement facilitates the identification of specific T-cell subsets that are associated with favorable prognoses, such as CD8+ tissue-resident memory T cells [220]. Dendritic cells can become inhibited in their antigen-presenting functions, leading to an impaired capacity to effectively activate T cells. Moreover, these cells may promote the differentiation of Tregs, thereby facilitating tumor immune escape [221, 222]. Similarly, B cells contribute to anti-tumor immune responses through the production of antibodies and the formation of tertiary lymphoid structures, as well as by activating T cells. However, they also secrete immunosuppressive factors, playing a role in immune evasion [223]. The presence of MDSCs and Tregs exacerbates the immunosuppressive state, significantly increasing tumor metastasis rates [193].
Understanding the dual roles of immune-infiltrating cells and their dynamic interactions within the TME is essential for developing therapeutic strategies that target these processes to inhibit cancer progression.
6.3 TME Heterogeneity
However, the composition and behavior of the TME exhibit significant variability across different cancer types, ultimately influencing the metastatic patterns and treatment responses unique to each tumor. In breast cancer, tumors frequently establish a pre-metastatic niche through interactions with fibroblasts and immune cells. TAMs predominantly adopt an M2-like phenotype, which fosters immunosuppression and angiogenesis, thereby facilitating metastasis to the lungs and bones [224, 225]. Conversely, CRC is characterized by heightened activity of CAFs, which promote ECM remodeling that supports liver metastasis. Notably, CRC cells secrete TGF-β, which drives the transformation of neutrophils into pro-tumor phenotypes, enhancing metastatic dissemination [5, 47]. Prostate cancer cells have a distinct propensity for bone metastasis, a phenomenon attributed to their capacity to exploit the osteoblastic niche. These malignant cells engage with osteoblasts and osteoclasts, thereby altering bone remodeling processes to form metastatic lesions [226, 227]. Recent advancements introduced by Zuo et al. have introduced a deep learning approach termed stKeep, designed to parse TME heterogeneity from spatial omics data. This innovative methodology integrates multimodal and molecular network data, facilitating the construction of cell, gene, and communication modules, which allows for an in-depth analysis of tumor ecosystem heterogeneity [228]. The advancement of emerging technologies offers new perspectives for understanding the mechanisms by which the heterogeneity of the TME influences metastasis, thereby providing a scientific basis for future individualized treatments.
The heterogeneity of the TME not only dictates the mechanisms underlying metastasis among different cancer types but also profoundly impacts the microenvironmental differences observed in metastatic target organs within the same cancer type. The establishment of metastatic lesions in distant sites is supported by pre-metastatic niche, which display diverse regulatory functions that promote metastasis across various tissues [229]. Lymphatic metastasis generally involves lymphangiogenesis and the remodeling of high endothelial venules. In contrast, pulmonary metastasis may promote the generation of Tregs, thereby creating an immunosuppressive microenvironment that further supports metastatic dissemination [230]. In contrast, pulmonary metastasis may engage alveolar epithelial cells to promote the generation of Tregs, forming an immunosuppressive microenvironment [231]. The liver's abundant vascular supply enhances angiogenic pathways, increasing the likelihood of metastasis [232]. In comparison, brain metastasis is closely associated with the activity of microglial cells and extracellular vesicles (Evs), while bone metastasis relies on the physiological activities of osteoclasts and osteoblasts [233-235]. Researchers often use target organ metastasis models based on the specificity of different cancer types to explore the mechanisms of tumor metastasis, for example, lung and breast cancers tend to metastasize to the brain [236, 237], whereas prostate cancer primarily metastasizes to the bone [238]. Commonly, there are in vitro models, such as cell lines and organoid systems, which can simulate the interaction between tumor cells and the host microenvironment. Studies have shown that breast cancer cells exhibit specific growth and metastatic abilities in brain-like microenvironments [239]. Meanwhile, in vivo models (e.g., xenograft models and genetically engineered mouse models) allow observation of the behavior of tumor cells in vivo, providing an opportunity to study the gene regulatory mechanisms of prostate cancer in skeletal metastasis [240]. However, each of these models has its own advantages and disadvantages: in vitro models may not be able to fully mimic the complex microenvironment in vivo, while in vivo models, although biologically relevant, are costly and cumbersome. Therefore, integrating the results from different models will contribute to a comprehensive understanding of tumor metastasis mechanisms and provide new perspectives and directions for subsequent studies.
7 Biomarker of Metastasis
Tumor markers, generated by neoplastic cells or induced by the host in response to neoplastic stimuli, are vital biomolecules that serve as objective indicators for assessing normal physiological processes, pathogenic mechanisms, and therapeutic responses (Figure 6). These biomarkers possess distinct molecular, histological, radiological, and physiological attributes, enabling precise and quantitative evaluation [241].
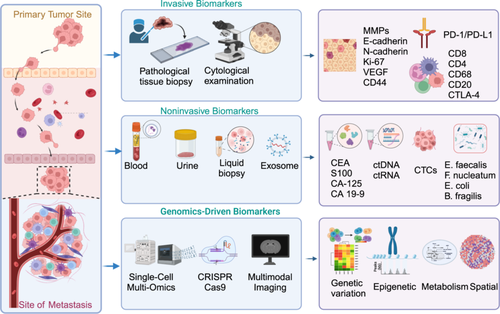
7.1 Applications of Biological Markers
Tumor markers, whether produced by neoplastic cells or triggered by the host in response to neoplastic stimuli, are critical biomolecules that act as objective indicators in the assessment of normal physiological processes, pathogenic mechanisms, and therapeutic responses. These markers, with their distinct molecular, histological, radiological, and physiological characteristics, enable precise and quantitative evaluations [241]. Tumor markers have become essential tools in clinical practice, particularly for monitoring treatment efficacy and detecting metastatic recurrence, making them a key component in dynamic observation and guiding clinical decision-making.
Biomarkers specific to tumor metastasis not only facilitate early disease detection but also inform the development of targeted therapies, thereby providing significant clinical value. Circulating biomarkers such as cell-free mitochondrial DNA (mtDNA), cell-free viral DNA, RNA, and EVs are commonly employed as tumor indicators. For example, a pan-gastrointestinal cancer early detection model, based on circulating free DNA (cfDNA) methylation analysis from 1781 tumors, demonstrated superiority over conventional detection methods [242]. Chemokines and their receptors are essential in the metastatic process, aiding in the identification of metastatic sites and the establishment of pre-metastatic niches [243]. Additionally, matrix metalloproteinases, which are involved in remodeling the TME, serve as important biomarkers for metastasis [5]. Despite the growing application of biomarkers in clinical diagnosis and treatment, challenges such as specificity and variability remain.
Immunotherapy has made significant advances in treating metastatic tumors, yet certain limitations persist, resulting in suboptimal outcomes for some patients. Biomarkers like PD-L1 expression and tumor mutational burden are currently used to predict responses to immunotherapy, but their predictive power is still under investigation [244, 245]. There is a critical need for more precise biomarkers to accurately predict immune responses, identify resistance, and foresee potential side effects. Emerging biomarkers in this field include tumor-specific antigens, immune checkpoint proteins, and immune cell subset characteristics, all of which show potential for improving predictive accuracy and therapeutic optimization. A recent study identified CD8-fit T cells as predictive biomarkers for immunotherapy response through single-cell functional assessment [246]. The development of novel biomarkers for predicting immunotherapy outcomes, particularly using next-generation sequencing technologies, is essential for advancing metastatic tumor treatments in the future.
7.2 Noninvasive Biomarker of Metastasis
Tissue-based molecules face significant limitations due to tumor heterogeneity, making the reliance on single-tissue biopsies potentially inadequate for guiding treatment decisions [247]. The American Society of Clinical Oncology assessed the prognostic utility of various molecular biomarkers from prostate cancer tissue but refrained from recommending their routine use. The lack of prospective validation and insufficient evidence of improved long-term outcomes were key reasons behind this decision [248]. In June 2016, the United States Food and Drug Administration (US FDA) approved the first liquid biopsy to detect EGFR mutations in patients with non-small cell lung cancer, marking the beginning of a new era in tumor liquid biopsies [249].
In response to the growing need for noninvasive diagnostic tools, several biomarkers have been explored, including protein-based, DNA-based, mRNA-based, and circulating miRNAs [250, 251]. A recent study developed and validated a noninvasive multidimensional biomarker assay combining pretreatment circulating tumor DNA (ctDNA) and peripheral T cell signatures to predict immunotherapy response in advanced non-small cell lung cancer [252]. Moreover, findings from the TRACERx study using ctDNA to track early metastatic dissemination in lung cancer have supported advancements in (neo)adjuvant trials and provided insights into metastasis detection through low-ctDNA-level liquid biopsies [253]. The noninvasive 5hmC-Seal technique, which evaluates 5hmC in cfDNA, has also shown promise as a tumor marker for glioma screening and disease monitoring [254]. Additionally, plasma snoRNA SNORD33 has been identified as a predictor of sensitivity to platinum-based chemotherapy in triple-negative BC, aiding in the selection of effective treatments [255]. Innovations like fluorine isotope 19 MRI have enabled the identification of TAMs in glioma genesis and allowed for prognosis monitoring and drug resistance tracking, previously achievable only through invasive methods [256]. Furthermore, a fecal microbial-based classification model combined with CA19-9 has shown 94% accuracy in pancreatic cancer identification, highlighting the potential of noninvasive screening methods [257]. Despite these advantages, large-scale clinical trials are still needed to substantiate the accuracy and cost-effectiveness of these biomarkers.
Exosomes, due to their transfer properties, hold significant promise for developing therapeutic vectors in oncology and drug applications. Their ability to cross the blood–brain barrier makes them particularly valuable for diagnosing central nervous system (CNS) diseases and monitoring CNS status [258]. Consequently, exosomes are emerging as promising diagnostic biomarkers in immunotherapy contexts [259]. The exosome protein hyaluronan-binding protein has been identified as a predictor of brain metastasis progression and patient survival, offering a novel target for preventing and treating brain metastases [260]. Although exosomes demonstrate remarkable transcellular permeability and biocompatibility, which are advantageous for drug delivery, the cost of exosome detection remains high, and evidence supporting co-targeted assays is still limited.
Research into metabolic products in tumor metastasis is unveiling new biological mechanisms and offering critical insights for developing novel diagnostics and therapies. Studies show that alterations in specific metabolic pathways, along with the activation of related signaling pathways, are closely linked to tumors’ invasive and metastatic capabilities, as well as their survival in distant organs. For example, changes in levels of lactate [261], fatty acids [262], and pyruvate [263] can reflect the metabolic state of metastasizing tumors and serve as biomarkers for tumor dissemination.
8 Therapy of Metastases
As understanding of the molecular mechanisms and signaling pathways driving tumor metastasis deepens, the development of treatments for metastatic disease is advancing more rapidly and effectively than traditional methods (Figure 7). This progress includes the swift emergence of new biomarkers and drugs, with the ultimate aim of delivering patient-specific therapies. These advancements are expected to greatly improve outcomes for metastatic diseases by offering personalized therapeutic options. Historically, the diversity and prevalence of cancer have posed substantial challenges to the development of effective anticancer drugs [264, 265].
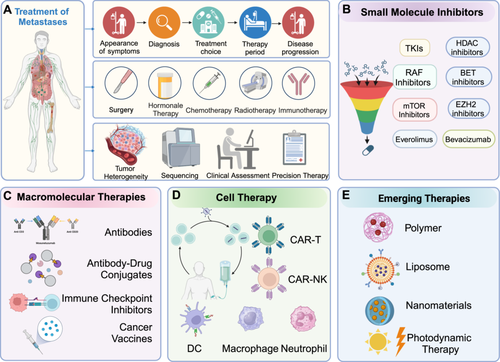
8.1 Small Molecule Drugs
Small molecule inhibitors, recognized for their selectivity, small size, and ability to target intracellular molecules, are considered an ideal first-line treatment for relapse or metastasis due to their minimal side effects [266]. However, the cost of developing a new drug that gains US FDA approval can exceed US$2 billion, making drug development a costly and intricate process requiring extensive efficacy screening [267]. Fortunately, discovering new functions and indications for existing drugs offers a cost-effective alternative to de novo drug synthesis [268]. Table 1 compares small-molecule inhibitors and biologic drugs across key factors like mechanism of action, delivery methods, and clinical use, offering insight into their roles in cancer treatment and metastasis.
| Category | Small-molecule Inhibitors | Biologic drugs | Reference |
|---|---|---|---|
| Mechanism of Action | Target intracellular enzymes or proteins to modulate tumor progression, gene expression, and cell cycle | Act on extracellular targets such as receptors or immune checkpoints, or modulate immune responses to enhance anti-tumor activity | |
| Examples | DNMTi, HDACi, BETi, IDH inhibitors, TKIs, etc. | Anti-PD-1, Anti-HER2, CAR-T Cell Therapies, antibody-drug conjugate (ADC), monoclonal antibodies, etc. | |
| Metastasis Stage | Early stages: invasion and migration | Later stages: Immune modulation and secondary tumor growth | [269] [270] |
| Efficacy | Inhibit invasion, motility, angiogenesis | Enhance immune response, prevent metastasis | [271] |
| Delivery Method | Oral or intravenous, good tissue penetration | Typically administered via subcutaneous or intravenous injection, targeted delivery | |
| Side Effects | Systemic toxicity, organ damage | Immune-related side effects (e.g., inflammation) | |
| Resistance | Gene mutations, pathway activation | Immune evasion, alternative checkpoint activation | |
| Clinical Use | Approved for AML, colorectal cancer, NSCLC, etc. | Used in melanoma, NSCLC, breast cancer, etc. | [272] |
| Combination Potential | Synergizes with biologics and chemotherapy | Combined with chemotherapy or small molecules for synergy |
- Abbreviations: AML, acute myeloid leukemia; BETi, bromodomain and extraterminal domain inhibitor; DNMTi, DNA methyltransferase inhibitor; HDACi, histone deacetylase inhibitor; HER2, human epidermal growth factor receptor 2; IDH, isocitrate dehydrogenase; NSCLC, non-small-cell lung cancer; TKIs, tyrosine kinase inhibitors.
Drug repositioning has opened new possibilities for anticancer treatments, as demonstrated by several cases. For instance, researchers identified the transcription factor KLF11 as a negative regulator of sarcoma CSCs through a CRISPR-CAS9 library screen, while thiazolidinedione, an oral hypoglycemic agent, has shown promise in combination therapies for multiorgan sarcomas [273]. Similarly, the tyrosine kinase receptor inhibitor mubritinib, targeting ERBB2, appears to be a viable treatment option for acute myeloid leukemia [274]. Additionally, recent research identified neurokinin-1 receptor (NK-1R) as a target for combating drug resistance and recurrence in colorectal cancer, with alprazolam acting as a NK-1R antagonist [275]. The antimalarial drug Atovaquone has been shown to inhibit the growth of primary and drug-resistant BC by blocking human epidermal growth factor receptor 2 (HER2)/β-catenin signaling [276]. Despite strong evidence supporting its therapeutic potential, regulatory approval and intellectual property issues have delayed its market entry [277].
Combining epigenetic drugs with conventional oncology therapies offers a promising approach to cancer treatment. Several classes of epigenetic modulators are currently being investigated for their potential in targeting metastasis pathways. Among them, histone deacetylase inhibitors (HDACis), BET protein inhibitors, and EZH2 inhibitors are at the forefront of clinical trials (Table 2). These agents work by modulating chromatin structure and gene expression to interfere with key processes involved in metastasis, such as EMT, cell migration, and invasion. Table 2 summarizes the ongoing clinical trials of epigenetic drugs targeting tumor metastasis pathways [281, 282]. Additionally, isocitrate dehydrogenase (IDH) enzyme inhibitors have been approved by the US FDA for the treatment of acute myeloid leukemia (AML) patients with IDH1 and IDH2 mutations, but these drugs have not been evaluated in the context of metastatic solid tumors [283]. Similarly, although BRD4 inhibitors such as OTX015 [284] and TEN-010 [285] have shown promise in preclinical models of solid tumors, their role in inhibiting metastasis has not yet been extensively tested in clinical trials targeting metastatic disease [286].
| Epigenetic Drug | Cancer type(s) | Combination with | Clinical trial ID | Phase | Status | Reference |
|---|---|---|---|---|---|---|
| HDAC inhibitors | Mechanism of action | Targets histone deacetylase (HDAC) enzymes | ||||
| Targeted pathway(s) | Cell cycle arrest (G1/S phase), apoptosis induction, differentiation of tumor cells, inhibition of metastasis (EMT suppression). | |||||
| Romidepsin | Carcinoma, renal cell, neoplasm metastasis | NCT00106613 | II | |||
| Prostate cancer, metastases | NCT00106418 | II | Completed | |||
| Lung cancer, metastatic cancer | NCT01302808 | I/II | Completed | |||
| Adult alveolar soft-part sarcoma | NCT00112463 | II | Completed | |||
| Male breast cancer, Recurrent breast cancer, Stage IV breast cancer | NCT00098397 | II | Completed | |||
| Metastatic squamous cell carcinoma of the head and neck | NCT00084682 | II | Completed | |||
| Unresectable locally advanced or metastatic colorectal cancer | NCT00077337 | II | Completed | |||
| Entinostat (MS-275) | Metastatic cholangiocarcinoma and pancreatic adenocarcinoma | Nivolumab | NCT03250273 | II | Completed | [278] |
| Abexinostat | Advanced solid tumor malignancies |
Pembrolizumab | NCT03590054 | I/II | Completed | |
| Pracinostat (SB939) | Translocation-associated recurrent, metastatic sarcomas | NCT01112384 | II | Completed | [279] | |
| Quisinostat (JNJ-26481585) | Ovarian cancer | Carboplatin | NCT02948075 | II | Completed | |
| Ricolinostat (ACY-1215) | Metastatic breast cancer, breast carcinoma | Nab-paclitaxel | NCT02632071 | I | Completed | [280] |
BET inhibitors |
Mechanism of action | Block BET protein interaction with acetylated histone lysines. | ||||
| Targeted pathway(s) | Epigenetic regulation: Disrupt histone acetylation; NF-κB signaling: Inhibit inflammation and tumor progression; Cell cycle: Affect proliferation; Metastasis: Suppress EMT and invasion. | |||||
| SF1126 | Advanced or metastatic solid tumors, cancer, solid cancers | NCT00907205 | I | Completed | ||
| ZEN-3694 | Metastatic castration-resistant prostate cancer | NCT02705469 | I | Completed | ||
| Metastatic castration-resistant prostate cancer | Enzalutamide | NCT02711956 | Ib/IIa | Completed | ||
| EZH2 inhibitors | Mechanism of action | Blocks EZH2, a histone methyltransferase that adds methyl groups to H3K27, repressing gene expression. | ||||
| Targeted pathway(s) | Histone methylation regulation, reprogramming cell differentiation, reactivating tumor suppressor genes, and modulating Notch signaling. | |||||
| PF-06821497 | Metastatic castration-resistant prostate cancer | Placebo enzalutamide | NCT06629779 | III | Recruiting | |
| CPI-1205 | Metastatic castration-resistant prostate cancer (mCRPC) | Enzalutamide abiraterone prednisone | NCT03480646 | Ib/II | Recruiting | |
- Note: Data sources (https://clinicaltrials.gov/).
- Abbreviations: BET, bromodomain and extraterminal domain; EZH2, enhancer of zeste homolog 2; H3K27, histone H3 at lysine 27; NF-κB, nuclear factor kappa-B.
The future of small molecule inhibitors looks promising, particularly with the use of predictive non-clinical models to identify potential drug candidates for human trials. Additionally, dynamic indicators from liquid biopsies could play a vital role in monitoring tumor recurrence and metastasis, complementing research into drug-refractory targets. In summary, these advancements may significantly enhance the effectiveness of small molecule inhibitors in cancer treatment [287].
8.2 Macromolecular Drugs
Recent advancements in cancer therapy have underscored the potential of chimeric antigen receptor T (CAR-T) cell therapy and other innovative strategies in addressing various malignancies. A screening study utilizing a protein antibody library has identified a promising CAR-T target for multiple myeloma [288]. Additionally, MSA-2, a non-nucleotide agonist of human STING, was discovered through high-throughput screening and has shown encouraging results in tumor regression and the induction of durable anti-tumor immune responses [289]. Comprehensive knowledge of a tumor's systemic metastatic status is vital for effective drug development. To address this, researchers developed the DeepMACT pipeline, an automated system that quantifies tumor metastasis and evaluates therapeutic antibody targeting, systematically detailing tumor size, shape, spatial distribution, and targeting efficiency [290]. Advancing these treatments requires a multidisciplinary approach that integrates protein engineering, tumor serology, and a deep understanding of immune-tumor cell interactions [291, 292]. Future improvements must focus on enhancing target precision, increasing cytotoxic payloads, refining antibody-drug linker technology, and optimizing combination therapies, including immunotherapy, once underlying mechanisms are fully understood [291].
Nanotechnology is playing a pivotal role in advancing drug delivery systems. For instance, the synthesis of nanostructured MUV-2, a hierarchical mesoporous iron-based metal-organic framework, enables high payload storage of the macromolecular drug paclitaxel, improving its selectivity for HeLa cancer cells while sparing noncancerous HEK cells. NanoMUV-2 also enhances cell permeation, cytosolic delivery, and cancer-targeting effects, thereby increasing paclitaxel's efficacy against cancer [293]. Furthermore, bispecific antibodies targeting CD3 and CAR-T cell therapies are capable of redirecting T cells toward B cell maturation antigen (BCMA)-expressing multiple myeloma (MM) cells, inducing cytotoxicity in target cells [294].
Despite the success of antibody-based therapies targeting programmed cell death protein 1 (PD-1)/PD-L1, cytotoxic T lymphocyte-associated antigen-4, HER2, CD20, and CD38 some treatments have been linked to serious adverse effects [295]. Recent studies have shown that tumor-infiltrating immune cells and CD8 + T cell function exhibit circadian rhythmic fluctuations, with tumor endothelial cells regulating immune cell infiltration through intercellular cell adhesion molecule-1 expression. The research also found that altering the timing of cell therapy and anti-PD-1 antibody treatment, especially in the evening, can significantly enhance therapeutic efficacy. By optimizing treatment timing, the effectiveness of immunotherapies can be improved, providing new insights for personalized treatment and clinical trial design [296]. Consequently, ongoing monitoring of both tumor and systemic immune responses following immune checkpoint inhibitor treatment is essential to gain deeper insights into the mechanisms of efficacy and resistance. Such knowledge can guide the development of novel immunotherapies or combination treatments with broader, more durable efficacy and improved safety profiles [295].
8.3 Cellular Therapy
The field of cellular therapy, particularly CAR-T cell therapy, has transformed cancer treatment, with approvals for refractory pre-B-cell acute lymphoblastic leukemia and diffuse large B-cell lymphoma (DLBCL) [297]. Clinical trials have demonstrated the effectiveness of CAR-T cell therapy in adult patients with refractory or relapsed DLBCL [298, 299]. Notably, the US FDA has approved CD19 CAR-T cell therapy Yescarta (axicabtagene ciloleucel) for treating adult patients with large B-cell lymphoma (LBCL) who are refractory to or have relapsed within 12 months of first-line chemoimmunotherapy. Despite these achievements, CAR-T therapy faces significant obstacles, including life-threatening toxicity, limited antitumor efficacy, antigen evasion, and restricted tumor interactions [300].
Macrophages are also emerging as promising targets for cancer immunotherapy. Sipuleucel-T (Provenge, Dendreon), the first FDA-approved therapeutic cancer vaccine for metastatic castration-resistant prostate cancer, uses a fusion protein that combines a tumor-targeting antigen with granulocyte-macrophage colony-stimulating factor to stimulate antigen-specific T cells and boost antitumor activity [301]. A notable development in this field is the recent initiation of clinical trials for HER2-targeted chimeric antigen receptor macrophage (CAR-M) therapy by Carisma Therapeutics [302]. NK cells, essential for tumor destruction, are also being explored in CAR-based therapies. CAR-NK therapy offers several advantages over CAR-T, including increased safety and the ability to activate multiple cytotoxic mechanisms [303, 304]. In a clinical trial using HLA-mismatched CAR-NK cells derived from cord blood, 11 patients with relapsed or refractory CD19-positive cancers responded positively to the treatment without significant toxicities [305]. CAR-NK therapy leverages the principles of CAR-T therapy while incorporating the innate biological advantages of NK cells, attracting significant attention in scientific and industrial circles. Neutrophils, as frontline defenders and key immune regulators, play dual roles in cancer, either promoting or inhibiting tumor growth through N1/N2 polarization, a topic of substantial interest. Recent studies have shown that CAR-engineered neutrophils significantly enhance antitumor activity and can act as vehicles for targeted drug delivery [306]. Additionally, targeting NETs has emerged as a promising strategy to combat cancer metastasis [307], although effective systemic delivery of these therapies remains a considerable challenge.
8.4 Other Drugs
Traditional cytotoxic antitumor drugs exert their effects by disrupting DNA synthesis or repair in cancer cells, thereby inhibiting cell division and proliferation. Although chemotherapy has broad antitumor efficacy, it is often associated with significant side effects and the development of drug resistance, which can ultimately lead to tumor metastasis. In contrast, endocrine therapy has become the standard of care for estrogen receptor (ER)-positive BC and has achieved substantial clinical success. When combined with cyclin-dependent kinases 4/6 (CDK4/6) inhibitors, it demonstrates direct antitumor activity; however, resistance and distant metastasis still occur in some patients [308]. Increasing evidence underscores the importance of immune modulation in overcoming treatment resistance. Recent advances in immuno-nanomaterials—either with intrinsic immunogenicity or designed to efficiently deliver immune modulators—have shown promise in enhancing immune responses and effectively targeting metastatic tumors [309]. Manganese-based and virus-like nanomaterials, in particular, have been found to significantly improve antitumor immune responses against metastatic disease [310, 311]. Despite these advancements, emerging chemical immune modulators require further optimization to improve targeting specificity and minimize side effects.
8.5 Emerging Therapies and Combination Approaches
The evolution of precision medicine has spurred the development of novel therapies and combination strategies designed to overcome the limitations of conventional treatments in metastatic cancers. Recent advances have focused on targeting new molecular pathways, including those involved in TME and metabolic reprogramming, which have shown promising therapeutic potential. Immunotherapies, particularly immune checkpoint inhibitors, when combined with targeted therapies or chemotherapy, have demonstrated significant improvements in efficacy and the potential to reduce resistance. For instance, the combination of pembrolizumab with chemoradiotherapy has notably enhanced progression-free survival in patients with newly diagnosed, high-risk, locally advanced cervical cancer [312]. Similarly, the NALIRIFOX regimen, which combines liposomal irinotecan, oxaliplatin, fluorouracil, and leucovorin, was recently approved by the FDA as a first-line treatment for metastatic pancreatic cancer (NCT04083235) [313]. Furthermore, metabolic reprogramming of both cancer cells and the immune cells within the TME is emerging as a promising therapeutic approach [314, 315], and combination therapies utilizing oncolytic viruses have emerged as a promising strategy for advancing innovative and effective tumor treatments in the future [316]. While these strategies are in early stages of development, they offer substantial potential for enhancing therapeutic outcomes. However, no combination therapy involving metabolic modulators and immunotherapy has yet received FDA approval. To optimize clinical trial designs and accelerate the drug discovery process, the integration of robust biomarker-driven patient stratification is critical.
Artificial intelligence (AI) is increasingly recognized as a powerful tool in identifying novel therapeutic targets, improving patient selection, and refining clinical trial methodologies, which could significantly enhance the success rates of emerging therapies [312, 317, 318]. AI has significantly increased the efficiency of drug discovery, especially in handling large-scale experimental data. It helps researchers identify potential bioactive compounds from high-throughput screening. By utilizing public databases and machine learning for virtual screening, AI can identify molecules from various compound libraries that may bind to target proteins, thereby accelerating the translation of computational predictions into viable clinical candidates [319].
9 Conclusions and Perspectives
Advances in personalized medicine and clinical oncology have greatly enhanced our understanding of the metastatic cascade, resulting in promising developments for managing metastatic tumors. The rise of molecularly targeted therapies and immunotherapies has created new pathways for treating these malignancies. However, the high cost of these treatments has limited access for some patients [320]. While most therapeutic agents rely on target engagement, the identification of novel targets through phenotypic screening marks a significant technological breakthrough [321]. These innovations underscore the potential of genetic-based approaches in developing targeted therapies and highlight the necessity of a comprehensive strategy that integrates diverse data for effective drug discovery and development [320, 322].
Several key areas require focus to achieve significant progress in treating metastatic tumors. First, further research is needed to elucidate the mechanisms that govern the site-specific timing and interaction of various stages in metastasis. Second, more accurate experimental models are essential for simulating the metastatic process and validating the therapeutic efficacy of potential targets and related compounds. Models for studying metastasis include in vitro models such as 2D and 3D cell cultures, which allow the study of cell behavior but lack the complexity of the TME. Notably, 3D cell culture models (such as spheroids, organoids, and tumor-on-a-chip systems) better simulate the TME, providing conditions closer to those in vivo [323]. These models allow for the study of cell–matrix interactions, drug permeability, and metastatic potential. In vivo models such as xenografts, genetically engineered mice, and patient-derived xenografts (PDX) [324] offer deeper insights into metastasis within a living organism. Additionally, humanized mouse models, where mice are engrafted with human immune cells, are increasingly used to study human-specific immune responses and tumor-immune interactions in metastasis [325]. Imaging technologies also enable real-time tracking of metastatic spread [326]. While these models are valuable for understanding metastasis, they still face challenges in fully replicating human tumor biology and immune responses, highlighting the need for more advanced models to improve cancer therapies. Additionally, clinical trials for anti-metastatic drugs face significant challenges, including the absence of complementary trials to monitor drug efficacy throughout tumor progression and factors beyond the disease itself.
Extensive research has been conducted on using multi-omics data to develop tumor prognostic risk models, which leverage genome-wide information or clinical histology data to track patients over time. However, intratumor heterogeneity often leads to acquired drug resistance, complicating targeted therapy and preventing metastasis [327]. Recent developments in pan-cancer proteogenomics, driven by multi-omics analyses, highlight the value of comprehensive proteogenomic approaches for understanding oncogenic drivers and their roles in cancer development [328]. Stratifying treatment and prognosis based on gene mutations has the potential to significantly improve outcomes and risk assessment for metastasis. Effectively addressing these challenges and harnessing big data in medicine could pave the way for more effective treatments for metastatic tumors [329, 330].
While drug development requires substantial resources from inception to approval, drug repurposing strategies have emerged as a promising alternative. Extensive research across multiple countries has demonstrated that many approved drugs have repurposing potential, although few oncology drugs have successfully transitioned to new indications [331]. Nevertheless, numerous studies provide compelling evidence for the anti-cancer properties of non-oncology drugs, highlighting their potential in treating tumor metastasis. For instance, antipsychotic drugs like chlorpromazine and trifluoperazine hydrochloride have been shown to induce autophagic cell death and inhibit triple-negative BC growth and brain metastasis by triggering G0/G1 arrest and apoptosis [332]. During immune activation, macrophage metabolic reprogramming generates various small-molecule metabolites that induce PTMs of proteins, which can serve as therapeutic targets, particularly in neurodegenerative disorders [333]. Chemical proteomics techniques offer a systematic way to detect functional PTMs triggered by immune metabolism [334]. Different enzymes responsible for PTMs can be exploited as therapeutic targets to reverse neurological deficits [335], while dynamic regulation of protein phase separation through PTMs adds another layer of therapeutic potential [336, 337]. The COVID-19 pandemic has accelerated efforts to develop effective treatments and vaccines [338]. Among the growing pipeline of immunotherapy-based anti-cancer drugs, cellular therapies lead the way, followed by immunomodulators that target T cells [339]. Key challenges in tumor immunotherapy include early assessment of the efficacy of immune combination therapies and the identification of personalized biomarkers to guide treatment [340]. For instance, combination therapies pairing PD-1 inhibitors with chemotherapy have shown significant promise [341]. The future of personalized medicine lies in optimizing these combinations to tailor immunotherapy for individual patients. Overall, these findings emphasize the potential of drug repurposing strategies and provide insights into the anti-cancer mechanisms of non-oncology drugs, potentially paving the way for novel therapeutic approaches in oncology.
Clinical trial failures frequently result from factors such as interactions with multiple drug targets. Thus, a thorough understanding of the complete drug target profile is critical for accurately assessing therapeutic potential [342]. In vivo gene editing therapies offer significant promise for treating tumor metastasis, with delivery technologies such as viral vectors, lipid nanoparticles (LNPs), and virus-like particles serving as key enablers [343-345]. Targeted immune-specific gene therapy, combined with genome editing, holds potential to enhance cancer immunotherapy by targeting multiple genes in T cells through advanced gene editing techniques [346]. LNPs, used as non-viral vectors, primarily facilitate the transport of nucleic acids, including siRNAs and therapeutic mRNAs [347]. The US FDA has approved LNP-based delivery systems for therapeutic siRNAs targeting human hepatocytes via intravenous injection [348, 349]. As gene editing therapies and delivery technologies continue to advance, these innovations are poised to become reliable tools and a foundational component of clinical treatments.
Looking ahead, several critical research challenges must be addressed to improve outcomes for metastatic cancer. These include overcoming tumor heterogeneity and resistance mechanisms, understanding the metastatic microenvironment and its role in organ-specific colonization and developing more effective experimental models for drug testing. Additionally, integrating multi-omics data and leveraging AI-driven insights could help identify novel biomarkers and therapeutic targets. Advancing gene editing and immunotherapy combinations, as well as expanding drug repurposing efforts, offer promising strategies for tackling metastasis. Addressing these challenges will be key to developing more effective treatments and improving patient outcomes. In conclusion, while significant progress has been made in understanding metastasis and developing therapeutic strategies, continued research into tumor biology, drug resistance, and the complex interactions within the metastatic microenvironment will be crucial. Addressing these challenges, along with leveraging new technologies such as AI, gene editing, and multi-omics, will drive the next generation of therapies and improve outcomes for metastatic cancer patients.
Author Contributions
Lin Tang: conceptualization (equal), writing–original draft (lead), writing–review and editing (lead). Shao-Cong Peng: conceptualization (equal), writing–original draft (lead), writing–review and editing (lead). Xiao-Wan Zhuang: conceptualization (equal), writing–original draft (lead), writing–review and editing (lead). Yan He: conceptualization (equal), writing–original draft (lead), writing–review and editing (lead). Yu-Xiang Song: conceptualization (equal), writing–original draft (lead), writing–review and editing (lead). Hao Nie: conceptualization (equal), writing–original draft (equal), writing–review and editing (equal). Can-Can Zheng: conceptualization (equal), writing–original draft (equal), writing–review and editing (equal). Zhen-Yu Pan: conceptualization (equal), writing–original draft (equal), writing–review and editing (equal). Alfred King-Yin Lam: conceptualization (equal), writing–original draft (equal), writing–review and editing (equal). Ming-Liang He: conceptualization (equal), writing–original draft (equal), writing–review and editing (equal). Xing-Yuan Shi: conceptualization (lead), project administration (lead), supervision (lead), writing–original draft (equal), writing–review and editing (equal). Bin Li: conceptualization (lead), project administration (lead), supervision (lead), writing–original draft (equal), writing–review and editing (equal). Wen Wen Xu: conceptualization (lead), project administration (lead), supervision (lead), writing–original draft (equal), writing–review and editing (equal). All authors have read and approved the final manuscript.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.





