Preventive Effect of Indian Gooseberry (Phyllanthus emblica L.) Fruit Extract on Cognitive Decline in High-Fat Diet (HFD)-Fed Rats
Abstract
Scope
Methylglyoxal (MG)-derived advanced glycation end products (AGEs) directly bind to the receptor for advanced glycation end products (RAGE), subsequently exacerbating obesity and obesity-induced cognitive decline. Indian gooseberry (Phyllanthus emblica L.) fruit has antiobesity properties. However, the underlying mechanism by which Indian gooseberry fruit prevents obesity-induced cognitive decline remains unclear.
Methods and results
This study aims to investigate the preventive effect of a water extract of Indian gooseberry fruit (WEIG) and its bioactive compound gallic acid (GA) on the obesity-induced cognitive decline through MG suppression and gut microbiota modulation in high-fat diet (HFD)-fed rats. Trapping MG, WEIG, and GA significantly ameliorate fat accumulation in adipose tissue and learning and memory deficits. Mechanistically, WEIG and GA administration effectively reduces brain MG and AGE levels and subsequently reduces insulin resistance, inflammatory cytokines, MDA production, and Alzheimer's disease-related proteins, but increases both antioxidant enzyme activities and anti-inflammatory cytokine with inhibiting RAGE, MAPK, and NF-κB levels in HFD-fed rats. Additionally, WEIG and GA supplementation increases the relative abundances of Bacteroidetes, Gammaproteobacteria, and Parasutterella, which negatively correlate with MG, inflammatory cytokine, and Alzheimer's disease-related protein expressions.
Conclusion
This novel finding provides a possible mechanism by which WEIG prevents obesity-induced cognitive decline through the gut-brain axis.
1 Introduction
Obesity is one of the most important public health challenges worldwide. Approximately 10% of the global population (7.5 billion people) has obesity, which imposes a substantial economic burden on individuals and society.[1, 2] In addition, the 2019 World Alzheimer's Report mentioned that the number of people living with dementia will increase to 152 million by 2050 and that the economic cost is expected to reach $9.12 trillion by 2050.[3, 4] Overall, obesity and Alzheimer's disease (AD) represent enormous challenges for the global society. Recently, accumulating evidence suggests that aging individuals with obesity develop cognitive impairment and susceptibility to AD.[5] Thus, clarifying the relationship between obesity and AD is advantageous for developing prevention strategies.
According to reported evidence, obesity causes insulin resistance and hyperglycemia, risk factors for accelerating AD progression.[6] In addition, oxidative stress and inflammation play crucial roles in the molecular connection between obesity and cognitive decline.[7] Methylglyoxal (MG) levels are increased during hyperglycemia and it has been identified as the common etiological agent in obesity and AD.[8, 9] MG is the precursor of advanced glycation end products (AGEs), which activate the receptor for advanced glycation end products (RAGE) and downstream signaling pathways to subsequently deteriorate neuronal inflammation and cognitive decline.[10] Moreover, the activation of mitogen-activated protein kinases (MAPKs), such as extracellular signal‑regulated protein kinase1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), and p38, is closely related to the nuclear factor-κB (NF-κB) signaling and induces the pro-inflammatory mediators in neurons.[11] RAGE directly mediates MAPK/NF-κB-initiated neuroinflammation has been reported; for example, silencing RAGE mitigates neuroinflammation by suppression of p38/NF-κB signaling pathway in a mouse model of Parkinson's disease.[12] In addition, the pathological features of AD are amyloid-β peptide (Aβ) accumulation, synapse loss, and the deposition of hyperphosphorylated tau-based neurofibrillary tangles.[13] Moreover, insulin plays multiple roles in modulating lipid metabolism, inflammation, Aβ clearance, and reducing tau phosphorylation.[14] Accordingly, the amelioration of obesity by inhibiting MG and RAGE-associated signaling might effectively prevent AD development.
The gut microbiota is involved in maintaining homeostasis in health and diseases such as obesity, nonalcoholic liver disease (NAFLD), type 2 diabetes, cancers, cardiovascular disease, and brain disorders.[15] The microbiota is a key regulator that influences behavior and cognitive functions through dynamic bidirectional communication of the gut-brain axis.[16] Additionally, Western-style diets induce cognitive impairment by altering neurotransmitters and immune-related cytokines that can be rescued through gut microbiota modifications.[17] However, the linkage between the gut microbiota and high fat-induced MG production remains unclear.
Indian gooseberry (Phyllanthus emblica L.) fruit is cultivated in India, Turkey, Taiwan, and China. It is edible and has therapeutic benefits due to its abundant bioactive components, such as tannins, polyphenols, flavonoids, and vitamin C.[18] Moreover, according to our previous report, the administration of a water extract of Indian gooseberry fruit (WEIG) significantly ameliorates methionine and choline-deficient (MCD) diet-induced nonalcoholic steatohepatitis (NASH) through antioxidant and anti-inflammatory effects and reduces lipid peroxidation in C57BL/6 mice.[19] Additionally, the hepatoprotective effect of WEIG is observed in high-fat diet (HFD)-induced rats with nonalcoholic fatty liver disease.[20] Polyphenols effectively trap MG.[21] In addition, the dysbiotic gut microbiota and altered lipid metabolism are improved by administering a P. emblica extract to ob/ob mice, indicating the anti-obesity capacity of P. emblica through MG trapping and gut microbiota modulation.[22]
Interestingly, a potential neuroprotective algorithm (NPA) indicated that P. emblica extract is one of the top candidate anti-AD treatments from 23 medicinal plants.[23] However, few studies have explored the protective effects of WEIG on obesity and neurodegenerative disease development by reducing the MG content and modulating the gut microbiota. This study aimed to investigate whether WEIG administration effectively alleviated insulin resistance, gut microbiota dysbiosis, and obesity-induced cognitive decline by suppressing MG production in HFD-induced obese Sprague–Dawley (SD) rats.
2 Results
2.1 Bioactive Compounds in the WEIG
Indian gooseberry (P. emblica L.) possesses high antioxidant activity due to the abundance of polyphenols and flavonoids.[18] In this study, high total polyphenol (143.7 ± 3.9 GA equivalents mg g−1 extract) and total flavonoid (19.6 ± 0.2 catechin equivalents mg g−1 extract) contents were detected in WEIG. In addition, the phenolic composition of WEIG was identified using HPLC and calculated from the standard calibration curves for each compound. As shown in Figure 1, GA (1.69 ± 0.03 mg g−1 extract), ellagic acid (4.08 ± 0.08 mg g−1 extract), and β-glucogallin (6.87 ± 0.03 mg g−1 extract) were identified as the major phenolic components in WEIG.
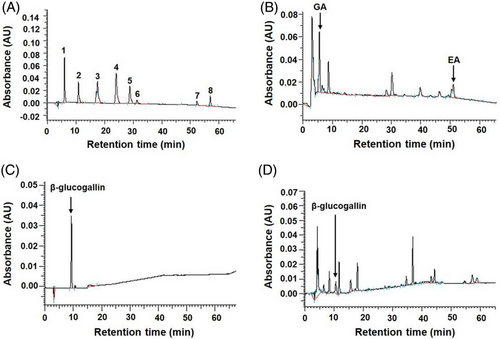
2.2 WEIG and its Bioactive Compound GA Effectively Reduced Fat Accumulation in HFD-Fed Rats
As shown in our previous study, WEIG significantly improves body weight and steatosis by enhancing adiponectin and PPAR-α production in SD rats with HFD-induced nonalcoholic fatty liver disease.[20] In addition, obesity causes MG and AGE accumulation, which are strongly correlated with cognitive impairment and AD.[24] Hence, the MG-trapping capacities of WEIG and its major phenolic components were evaluated. As shown in Figure 2A, ALA (effective MG inhibitor) exhibited a good MG-trapping ability, followed by GA, H-WEIG, EA, L-WEIG, and β-glucogallin. Accordingly, the effects of WEIG and GA on preventing cognitive decline were investigated in HFD-fed rats. The body fat ratio and average weights of adipose tissues (epididymal, perinephric, and mesenteric fat) were significantly increased in the HFD group compared with the control group (Figure 2B and Table 1). H-WEIG administration effectively reduced the body fat ratio and average weights of adipose tissues in HFD-induced rats (Figure 2B and Table 1). Moreover, the average weights of epididymal adipose tissue were significantly decreased by L-WEIG, H-WEIG, ALA, and GA supplementation compared to the HFD group (Figure 2B and Table 1).
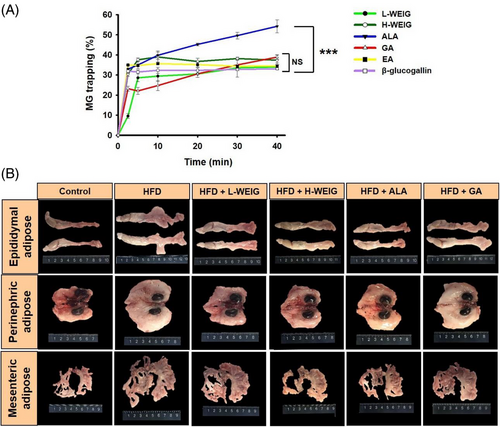
| a) | Control | HFD | HFD + L-WEIG | HFD + H-WEIG | HFD + ALA | HFD + GA |
|---|---|---|---|---|---|---|
| Epididymal adipose [g] | 4.98 ± 0.55c | 13.31 ± 1.24a | 9.78 ± 0.97b | 8.96 ± 0.6b | 10.61 ± 1.13b | 9.95 ± 0.75b |
| Perinephric adipose [g] | 6.75 ± 0.98c | 19.95 ± 1.66a | 17.98 ± 2.30ab | 14.20 ± 1.28b | 17.95 ± 2.14ab | 15.08 ± 1.04ab |
| Mesenteric adipose [g] | 4.73 ± 0.43c | 9.50 ± 0.68a | 7.80 ± 0.88ab | 6.67 ± 0.45bc | 7.97 ± 0.95ab | 7.87 ± 0.77ab |
| Body fat ratio [%] | 4.11 ± 0.34c | 8.02 ± 0.38a | 7.37 ± 0.60ab | 6.38 ± 0.30b | 7.19 ± 0.66ab | 7.36 ± 0.55ab |
- a) The rats were fed a normal diet without the administration of a high-fat diet designated as the control group. The other groups were orally administrated with a high-fat diet with L-WEIG, H-WEIG, ALA, and GA for 16 weeks. The animal received an oral administration of L-WEIG (250 mg kg−1 b.w.), H-WEIG (500 mg kg−1 b.w.), ALA (1 mg kg−1 b.w.), or GA (100 mg kg−1 b.w.). Water extract from the Indian gooseberry fruit, WEIG. Values that had not been indicated with the same alphabet differed significantly (p < 0.05). Tissue relative percentage (%) was calculated as follows: [Tissue weight (g) ÷ final body weight (g)] × 100.
Moreover, serum biochemical parameters were analyzed to confirm the protective effects of H-WEIG, L-WEIG, ALA, and GA administration on HFD-induced obese rats. The CHOL level was slightly increased in the HFD group and was significantly reduced by H-WEIG and ALA treatment (Table 2). Additionally, the TG level was substantially increased in HFD-fed rats and effectively decreased by L-WEIG, H-WEIG, ALA, and GA supplementation (Table 2). A similar trend was also observed for LDL levels (Table 2). Likewise, glucose and insulin levels were significantly increased in HFD-fed rats, but were decreased by L-WEIG, H-WEIG, ALA, and GA administration (Table 2). The HOMA-IR index was substantially increased in the HFD group, indicating that the insulin-resistant obese rat model was successfully established (Table 2). H-WEIG and GA administration effectively decreased the HOMA-IR index in the HFD-fed rats (Table 2).
| a) | Control | HFD | HFD + L-WEIG | HFD + H-WEIG | HFD + ALA | HFD + GA |
|---|---|---|---|---|---|---|
| CHOL [mg dL−1] | 47.50 ± 4.64abc | 55.43 ± 3.42a | 47.50 ± 3.65abc | 39.25 ± 1.62c | 44.37 ± 3.0bc | 51.75 ± 4.04ab |
| TG [mg dL−1] | 67.83 ± 12.13b | 117.71 ± 17.79a | 66.25 ± 6.22bc | 48.00 ± 5.44bc | 57.56 ± 5.63bc | 40.38 ± 2.80c |
| LDL [mg dL−1] | 4.76 ± 0.27b | 8.95 ± 1.08a | 6.61 ± 0.69b | 4.66 ± 0.39b | 5.83 ± 0.79ab | 5.50 ± 0.39b |
| Glucose [mg dL−1] | 157.50 ± 8.81b | 221.17 ± 18.01a | 177.50 ± 9.09b | 140.67 ± 9.58b | 164.33 ± 5.12b | 153.00 ± 7.44b |
| Insulin [µg L−1] | 0.61 ± 0.19bcd | 2.21 ± 0.32a | 1.23 ± 0.21b | 0.35 ± 0.11d | 1.16 ± 0.35bc | 0.46 ± 0.09cd |
| HOMA-IR index | 4.75 ± 1.11c | 22.99 ± 7.40a | 13.41 ± 2.78abc | 8.23 ± 2.03bc | 18.95 ± 4.05ab | 5.55 ± 0.92c |
- a) The rats were fed a normal diet without the administration of a high-fat diet designated as the control group. The other groups were orally administrated with a high-fat diet with L-WEIG, H-WEIG, ALA, and GA for 16 weeks. The animal received an oral administration of L-WEIG (250 mg kg−1 b.w.), H-WEIG (500 mg kg−1 b.w.), ALA (1 mg kg−1 b.w.), or GA (100 mg kg−1 b.w.). Water extract from the Indian gooseberry fruit, WEIG. Values that had not been indicated with the same alphabet differed significantly (p < 0.05).
2.3 WEIG and GA Administration Ameliorated Memory Deficits and Alterations in AD-Associated Biomarkers
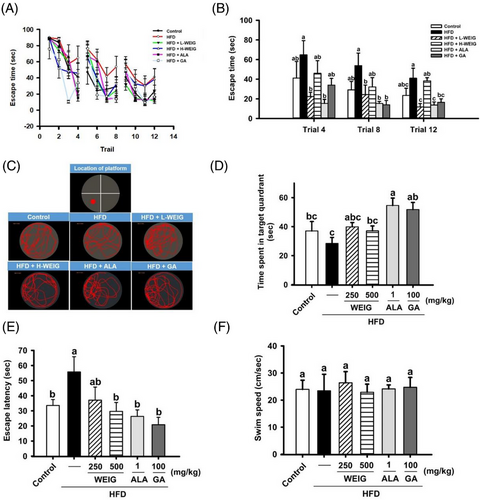
Furthermore, memory and learning capabilities were assessed using the Morris water maze test. In the reference memory task, HFD-fed rats spent more time finding the visible platform in trials 4, 8, and 12 than rats in the other groups (Figure 3A,B). L-WEIG, ALA, and GA administration significantly improved the memory ability of HFD-fed rats (Figure 3A,B). In the probe test, HFD-fed rats spent less time in the target quadrant, whereas ALA- and GA-treated rats spent more time in the same quadrant to find the hidden platform (Figure 3C,D). Additionally, the visible platform was relocated in the opposite quadrant to evaluate the escape latency. As anticipated, a longer escape latency was observed in the HFD group than in the control group (Figure 3E). Oral administration of H-WEIG, ALA, and GA significantly reduced the escape latency of HFD-treated rats (Figure 3E). Moreover, the average swim speed of rats in the Morris water maze test was not significantly different in each group (Figure 3F), indicating WEIG and GA administration restored the memory and learning capabilities that were impaired by HFD treatment.
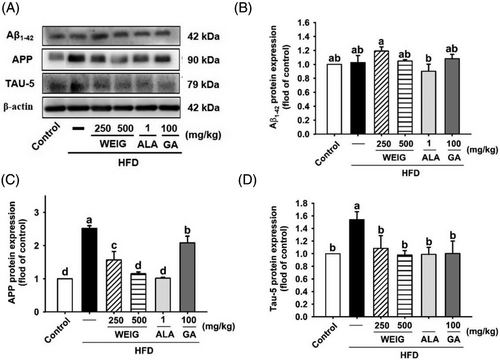
Furthermore, the cognitive impairment was confirmed by assessing the levels of AD biomarkers (Aβ1-42, APP, and TAU-5). Although the expression of the Aβ1-42 protein was not significantly altered in any of the groups, upregulation of APP was detected in the HFD group and was subsequently downregulated by H-WEIG, L-WEIG, ALA, and GA administration in rats (Figure 4). Additionally, TAU-5 protein levels were dramatically increased in the HFD group but decreased by all treatment (Figure 4).
2.4 WEIG and GA Administration Significantly Enhanced Brain MG and AGE Clearance in Rats with Obesity-Induced Cognitive Decline
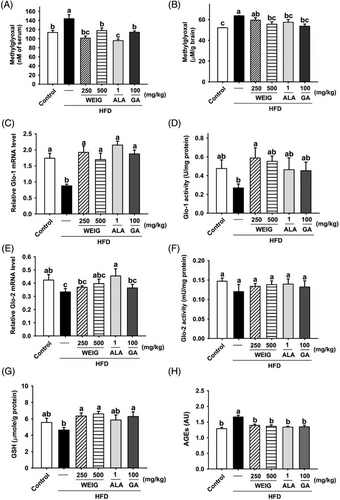
In addition, MG and AGE levels were validated to clarify whether MG and AGE accumulation in obese rats contribute to the pathology of cognitive decline. Interestingly, high serum MG levels were observed in HFD-fed rats during the experimental period (Figure 5A). Oral gavage of H-WEIG, L-WEIG, ALA, and GA effectively reduced the serum MG level compared to the HFD group (Figure 5A). Moreover, a similar trend for MG contents in the brain tissue was observed among the groups (Figure 5B). The glyoxalase system is composed of Glo-1 and Glo-2 enzymes and the coenzyme GSH, which promotes the metabolism of MG and MG-derived AGEs.[25] Based on this information, Glo-1, Glo-2, and GSH levels were evaluated. As shown in Figure 5C, Glo-1 mRNA levels were considerably decreased by HFD administration, whereas Glo-1 mRNA expression was increased by H-WEIG, L-WEIG, ALA, and GA treatments. Additionally, H-WEIG and L-WEIG supplementation significantly increased Glo-1 enzyme activity (Figure 5D). Unexpectedly, the mRNA and enzyme activities of Glo-2 were not altered among the groups (Figure 5E,F). Furthermore, GSH enzyme activity was significantly increased in the H-WEIG, L-WEIG, and GA groups compared with the HFD group (Figure 5G). Consistent with this finding, the content of AGEs in brain tissue was significantly increased in HFD-treated rats and decreased by H-WEIG, L-WEIG, ALA, and GA administration (Figure 5H).
2.5 WEIG and GA Administration Promoted Anti-Inflammatory and Antioxidant Activities with Suppressing the RAGE, MAPK, and NF-κB Levels
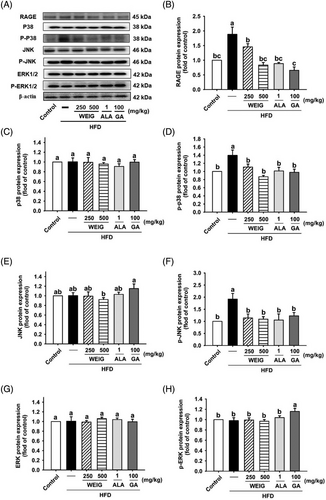
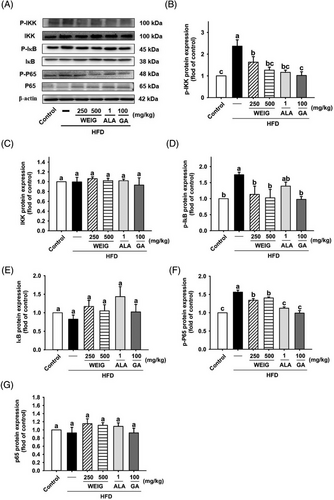
A recent cohort study showed that AGEs and their receptor (RAGE) were strongly associated with the development of cognitive decline in 3889 participants (p < 0.05).[26] We assessed whether RAGE and the activation of its signaling pathway were involved in the development of cognitive decline in HFD-fed rats. RAGE protein expression was significantly increased in the brain tissue of HFD-fed rats and decreased in the H-WEIG, L-WEIG, ALA, and GA groups (Figure 6A,B). RAGE engages with its ligands and subsequently triggers the activation of downstream p38, JNK, ERK1/2, and NF-κB signaling pathways linked to the inflammatory response, oxidative stress, and cognitive impairment.[27, 28] As shown in Figure 6C–F, the levels of p38 and JNK phosphorylation were significantly increased in the HFD group compared with the control group. Decreased p38 and JNK phosphorylation was observed in the L-WEIG, H-WEIG, ALA, and GA groups compared to the HFD group (Figure 6C–F). The levels of ERK1/2 were not significantly altered among the groups (Figure 6G–H). In addition, the levels of IKK, IκB, and p65 phosphorylation increased in the HFD group, whereas L-WEIG, H-WEIG, ALA, and GA administration decreased the levels of these phosphorylated proteins in HFD-fed rats (Figure 7).
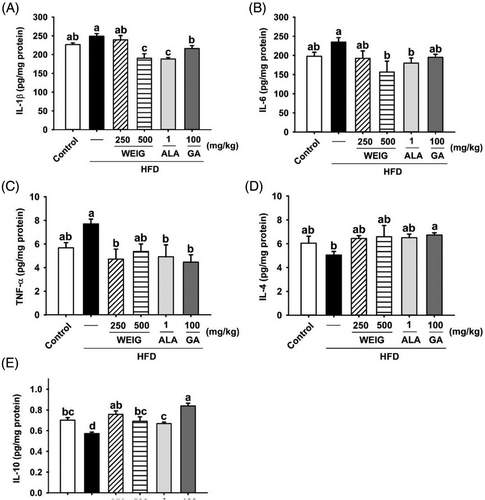
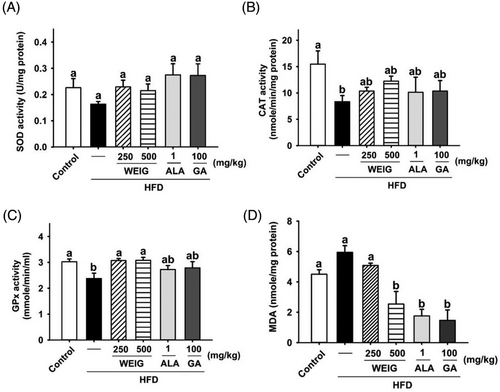
Previous evidence has shown that RAGE transports circulating Aβ into the brain, enhancing neuroinflammation and oxidative stress, and accelerating AD progression.[29, 30] Accordingly, inflammatory cytokines and antioxidant enzymes in the brain tissue of HFD-fed rats were evaluated to confirm the effects of RAGE and its signaling pathway. The levels of IL-Iβ, IL-6, and TNF-α were increased by HFD supplementation (Figure 8A–C, p > 0.05). Oral administration of H-WEIG, ALA, and GA effectively reduced IL-Iβ, IL-6, and TNF-α levels (Figure 8A–C, p < 0.05). Moreover, the secretion of TNF-α was suppressed by H-WEIG, L-WEIG, and GA oral gavage compared with the HFD group (Figure 8C). Furthermore, the levels of anti-inflammatory cytokines (IL-4 and IL-10) were decreased in the HFD group and significantly increased in the H-WEIG, L-WEIG, ALA, and GA groups (Figure 8D,E). In addition, CAT and GPx enzyme activities were significantly decreased in HFD-fed rats compared with control rats (Figure 9B,C). GPx activity was effectively increased by L-WEIG and H-WEIG supplementation (Figure 9B,C). In addition, a high MDA level was detected in the HFD group, whereas it was significantly reduced in the H-WEIG, ALA, and GA groups (Figure 9D).
2.6 WEIG and GA Supplementation Increased Abundances of Components of the Microbiome that Negatively Correlated with the Process of Obesity-Induced Cognitive Decline
Distinct clustering and principal coordinate analysis (PCA) of gut microbiota were performed by calculating Bray–Curtis distances to clarify the relationship between MG and the microbiota in rats with obesity-induced cognitive impairment. As shown in Figure 10A,B, the distinct clustering and PCA results showed that the control group formed a distinct cluster compared to the other groups. The PCA plots revealed that the H-WEIG and L-WEIG groups were closely clustered (Figure 10B), indicating that the microbiota compositions would be similar due to the WEIG treatment. Notably, the PCA data overlapped between the ALA and GA groups (Figure 10B).
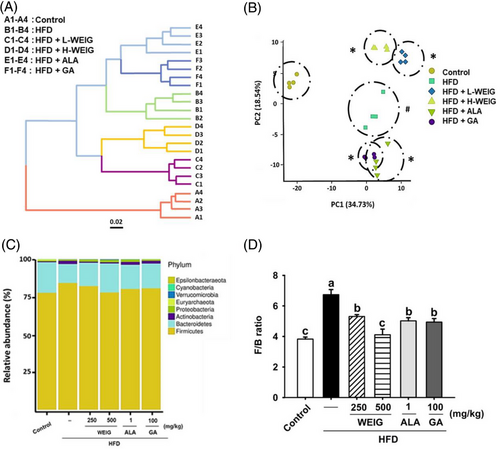
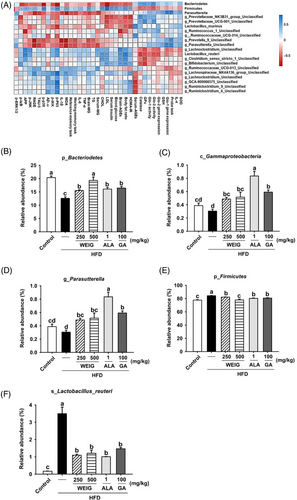
In addition, the relative abundance at the phylum level was analyzed using the Silva_132 database. A high relative abundance of Firmicutes with a low composition of Bacteroidetes was observed in HFD-fed rats compared to the control group (Figure 10C,D). The Firmicutes/Bacteroidetes (F/B) ratio was increased in the HFD group but significantly reduced by H-WEIG, L-WEIG, ALA, and GA supplementation (Figure 10C,D). In addition, the relationship between the results and gut microbiota composition was analyzed by calculating Spearman's rank correlation coefficient. As shown in Figure 11A, negative correlations were observed between protein levels (MG, AGEs, TAU-5, RAGE, MAPK, and NF-κB levels) and the abundance of Bacteroidetes, Gammaproteobacteria, and Parasutterella. Interestingly, high antioxidant and Glo-1 enzyme activities positively correlated with the abundance of Bacteroidetes, Gammaproteobacteria, and Parasutterella (Figure 11A). In addition, the relative abundance of Firmicutes and Lactobacillus reuteri showed negative correlations with antioxidant and Glo-1 activities and positive correlations with MG, AGE, and inflammatory cytokine levels (Figure 11A). Moreover, the five most abundant bacteria were recorded in all groups and compared. The relative abundance of Bacteroidetes at the phylum level was significantly decreased in the HFD group and increased in the H-WEIG, L-WEIG, ALA, and GA groups (Figure 11B). Similarly, low relative abundances of Gammaproteobacteria (class) and Parasutterella (genus) were observed in the HFD group, whereas they were effectively increased by H-WEIG, ALA, and GA administration in HFD-fed rats (Figure 11C,D). On the other hand, high relative abundances of Firmicutes (phylum) and L. reuteri (species) were observed in the HFD group, which were significantly reduced by H-WEIG, L-WEIG, ALA, and GA supplementation in HFD-fed rats (Figure 11E,F).
3 Discussion
Obesity is a chronic inflammatory disease with a considerable effect on the development of various illnesses. Although pharmacological interventions with weight-reducing drugs (liraglutide, orlistat, and lorcaserin) effectively relieve obesity, side effects such as insomnia, dizziness, dysgeusia, and headache are observed.[31] Recently, a growing and exciting field of research has been represented by the study of obesity-induced neurological disorders.[32] A HFD induces insulin resistance in the mouse brain, and cognitive impairment was documented.[33] Learning and memory capacities are reduced via gut microbiota regulation in a mouse model of HFD-induced obesity.[34] However, few reports have clarified the culprits involved in the underlying mechanism of obesity-induced cognitive decline. A high level of MG is formed and accumulates in patients with obesity and neuronal diseases,[35] indicating that MG might dominate the process of obesity-induced cognitive decline. In the present study, WEIG effectively reduced insulin resistance and cognitive impairment by inhibiting MG production in HFD-induced obese rats.
MG trapping is a critical process involved in suppressing obesity and associated disease development.[35] According to a previous study, GA administration significantly improves oxidative stress through MG trapping in mice with diabetic nephropathy.[36] Likewise, WEIG and its bioactive compound GA effectively trapped MG and reduced TG levels, LDL levels, glucose levels, insulin levels, HOMA-IR, and fat accumulation in the present study. Additionally, MG has been shown to directly modulate brain function and behavior related to Glo-1 activity, GSH levels, and AGE contents in rats.[37] Moreover, oral gavage of GA substantially reduces lipogenesis and hepatic steatosis in HFD-induced obese mice.[38]
GA improves MG-induced endoplasmic reticulum stress and fibrosis by upregulating GSH, Glo-1, and Nrf2 levels in MG-induced diabetic nephropathy mice,[36] indicating the antioxidant capacity of GA effectively suppresses MG and AGE formations. In this study, brain MG and AGE levels were increased considerably and reduced Glo-1 and GSH levels, ameliorating memory and learning deficits in HFD-fed rats. There still needs to be a knowledge gap regarding the mechanisms of GA for the prevention of cognitive decline. Recently, GA was identified as an α/β-secretase modulator that effectively rescues cognitive decline in amyloid β-protein precursor/presenilin 1 (APP/PS1) transgenic mice.[39, 40] GA has been identified as an AD attenuator due to its antioxidant, anti-inflammatory, and anti-amyloidogenic properties.[41] Consistently, our results recapitulated findings from previous studies revealing that WEIG and its bioactive compound GA significantly decreased the formation of MG- and AGE-related cognitive impairment by reducing brain APP and TAU-5 protein levels and enhancing the memory and learning abilities in HFD-fed rats.
RAGE is the primary receptor for AGEs that transports Aβ across the blood–brain barrier (BBB) and promotes neuroinflammation and oxidative stress via various signaling pathways.[29, 30] Interestingly, P. emblica L. extract exhibits strong MG scavenging and neuroinflammatory suppression activities.[23] In our study, WEIG and GA treatment significantly reduced the levels of neuroinflammatory cytokines (IL-1β, IL-6, and TNF-α) and MDA with downregulating RAGE and its-associated p38, JNK, NF-κB protein levels in HFD-fed rats. Additionally, an increase in the levels of anti-inflammatory cytokine (IL-10) and antioxidant enzyme (GPx) was observed in rats with obesity-induced cognitive decline following WEIG administration.
The microbiota-gut-brain axis has been identified as a new therapeutic strategy for obesity and related diseases.[42] A high Firmicutes/Bacteroidetes ratio is a possible biomarker of dysbiosis in obese individuals.[43] Interestingly, antiobesity treatment through gastric bypass surgery noticeably increases the relative abundance of Gammaproteobacteria and decreases the Firmicutes abundance.[44] In addition, Parasutterella has been defined as a core component of the gut microbiota in humans, mice, and rats.[45] The relative abundance of Parasutterella negatively correlates with the intake of glycolipids, phospholipids, and long-chain fatty acids in obese humans.[46] However, the relationship between associated biomarkers and the microbiota composition in obesity-induced cognitive decline remains unclear. The present study revealed that high relative abundances of Bacteroidetes, Gammaproteobacteria, and Parasutterella were negatively correlated with levels of MG, AGEs, TAU-5, RAGE, MAPK, and NF-κB levels. Nevertheless, considering the 3R (reduce, reuse, recycle) policy, six animals per group could be a limitation in the present study.
4 Conclusions
WEIG and GA effectively decreased insulin resistance, inflammation, oxidative stress, microbiota dysbiosis, and memory loss with suppressing MG production, RAGE, MAPK, and NF-κB levels in HFD-induced obese rats. This study is important because it provides possible mechanisms by which WEIG and its bioactive compound prevent obesity-induced cognitive decline.
5 Experimental Section
Materials
Indian gooseberry fruits were kindly provided by the Miaoli District Agricultural Research and Extension Station, Council of Agriculture, Executive Yuan (Miaoli, Taiwan). Glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT) kits were purchased from Cayman Chemicals (Ann Arbor, MI, USA). Glyoxalase I (Glo-1) and Glo-2 activity assay kits were purchased from BioVision (Milpitas, CA, USA). Interleukin (IL)-1β, IL-4, IL-6, TNF-α, and IL-10 ELISA kits were purchased from R&D Systems, Inc. (Minneapolis, MN, USA). The β-actin antibody was purchased from Novus Biologicals (Littleton, USA). Amyloid precursor protein (APP), amyloid beta1-42 (Aβ1-42), TAU-5, and RAGE antibodies were obtained from Abcam (Cambridge, MA, USA). p38, JNK, and ERK1/2 antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies against the phosphorylated inhibitory subunit of NF-kappa-B alpha (pIκBα), phospho-NF-kappa-B p65 subunit (pP65), and IκB kinase (IKK) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, syringic acid, epicatechin, ellagic acid, GA, naringin, β-glucogallin, and MG were purchased from Sigma–Aldrich (St. Louis, MO, USA).
Extraction of Indian Gooseberry Fruit
Fresh Indian gooseberry fruits were hot-air dried at 60 °C and then powdered using a high-speed grinder. The powder was soaked in deionized water at a ratio of 1:40 overnight. The solution was filtered, lyophilized, and stored at −20 °C for later use. The extraction rate of the water extract of Indian gooseberry fruit (WEIG) was approximately 16.9 ± 0.2%.
Measurements of the Total Polyphenol and Total Flavonoid Contents
The total polyphenol and flavonoid contents in WEIG were determined using spectrophotometry with a previously reported method.[47] The total polyphenol and total flavonoid contents were calculated and calibrated using gallic acid (GA) and catechin as standards, respectively.
Phenolic Compound Analysis
The WEIG was diluted with ddH2O to an appropriate concentration and filtered through a 0.22 µm filter. Then, phenolic compounds in WEIG were analyzed using a Chromaster HPLC system (Hitachi, Japan) equipped with a pump (no. 5110), autosampler (no. 5210), column oven (no. 5310), and diode array detector (no. 5430). A LiChrospher 100 RP-18 column (Merck KGaA, Darmstadt, Germany) was used for separation, and the mobile phase consisted of buffer (A) 2% v/v acetic acid in ddH2O and (B) 0.5% v/v acetic acid in ddH2O and acetonitrile (50:50, v/v). The analytical gradient was as follows: 5–10% B at 0–10 min, 25% B at 10–40 min, 50% B at 40–60 min, 70% B at 60–65 min, 100% B at 65–70 min, 50% B at 70–75 min, 30% B at 75–80 min, and 2% B at 80–82 min. The flow rate (0.8 mL min−1) and temperature (40 °C) were set. The sample injection volume was 20 µL, and the absorption wavelength was 254 nm. Standards of GA, protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, syringic acid, epicatechin, ellagic acid, and naringin were used to identify the components of the WEIG. In addition, β-glucogallin was analyzed under other conditions using HPLC. Solution A (1% formic acid in ddH2O) and solution B (1% formic acid in methanol) were used. The analytical gradient was as follows: 2% B at 0–10 min, 37% B at 10–37 min, 37% B at 37–42 min, 37–40% B at 42–60 min, 40–60% B at 60–70 min, 60–100% B at 70–90 min, 100% B at 90–104 min, 100–2% B at 104–105 min, and 2% B at 105–112 min. The flow rate (1 mL min−1) and temperature (35 °C) were set. The sample injection volume was 10 µL, and the absorption wavelength was 280 nm. The data were reported as the means ± standard deviations (mg g−1 extract).
Animal Treatment
Five-week-old male SD rats were purchased from BioLASCO Experimental Animal Center (Taipei, Taiwan) and housed under controlled environmental conditions (22 ± 2 °C, 65 ± 5% humidity, and a 12 h regular light-dark cycle). All rats were fed a normal chow diet (LabDiet 5001) and allowed to acclimate to the environment for 1 week. The animal study was approved by the IACUC of National Chung Hsing University (approval no: 108-095) and carried out according to the National Research Council's Guide for the Care and Use of Laboratory Animals.
In this study, the effect of the WEIG on the prevention of cognitive decline was evaluated in a high-fat diet (HFD)-fed rat model. The rats were divided into six groups (n = 6). Dosage information included Control (LabDiet 5001), HFD (LabDiet 5001 + 40% butter, total 60% kcal), L-WEIG (250 mg kg−1 b.w./daily), H-WEIG (500 mg kg−1 b.w./daily), alagebrium chloride (ALA as positive control, 1 mg kg−1 b.w./daily), and GA (pure GA, 100 mg kg−1 b.w./daily) groups. Except for the control group, all rats were fed a HFD for 112 consecutive days, and treatments were administered daily by oral gavage.
Morris Water Maze Test
The water maze device was a circular swimming pool with a diameter of 180 cm and a height of 45 cm. The swimming pool was filled with water to a height of 27 cm and contained a movable rest platform in one of the four quadrants (the diameter of the platform was 10 cm, and the height was 25 cm). The Morris water maze tests included a reference memory task, probe test, and working memory task to evaluate the rats' memory and learning abilities, as described in a previous report.[48] Briefly, a reference memory test was performed from day 109 to day 111 of the animal experiment. An escape platform was placed in quadrant IV of the pool. Rats were put into the pool from the side, in a random location, four times each day. Latency, path, and location in the pool were recorded for each rat. The maximum time for each trial was 90 s; rats that failed to reach the platform within 90 s were guided there. Each rat performed 12 trials in total over 3 days. The probe test was performed immediately after the reference memory task on day 111. The escape platform was removed from the pool, where the rats were placed to perform a spatial probe test for 120 s. Time spent in quadrant IV, where the platform had previously been located, and in the other quadrants was examined to investigate memory and learning ability. A working memory task was performed on day 112. Following the training procedure used in the reference memory task, rats were placed in the pool. However, the location of the escape platform was altered (quadrant III), and each rat had an extra training trial before the four experimental trials. Latency to reach the platform was recorded for each trial and assessed by mean performance. All data were analyzed using EthoVision XT 7 software (Noldus Information Technologies, Wageningen, the Netherlands).
Analysis of Biochemical Parameters
Methylglyoxal (MG) Analysis
As described in a previous report, the concentrations of MG in serum, brain tissue, and feces were analyzed using HPLC.[49] Briefly, o-phenylenediamine (OPD) was reacted with samples at 37 °C for 24 h to form the product 2-methylquinoxaline (2-MQ). After derivatization, the solid phase extract (SPE) of the mixtures was collected using a commercial column (InertSep Pharma, GL Sciences, Tokyo, Japan) and analyzed using HPLC.
Brain Tissue Homogenization and Protein Analysis
Brain tissue homogenization and Western blot analysis were performed using previously described methods.[50] Briefly, brain tissue was homogenized using a tissue homogenizer (SH-100, KURABO, Osaka, Japan). Then, the protein levels of Aβ1-42, APP, TAU-5, RAGE, pP38, pJNK, pERK1/2, pIKK, pIκB, pP65, and β-actin were assessed using Western blot analysis. Protein levels were detected and quantitated using a Biospectrum AC Imaging System (UVP, Upland, CA, USA).
Analyses of Antioxidant Enzyme Activities and Malondialdehyde Level
The activities of antioxidant enzymes such as SOD, CAT, and GPx in the brain were analyzed according to the operation manuals provided by Cayman Chemicals. In addition, the malondialdehyde (MDA) level in brain tissue was measured by performing a thiobarbituric acid reactive substance (TBARS) assay using a previously reported method.[51]
Glyoxalase System Enzymes and Gene Analysis
Glo-1, Glo-2, and glutathione (GSH) activities in the brain were measured using commercial kits according to the manufacturer's instructions. In addition, Glo-1 and Glo-2 gene expression levels were measured quantitatively using 7300 Real-time PCR Systems (Applied Biosystems, Waltham, MA, USA) with SYBR Green real-time PCR master mix (Toyobo, Co., Ltd., Japan). The procedure used to analyze the expression of the Glo-1 and Glo-2 genes was performed according to the standard method provided by Applied Biosystems. The relevant parameters and primers were as follows: hold for 1 min at 95 °C and 50 cycles of 95 °C for 15 s and 56 °C for 15 s with data collection during the extension step. Glo-1 primers (forward: CAGCGTGGGCTTTTTCCA; reverse: TCAGTGCCCCAGTTGTGTGT), Glo-2 primers (forward: AGGGAACCGCAGACGAGAT; reverse: GAGGAAGCCGGCCTAAGACT), and GAPDH primers (forward: ACGGACCAGAGCGAAAGCAT; reverse: TGTCAATCCTGTCCGTGTCC) were used.
Analysis of Advanced Glycation End Product (AGE) Contents
AGE contents in animal feces and brain tissue were analyzed using a FLUO star galaxy spectrophotometer (BMG Labtech, Offenburg, Germany) by detecting the autofluorescence characteristic of AGEs. Briefly, feces (1 g) were homogenized with 10 mL of PBS and centrifuged. The supernatant was collected for the AGE analysis. The brain tissue (100 mg) was homogenized with 1 mL of NaOH and then centrifuged to obtain a brain tissue homogenate. A 0.2 M boric acid solution was added to the supernatant until the pH reached 8.5. The supernatants (from feces or brain tissue) were measured at 355 nm (excitation) and 405 nm (emission) using a FLUO star galaxy spectrophotometer. The relative fluorescence intensity of AGEs was calibrated against a native BSA solution (its value was defined as 1 AU).
Determination of Immune-Related Cytokine Levels
The levels of cytokines in the brain homogenates, including IL-1β, IL-6, TNF-α, IL-4, and IL-10, were analyzed using commercial assay kits according to the manufacturer's instructions.
Microbiota Analysis
Rat fecal DNA was extracted with the AllPure Genomic DNA Extraction Kit (Allbio, Taichung, Taiwan) according to the manufacturer's instructions. The V3 and V4 hypervariable regions of prokaryotic 16S rDNA were amplified by PCR using the forward primer “CCTACGGRRBGCASCAGKVRVGAAT” and the reverse primer “GGACTACNVGGGTWTCTAATCC.” Next-generation sequencing (NGS) and sequencing of the DNA library were based on the Silva_132 database and performed by AllBio Science Incorporated (Taichung, Taiwan).
Statistical Analysis
The comparison of variables within the group was analyzed through the one‑way ANOVA followed by Duncan's post hoc test using Social Science (SPSS) software 2.0 statistical software (IBM Corporation, Armonk, NY, USA) and presented as the mean ± SEM (n = 6). p-Values < 0.05 were considered significant.
Acknowledgements
This research work was supported in part by the grant MOST 110-2320-B-005-004 and MOST 111-2320-B-005-008 from the Ministry of Science and Technology, Taiwan.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
Y.-Y.C. and S.-Y.C. contributed equally to this work. Y.Y.C. and G.C.Y. conceived and designed the experiments. Y.Y.C. performed the experiments and analyzed the data. S.Y.C. analyzed the data and wrote the manuscript. J.A.L. checked data curation. G.C.Y. supervised the project and edited the manuscript. All authors read and approved the final manuscript.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




