Diet Supplementation of Luteolin before Fatty Liver Formation Improves Hepatic Steatosis in Obese Mice by Inhibiting Visceral Adipose Tissue Lipolysis
Abstract
Scope
Serotonin (5-HT)-induced visceral adipocyte lipolysis is essential for the development of obesity-related complications. Diet supplementation of luteolin prevents high-fat diet (HFD)-fed mice against obesity and associated fatty liver. However, independent of the body weight loss, whether dietary luteolin can substantially reduce hepatic steatosis remains unclear.
Methods and results
In differentiated 3T3-L1 cells, 5-HT treatment promotes adipocyte lipolysis, while luteolin significantly inhibits 5-HT-induced lipolysis, Ca2+-PKG cascade, and SIRT1/FoxO1/AMPKα signaling through binding to 5-HT receptor HTR2B. Further, 5-week-old mice are fed with an HFD for 16 weeks. At the 6th, 8th, or 10th weeks of HFD feeding, some mice are switched to a luteolin-containing HFD, respectively. In all HFD-fed mice, body weight gain and body component are unaffected by dietary luteolin. However, diet supplementation of luteolin at the 6th or 8th, rather than at the 10th weeks, alleviates hepatic steatosis. Meanwhile, dietary luteolin reduces epididymal adipose tissue (EAT) lipolysis, and represses the level of lipolytic enzyme, the expression of Htr2b, and the activation of PKG and SIRT1/FoxO1/AMPKα signaling in EAT.
Conclusions
Diet supplementation of luteolin before the formation of fatty liver protects HFD-fed mice against ectopic lipid deposition in liver by inhibiting visceral adipocyte lipolysis.
1 Introduction
Diet-induced obesity is caused by chronic overnutrition and characterized by excessive body fat storage, which is associated with the development of metabolic diseases, including nonalcoholic fatty liver disease (NAFLD).[1, 2] In the early stage of obesity, excess energy was preserved in the form of triglyceride (TG) in lipid droplets and leads to adipocyte hyperplasia.[3] Further, visceral adipocyte expansion reaches the maximum and promotes adipocyte dysfunction and lipolysis. Adipocyte lipolysis releases high levels of non-esterified fatty acids (NEFA), which are unable to be sufficiently absorbed and transformed in adipocytes due to adipocyte dysfunction,[4] and eventually results in the ectopic fat deposited in hepatocytes and the development of NAFLD.[5, 6]
Lipolysis, i.e., neutral hydrolysis of TGs to NEFA and glycerol, requires the involvement of three neutral lipases, including adipose triglyceride lipase (ATGL), hormone sensitive lipase (HSL), and monoacylglycerol lipase (MGL). In adipose tissue, ATGL and HSL are responsible for more than 90% of TG hydrolysis.[7] Sirtuin 1 (SIRT1) regulates the acetylation status and functional activity of Forkhead box O1 (FoxO1), which directly binds to the ATGL promoter and drivers the activation of adipocyte lipolysis.[8] The activation of lipolysis triggers the triglyceride hydrolysis and elevates the phosphorylation of Adenosine 5’-monophosphate-activated protein kinase (AMPK).[9] In addition, intracellular Ca2+ accumulation promotes lipolysis by increasing cGMP level.[10] In mouse studies, epididymal adipose tissue (EAT) is the most commonly used visceral fat depot. In EAT from the mice fed with a high-fat diet (HFD), the elevated serotonin (5-HT) receptor 2B (HTR2B) signaling promotes Ca2+-cGMP-PKG cascade and adipocyte lipolysis, which leads to the development of obesity-related complications.[11] Therefore, 5-HT signaling may drive adipocyte lipolysis through promoting the activation of Ca2+-cGMP-PKG cascade and SIRT1/FoxO1/AMPK pathway, which ultimately leads to ectopic fat deposit in hepatocytes.
Luteolin (3´,4´,5,7-tetrahydroxy flavone) is a natural flavonoid, widely distributed in a variety of fruits and vegetables, such as chrysanthemum flowers, celery, broccoli, and parsley.[12] Some studies have demonstrated that dietary luteolin reduces body weight gain and associated hepatic steatosis in HFD-fed animals.[13, 14] Thus, the improvement of fatty liver may be a secondary and concomitant outcome of body weight loss. Independent of the body weight loss, whether dietary luteolin can substantially reduce hepatic steatosis need to be further investigation.
In this study, the effect of luteolin on 5-HT-induced lipolysis was firstly assessed in mature 3T3-L1 adipocytes. To investigate the mechanism by which luteolin inhibits 5-HT-induced adipocyte lipolysis, we detected Ca2+-PKG cascade and SIRT1/FoxO1/AMPK signaling and simulated the binding of HTR2B and luteolin utilizing a molecular docking program. Next, the mice were fed with a low-fat diet (LFD) or an HFD for 16 weeks. Meanwhile, some HFD-fed mice were switched to a luteolin-containing HFD at the 6th, 8th, or 10th weeks of HFD feeding. Further, the substantial action of dietary luteolin on hepatic steatosis, visceral adiposity, and lipolytic molecules were identified in HFD-fed mice.
2 Results
2.1 Luteolin Addition Suppressed 5-HT-Induced Adipocyte Lipolysis
The elevated HTR2B signaling promotes visceral adipocyte lipolysis and leads to the development of obesity-related complications.[11] Here, to examine whether 5-HT directly induce adipocyte lipolysis, 3T3-L1 pre-adipocytes were fully differentiated to mature adipocytes, and subsequently treated with 5-HT at physiological concentration (Figure 1A). In differentiated 3T3-L1 adipocytes, 5-HT treatment significantly reduced intracellular fat accumulation (Figure 1B,C). Along with the alternation of intracellular lipid level, 5-HT treatment also significantly elevated the release of free glycerol and NEFA from 3T3-L1 cells (Figure 1D,E). Meanwhile, the expression of lipolytic enzyme gene Atgl, Hsl, and Mgl (Figure 1F–H), the protein level of ATGL (Figure 1I,J), and the phosphorylation of HSL (Figure 1I,K) were upregulated by 5-HT treatment. Additionally, the expression of lipogenesis-related gene Dgat1, Dgat2, Gpat3, and Mogat2 was unaffected by 5-HT treatment (Figure S1, Supporting Information). The results affirmed that 5-HT treatment induced the lipolysis in 3T3-L1 adipocytes.
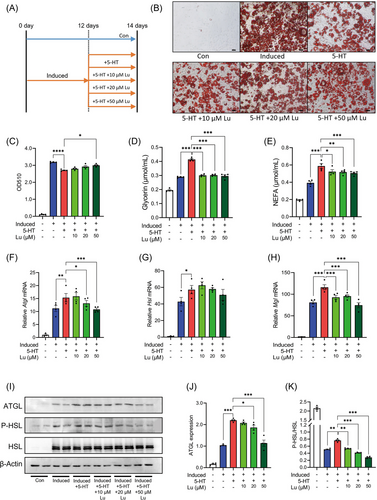
Further, to assess the effect of luteolin on 5-HT-induced adipocyte lipolysis, as shown in Figure 1A, 10, 20, or 50 µM luteolin were added. In the context of 5-HT treatment, luteolin addition increased the intracellular lipid levels, although the increase did not reached the statistical significance after 10 or 20 µM luteolin addition (Figure 1B,C). Consistent with the enhancement of intracellular lipid levels, luteolin addition successfully abolished the effects of 5-HT on the release of glycerol and NEFA (Figure 1D,E), the expression of Atgl and Mgl (Figure 1F–H), the protein level of ATGL (Figure 1I,J), and the phosphorylation of HSL (Figure 1I,K). Additionally, in 3T3-L1 adipocytes without 5-HT treatments, the release of glycerol and NEFA and the expression of lipolytic genes were unaffected by luteolin addition (Figure S2, Supporting Information). Therefore, luteolin addition inhibited 5-HT-induced adipocyte lipolysis.
2.2 Luteolin Inhibited 5-HT-Induced Adipocyte Lipolysis via SIRT1/FoxO1/AMPKα Pathway
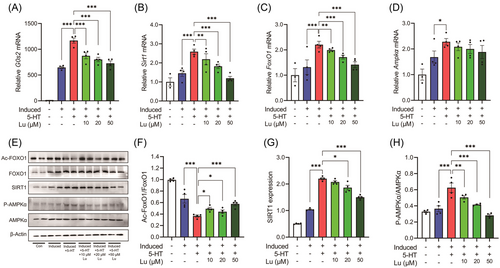
SIRT1/FoxO1/AMPKα pathway has been implicated in adipocyte lipolysis and FoxO proteins promote triacylglycerol catabolism through regulating ATGL and its inhibitor G0/S1 switch 2 (G0S2).[8, 15] Along with the induction of lipolysis, 5-HT treatment promoted the expression of G0s2, Sirt1, FoxO1, and Ampkα in 3T3-L1 adipocytes (Figure 2A–D). In addition, 5-HT treatment inhibited FoxO1 acetylation, increased the level of SIRT1 and phosphorylated-AMPKα (Figure 2E–H). Except for the expression of Ampkα, luteolin addition totally abated the effects of 5-HT on the aforementioned molecules involved in adipocyte lipolysis (Figure 2), suggesting that luteolin inhibited 5-HT-induced adipocyte lipolysis via SIRT1/FoxO1/AMPKα pathway.
2.3 Luteolin Competitively Bound to HTR2B and Blocked Downstream Ca2+-PKG Cascade
5-HT exerts specific biological activities through the different 5-HT receptors (HTRs). Of the HTRs, HTR1A and HTR1B play the primary roles at the neural central, whereas HTR2A and HTR2B are mainly expressed in peripheral tissue and have been implicated in the regulation of metabolic homeostasis.[11, 16, 17] Here, 5-HT treatment upregulated the expression of Htr1a and Htr2b, but not Htr1b and Htr2a, in 3T3-L1 adipocytes (Figure 3A–D). Luteolin addition reversed the upregulation of Htr1a and Htr2b (Figure 3A,D). To identify the possible interaction between luteolin and HTRs, we performed a molecular docking simulation using publicly available molecular structures of proteins and found that the binding energy of HTR2B to luteolin was the highest in the tested HTRs (Figure 3E and Figure S3, Supporting Information). Like 5-HT, luteolin also bound to the active pocket 1 of HTR2B (Figure 3E). And even, relative to 5-HT, luteolin possessed the smaller molecular distance and the higher binding energy to HTR2B (Figure 3F,G). These results suggest that luteolin can competitively space-occupy the active pocket 1 of HTR2B to block 5-HT binding to HTR2B.
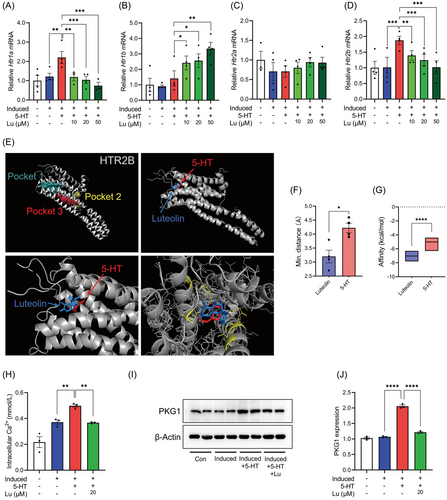
As a G protein-coupled receptor, HTR2B activation leads to intracellular Ca2+ accumulation and cGMP production, which in turn induces PKG activation and adipocyte lipolysis.[11, 18] As expected, 5-HT treatment significantly increased intracellular Ca2+ levels and PKG1 expression (Figure 3H–J). Luteolin addition blunted 5-HT-induced intracellular Ca2+ influx and PKG1 expression (Figure 3H–J). Thus, luteolin competitively binds to HTR2B to suppress Htr2b expression and blunt intracellular Ca2+-PKG1 cascade, and thereby inhibits the 5-HT-induced adipocyte lipolysis.
2.4 Diet Luteolin Supplementation before the Formation of Fatty Liver Alleviates Hepatic Steatosis in HFD-fed Mice
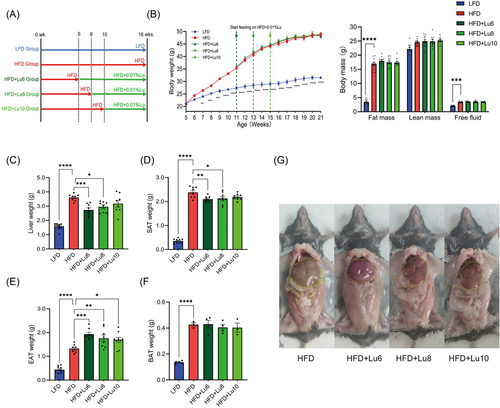
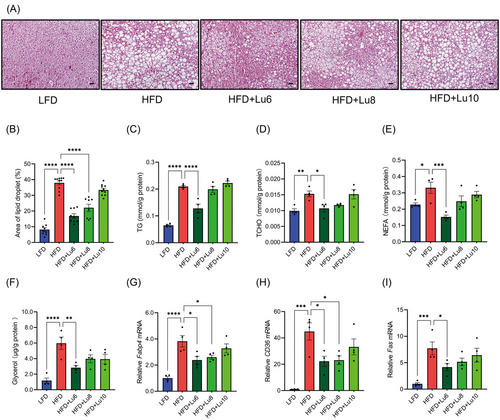
To assess the effect of dietary luteolin on obesity-related hepatic steatosis, we firstly detected the lipid droplet and triglyceride content in liver in HFD-fed mice and identified that fat liver was formed since the 10th week of HFD feeding (Figure S4A–C, Supporting Information). Next, the other 5-week-old mice were randomly divided into five groups: LFD, HFD, HFD+Lu6, HFD+Lu8, and HFD+Lu10 groups (Figure 4A). In HFD-fed mice, body weight gain and body component were unaffected by dietary luteolin (Figure 4B). However, relative to those in the HFD group, HFD+Lu6 and HFD+Lu8 groups had the lower liver and SAT weights (Figure 4C,D). Intriguingly, EAT weights (Figure 4E) were obviously elevated in all luteolin-treated groups and BAT weights (Figure 4F) were unaffected by dietary luteolin. Along with the reduction of liver weights, the livers from HFD+Lu6 and HFD+Lu8 groups were redder and more elastic (Figure 4G) and contained smaller fat vacuoles (Figure 5A,B). Additionally, diet luteolin supplementation at 6th week of HFD feeding significantly reduced the liver levels of TG, TCHO, NEFA, and glycerol (Figure 5C–F), and down-regulated the expression of gene (Fabp4, CD36, and Fas) involved in lipid transport and synthesis (Figure 5G–I). Consistently, serum TG, LDL-c, NEFA, glycerol, and ALT levels were also improved in HFD+Lu6 group (Table 1). Thus, independent of the body weight loss, diet supplementation of luteolin before fatty liver formation improves hepatic steatosis in HFD-fed mice.
| Parameter | LFD | HFD | HFD+Lu6 | HFD+Lu8 | HFD+Lu10 |
|---|---|---|---|---|---|
| (N = 10) | (N = 10) | (N = 10) | (N = 10) | (N = 10) | |
| ALT [U L−1] | 8.100 ± 0.314**** | 21.800 ± 2.225 | 14.000 ± 1.795* | 17.700 ± 2.864 | 19.400 ± 2.045 |
| AST [U L−1] | 29.100 ± 2.718 | 34.100 ± 4.212 | 28.400 ± 3.205 | 30.000 ± 3.204 | 32.600 ± 3.618 |
| ALP [U L−1] | 18.600 ± 0.702** | 30.400 ± 3.413 | 27.400 ± 2.141 | 24.800 ± 2.453 | 24.300 ± 1.826 |
| TG [mmol L−1] | 0.398 ± 0.025 | 0.403 ± 0.031 | 0.322 ± 0.020* | 0.347 ± 0.018 | 0.344 ± 0.010 |
| TCHO [mmol L−1] | 0.530 ± 0.028**** | 1.572 ± 0.071 | 1.617 ± 0.060 | 1.537 ± 0.054 | 1.562 ± 0.074 |
| LDL-c [mmol L−1] | 0.078 ± 0.002**** | 0.237 ± 0.010 | 0.1763 ± 0.013*** | 0.181 ± 0.007*** | 0.197 ± 0.011* |
| HDL-c [mmol L−1] | 0.421 ± 0.013**** | 1.148 ± 0.046 | 1.196 ± 0.040 | 1.142 ± 0.033 | 1.133 ± 0.050 |
| Glycerol [µg mL−1] | 277.900 ± 20.471**** | 446.844 ± 12.020 | 384.511 ± 12.354* | 428.822 ± 7.910 | 430.156 ± 21.582 |
| NEFA [mmol L−1] | 0.388 ± 0.016**** | 0.691 ± 0.029 | 0.590 ± 0.029* | 0.620 ± 0.020 | 0.631 ± 0.033 |
- Note: ATL, Alanine transaminase; AST, Aspartate transaminase; ALP, Alkaline phosphatase; TG, Triglycerides; TCHO, Total cholesterol; LDL-c, Low density lipoprotein cholesterol; HDL-c, High density lipoprotein cholesterol; NEFA, Non-esterified fatty acid.
- Each group was compared with the HFD group, using one-way ANOVA test.
- * p < 0.05;
- ** p < 0.01;
- *** p < 0.001;
- **** p < 0.0001.
2.5 Dietary Luteolin Inhibits EAT Lipolysis and Associated Lipolytic Signaling
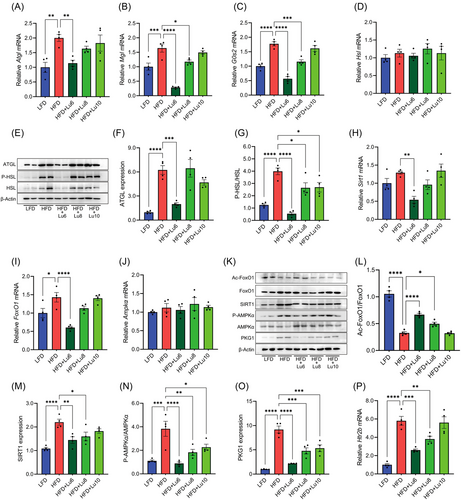
In HFD-fed mice, the liver weights and lipid levels are negatively correlated with EAT weights during the development of obesity-induced fatty liver. The activation HTR2B signaling and associated adipocyte lipolysis in EAT leads to ectopic lipid deposition in liver and the development of fatty liver.[11] Given diet supplementation of luteolin before fatty liver formation elevated EAT weights (Figure 4E) and improved hepatic steatosis (Figure 5), the levels of lipolytic enzymes and the activation of lipolytic signaling molecules should be depressed in EAT in the mice fed with a luteolin-containing HFD. Consistent with this notion, relative to those from HFD group, the mice from HFD+Lu6 group had the lower expression of Atgl, Mgl, and G0s2, protein level of ATGL, and phosphorylation of HSL (Figure 6A–G). Although EAT 5-HT levels were unaffected by dietary luteolin (Figure S5, Supporting Information), diet luteolin supplementation at the 6th week of HFD feeding reversed the action of HFD on the expression of Sirt1, FoxO1, and Htr2b, the acetylation of FoxO1, the level of SIRT1 and PKG, and the phosphorylation of AMPKα (Figure 6H–P). Altogether, diet supplementation of luteolin before fatty liver formation improves hepatic steatosis in obese mice by inhibiting visceral adipose tissue lipolysis and associated Ca2+-PKG cascade, and SIRT1/FoxO1/AMPKα signaling.
3 Discussion
It has been reported that diet supplementation of luteolin at start time of HFD feeding could protect the mice against diet-induced obesity and related complications, such as hepatic steatosis and insulin resistance.[14, 19, 20] However, diet supplementation of luteolin at the 10th week of HFD feeding does not affect mouse body weight gain but ameliorates obesity-related insulin resistance.[20] Thus, the improved action of luteolin on diet-induced obesity and associated metabolic diseases may greatly depend on the start time and duration of dietary luteolin intervention. The study reveals that diet supplementation of luteolin before fatty liver formation improves hepatic steatosis in HFD-fed mice by inhibiting visceral adipose tissue lipolysis, suggesting a reasonable therapeutic and interventional course of dietary luteolin for HFD-induced hepatic steatosis.
Luteolin mainly exists in the glycoside form in various edible plants and is primarily metabolized and absorbed in the intestinal tract.[21] When rats are orally administrated with luteolin-rich Chrysanthemum extract (approximately equivalent to 200 mg luteolin per kg body weight), plasma luteolin level reaches to 4 µg mL−1 (13.9 µM).[22] Serum luteolin level is higher than 14.1 µM after the gavage of 5 µmol kg−1 (approximately equivalent 14.3 mg kg−1 body weight) luteolin.[22] At the similar gavage dosage (14.7 mg kg−1 body weight), the maximum plasma concentration of luteolin is 100 µM.[22, 23] Therefore, in this study, 0.01% luteolin (approximately equivalent to 10 mg kg−1 body weight) used as the dosage of dietary intervention not only refers to human recommended daily intake of luteolin (2–125 mg day−1),[24] but also is comparable with the dose of the previous animal studies.[22, 23, 25]
Physiological utilization of luteolin is dependent on the degradation and transformation of intestinal microorganisms.[26] However, HFD feeding for more than 4 weeks significantly changes mouse intestinal microbial population,[27] and the post-dieting dysbiosis leads to the degradation of dietary flavonoids, which in turn interferes with host energy expenditure.[28] Supporting the notions, our previous study[24] and this study show that diet luteolin supplementation after the 6–10 weeks of HFD feeding does not affect body weight gain of HFD-fed mice (Figure 4B). Intriguingly, the improvement of dietary luteolin on obesity-associated insulin sensitivity[20] and hepatic steatosis (Figures 4 and 5) does not depend on the change of mouse body weight in HFD-fed mice. The observations suggest that some microbiome-degraded products of luteolin may possess the substantial activities to combat insulin resistance and hepatic steatosis, a hypothesis that needs further investigation.
Adipose tissue gradually loses its function in the progress of obesity. In the early stage of HFD-induced obesity, basal adipocyte lipolysis is reduced. However, in the late stage of HFD-induced obesity, basal lipolysis is significantly elevated in white adipose tissue and finally leads to the ectopic deposition of lipids.[11, 29] As the lipolysis regulator and energy sensor, the activation of SIRT1/FoxO1/AMPKα signaling is also consistent with the lipolysis level in EAT. In our prior study, 12 weeks of HFD feeding prominently depresses EAT lipolysis and SIRT1/FoxO1/AMPKα signaling, and dietary luteolin reverses the action of HFD feeding.[30] In this study, EAT lipolysis and SIRT1/FoxO1/AMPKα signaling are remarkably induced by 16 weeks of HFD feeding and are inhibited by dietary luteolin. These results support the regulated synchronism between the level of visceral adipocyte lipolysis and the activation of SIRT1/FoxO1/AMPKα signaling.
Although we demonstrate the regulation of luteolin on 5-HT-induced 3T3-L1 lipolysis and HFD-induced hepatic steatosis, this study possesses some potential limitations. Firstly, besides HTR2B signaling, several classical stimulations, such as insulin[31, 32] and cold[33, 34] also induce adipose tissue lipolysis. As a multi-functional compound, luteolin may also affect adipocyte lipolysis and the development of fatty liver through regulating other signaling pathways in response to the stimulations, which needs future research. Additionally, although the improvements of dietary luteolin on hepatic steatosis in HFD+Lu6 and HFD+Lu8 groups are more significant than those in HFD+Lu10 group, the durations of luteolin treatments in HFD+Lu6 and HFD+Lu8 groups are also longer than those in HFD+Lu10 group. Since the levels of some EAT lipolytic molecules, including the phosphorylated levels of HSL and AMPK and the protein expression of PKG1, are altered in the mice from HFD+Lu10 group (Figure 6), we cannot exclude the presumption that the mice may have the improvement of fatty liver when the duration of diet luteolin supplementation is prolonged. However, our study shows that the earlier supplementation of diet luteolin is more beneficial to protect HFD-fed mice against hepatic steatosis.
In conclusion, diet luteolin supplementation before the formation of fatty liver alleviates HFD-induced hepatic steatosis by inhibiting EAT lipolysis, suggesting a reasonable therapeutic and interventional course for obesity-related fatty liver.
4 Experimental Section
Mice and Diets
Three-week-old male C57BL/6N mice were purchased from Vital River Laboratory Animal Technology Co. (Beijing, China) and housed in ventilated cages in a pathogen free barrier facility with a 12-h light: 12-h dark cycle. After 2 weeks, the mice were divided into five groups (n = 10): 1) the LFD group, fed on a LFD (10 kcal% fat, D12450B, Research Diets, New Brunswick, NJ, USA) for 16 weeks; 2) the HFD group, fed on an HFD (45 kcal% fat, D12451, Research Diets) for 16 weeks; 3) the HFD+Lu6 group, fed on an HFD for 6 weeks and then switched to a luteolin-containing HFD (0.01% luteolin [≥98%, HPLC, Huayi Biotechnology, Shanghai, China]), 4) the HFD+Lu8 group, fed on an HFD for 8 weeks and then switched to a luteolin-containing HFD, 5) the HFD+Lu10 group, fed on an HFD for 10 weeks and then switched to a luteolin-containing HFD. The detailed formulation of all diets was provided in Table S1, Supporting Information. Mouse body component, including fat mass, lean mass, and free fluid distribution, was detected using a minispec live mouse component analyzer (Bruker, LF-50). At 21-week-old, the mice were sacrificed by the exposure to CO2 and the sera and tissues, including liver, EAT, subcutaneous adipose tissue (SAT), and brown adipose tissue (BAT) were harvested. Additionally, to identify the time of fatty liver formation in HFD-fed mice, the other 5-week-old mice (n = 30) were fed on an HFD and sacrificed at 0, 5, 10, 15, 20 weeks of HFD feeding, respectively (n = 6). All mouse protocols were approved by the Animal Committee of Hefei University of technology.
Cell Culture
3T3-L1 pre-adipocytes were purchased from the cell culture center of Peking Union Medical College, cultured in DMEM (Gibco, Carlsbad, CA, USA) with 10% newborn bovine serum (Gibco), and fully differentiated into mature adipocytes as previously described.[35, 36] 5-HT was dissolved in DMSO at 100 µg mL−1 as a stock solution and luteolin was dissolved in PBS at 10 mM as a stock solution. As previously reported[37] adipocyte lipolysis was induced by 100 ng mL−1 5-HT. To assess the effect of luteolin on adipocyte lipolysis, 10, 20, or 50 µM luteolin were added into the culture media of 3T3-L1 adipocytes for 48 h. The data were representative of three independent experiments.
Dosage Information
In mouse study, the dosage of dietary luteolin was 0.01% w/w, which was approximately equivalent to 10 mg kg−1 body weight per day.[38] In cell study, 10, 20, or 50 µM luteolin was added to 3T3-L1 culture medium according to the previous report.[39] According to blood level of luteolin after oral administration,[22, 23, 25] the dosage of luteolin in mouse study was comparable to those used in cell study.
Serum and Liver Lipid Analysis
To obtain the extract of liver glycerol, 100 mg liver tissue was fully homogenized in 0.9 mL saline, repeated freezing and thawing for three times, and centrifuged at 5000 × g for 10 min.[40] The total liver lipids were extracted using the Bligh and Dyer method.[41] 100 mg liver tissue were mixed with 1500 µL of chloroform: methanol (1:2) in a glass tube, and vortexed for 30 min at 4 °C. Subsequently, 500 µL of double-distilled water and 500 µL of chloroform were added to the tube, and the suspension was vortexed for 5 min and centrifuged at 5000 × g for 10 min. After centrifugation, 900 µL solution was collected from the chloroform layer and moved into a new glass tube to dry. Finally, the dry sediment was dissolved in the methanol. To obtain the extract of serum lipids, blood samples were placed at 4 °C for 4 h and centrifuged at 5000 × g for 15 min.[40]
The total cholesterol (TCHO), TG, high density lipoprotein cholesterol (HDL-c), low density lipoprotein cholesterol (LDL-c), alanine aminotransferase (ALT), NEFA, aspartate aminotransferase (AST), alkaline phosphatase (ALP) in plasma and liver were detected using a biochemical analyzer (Beckman Coulter, AU680). Free glycerol was detected using a glycerol assay kit (Sigma-Aldrich, Burlington, VT, USA) according to the manufacturer's instructions.
Protein Extraction and Western Blot Analysis
Cells or tissues were lysed in the radioimmunoprecipitation assay lysis buffer. The concentration of the total protein was detected using a Bicinchoninic Acid assay kit (Beyotime Institute of Biotechnology, Shanghai, China). The 20 µg protein lysates were separated on the SDS-polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane (Millipore, Burlington, VT, USA). The target proteins were detected with the following primary antibodies: ATGL (1:1000, Cell Signaling Technology, Danvers, MA, USA), HSL (1:500, Abcam, Cambridge, MA, USA), phosphorylated-HSL (1:500, Abcam), SIRT1 (1:1000, Cell Signaling Technology), AMPKα (1:1000, Cell Signaling Technology), phosphorylated-AMPKα (1:1000, Cell Signaling Technology), FoxO1 (1:500, BioLegend, San Diego, CA, USA), Acetyl-FoxO1 (1:500, Affinity Biosciences, Cincinnati, OH, USA), and PKG1 (1:1000, Boster Biological Technology, Wuhan, China) antibodies. HRP labeled goat anti-rabbit IgG (1:4000, KPL, Gaithersburg, MD, USA) was used as the secondary antibody. Chemiluminescence was detected by ECL western blot kit (BIO-RAD, Hercules, CA, USA) in a biomolecular imager (GE Healthcare, ImageQuant LAS 4000 mini).
Total RNA Isolation and Quantitative Real-Time PCR
Total RNA was isolated by Trizol reagent (Takara, Dalian, China) and reverse-transcribed into cDNA using a reverse transcription kit (Takara) according to the manufacturer's instructions. Quantitative real-time PCR was performed by iTaq universal SYBR Green supermix (BIO-RAD) in a real-time PCR detector (BIO-RAD, myiq2). The relative mRNA expression was measured by adopting ΔΔCT method and normalized to β-Actin. For cell study, the relative mRNA levels of genes per group were standardized to those in the undifferentiated 3T3-L1 cells. For mouse study, the relative mRNA levels of genes per group were standardized to those in the LFD group. The primer sequences were listed in Table S2, Supporting Information.
Hematoxylin and Eosin (H&E) Staining
The 7 µm tissue sections were dewaxed, rehydrated, and stained in hematoxylin and eosin solution. The stained sections were photographed under an optical microscope (Nikon, DS-U2). Five fields from each slice were randomly examined, and the level of liver fat accumulation was presented as the ratio of the area occupied by white vacuole to the total area of tissue section using Image-Pro Plus 6.0 software.
Oil Red O Staining of 3T3-L1 Cells
Intracellular lipid droplets were detected using Oil red O staining as previous reported.[42] Briefly, the 3T3-L1 cells were sequentially fixed in 4% formaldehyde solution, soaked in propylene glycol, and stained with 0.5% Oil red O solution (Sigma-Aldrich). After successively washing with 98% propylene glycol for once and with 85% propylene glycol for twice, the cells were photographed in PBS under the microscope (Nikon, DS-U2), and the intracellular lipid was dissolved in ethanol to detect the absorbance value at 510 nm.
Molecular Docking Simulation
The 3D molecular structures of the bioactive compounds were collected from TCMSP in mol2 format and transformed into PDBQT format. Protein Data Bank (PDB, http://www.rcsb.org/) was utilized for the collection of crystal structures of the core proteins (7e2x-Htr1a, 4iaq-Htr1b, 6wgt-Htr2a, and 6drx-Htr2b). AutoDock Tools (version 1.5.6 http://mgltools.scripps.edu/) were further used for removal of water and addition of hydrogen atoms to the crystal structures of core targets and the results were saved as PDBQT format. Molecular docking between the bioactive compounds and core targets was performed by AutoDock.[43] The binding pattern with the lowest binding energy was selected. Finally, the interactions between the bioactive compounds and the core targets were visualized as 3D diagrams using PyMol (version2.5.0a).
Statistical Analysis
All data were expressed as mean ± SEM. The study used a nonparametric Mann–Whitney U test to compare two-group data in molecular docking simulation. One-way ANOVA test was used for multiple group comparisons in cell and mouse experiments. GraphPad Prism 9.0 software was used for all statistical analyses and p < 0.05 was considered statistically significant.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (32070757 to J.L., 32200630 to X.Z.), the Industrial Innovation Funds for Linquan County-Hefei University of Technology (JZ2018QTXM0553 to J.L.), the University Synergy Innovation Program of Anhui Province (GXXT-2019-026 to J.L.), and the Fundamental Research Funds for the Central Universities (13020-03712022006 to X.Z.).
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
J.W. designed and performed experiments, analyzed data, generated the figure panels, and wrote the draft manuscript. Z.Z. helped to perform the experiments. Y.L., J.C., F.W., and L.W. helped to design experiments. J.L. and X.Z. led the project, designed the experiments, and revised the manuscript.
Open Research
Data Availability Statement
The published article includes all datasets generated or analyzed during this study and available upon request from the lead contact.




