A Synbiotic Ameliorates Con A-Induced Autoimmune Hepatitis in Mice through Modulation of Gut Microbiota and Immune Imbalance
Abstract
Scope
Changes in the intestinal flora are related to autoimmune hepatitis (AIH) development. The aim of this study is to investigate the synergistic effects of probiotics and prebiotics on liver injury induced by concanavalin A (Con A).
Methods and results
C57BL/6 mice are fed probiotics (Pro), prebiotics (Pre), synbiotic (Syn) for 7 days and then Con A is injected via tail veins to induce AIH. Additionally, methylprednisolone (MP) is gavaged 0.5 h after the Con A injection. It is found that both Pro, Pre, Syn, and MP decrease the levels of serum transaminase, liver F4/80+ macrophage cells, and hepatocellular apoptosis. Pro, Pre, and Syn decrease proinflammatory cytokines, elevate levels of anti-inflammatory as well as restored immune imbalance in AIH. Besides, Pro, Pre, and Syn not only reshape the perturbed gut microbiota, but also maintain intestinal barrier integrity, block the activation of lipopolysaccharide (LPS)/TLR4/NF-κB pathway in the liver. Interestingly, the effects of Syn are superior to Pro or Pre alone in Con A-induced acute liver injury.
Conclusions
Syn obviously facilitates AIH remission. The combined use of Pro and Pre is effective in improving Pro and Pre efficacy and can be an important tool for preventing and adjuvant treating patients for AIH.
1 Introduction
Autoimmune hepatitis is inflammatory of immune-mediated destruction of hepatocytes with unclear etiology. It occurs at all age and can progress to liver cirrhosis and failure if untreated or misdiagnosed.[1] AIH is characterized by serum autoantibody positivity, elevated aminotransferase levels, and hepatic pathologies with the presence of interface hepatitis.[2] A combination of corticosteroids and azathioprine is effective therapeutic strategies for AIH, but the prolonged use of steroids may cause significant side effects, such as immunosuppression, osteoporosis, and sodium retention, and the discontinuation of steroid treatment is followed by disease relapse in most patients.[3] Considering that, it is significantly important to develop more safer and effective treatment strategies.
Concanavalin A (ConA), a lectin originally extracted from the jack bean, predominantly stimulates T cells to induce acute liver injury. The typical characteristic of ConA induced acute hepatitis model was CD4+T cells, natural killer T, Kupffer cells were activated and released cytokines to cause inflammation and massive hepatocytes damage.[4] This model is a murine model that mimics human AIH. Emerging evidence has shown a strong linkage between intestinal flora and liver diseases. Germ free (GF) mice are resistant to acute AIH induced by Con A, in sharp contrast to specific pathogen free (SPF) mice.[5] Additionally, the abundance of probiotics, especially Bifidobacterium and Lactobacillus, decreased in the feces of AIH patients.[6] This results in intestinal barrier destruction and an increase in serum lipopolysaccharide (LPS), which caused endotoxemia or systemic low-grade inflammation in the patients.[7] The results of these studies indicate that the intestinal microbiota may play an important role in the pathogenesis of AIH.
Prebiotics include galactose oligosaccharides (GOS), fructo-oligosaccharides (FOS), and inulin, which are food ingredients that selectively stimulate to increase short-chain fatty acid concentration and abundance of Bifidobacteria and Lactobacilli in the gut microbiota that confer health benefits to the host.[8, 9] Bacteria of the genus Bifidobacterium and Lactobacillus are normal inhabitants in the gut and represent a significant part of the healthy microbial community. Data from animal studies revealed that Bifidobacterium longum R0175, Lactobacillus acidophilus LA14, or Lactobacillus reuteri DSM 17938 suppressed production of inflammatory factors and mitigated the histological injury to the liver and gut by ameliorating dysbiosis of the gut microbiota.[10-12] A clinical trial demonstrated that the 14 alive probiotic strains containing Bifidobacterium and Lactobacillus can significantly reduce liver fat, aminotransferase activity, and TNF-α and IL-6 levels in NAFLD patients.[13] What is more, Bifidobacterium and Lactobacillus strains could block of the IL-23 dependent Th17 differentiation pathway[14] and increase percentages of regulatory T cells (Treg) in peripheral blood leukocyte[15] showing the immunomodulatory effects. Our previous evidence has shown that the treatment of AIH in mice with the compound probiotic improved ileal barrier function while increasing the diversity of intestinal flora, which blocked TLR4/NF-κB pathway to product inflammatory factors.[16]
Synbiotic refer to mixture of prebiotics and probiotics for improvement of health. Probiotics can selectively utilize prebiotics as substrate for their growth.[17] Currently, increasing evidence has indicated that synbiotic supplementation could improve intestinal barrier function and microbial composition.[18] In addition, a study has suggested that compared with fermentable fiber, oral synbiotic can significantly reduce the level of serum LPS in patients with minimal hepatic encephalopathy.[19] In the HFD-fed mice, compared with Lactobacillus plantarum S58 or hull-less barley β-glucan, probiotics and prebiotics synergistically caused a greater inhibition in serum inflammatory markers and obviously reversed the HFD-induced reduction in the protein expression of zonula occludens-1 (ZO-1), occludin, and claudin-7.[20] These studies show that the effects of symbiotic is superior to probiotic or prebiotic alone.
Therefore, it is speculated that synbiotic supplementation could be a more effective preventative strategy for AIH. However, studies on the role of synbiotic in AIH are very limited and the underlying molecular mechanism remains to be elucidated. Thus, the major focus of the present investigation is to assess whether a mixture of probiotics and prebiotics could significantly ameliorate Con A-induced AIH in mice and compare it's efficacy with the probiotics and prebiotics alone.
2 Results
2.1 Probiotics, Prebiotics, and Synbiotic Relieved Con A-Induced Liver Injury
Con A induced AIH in mice, which was characterized by immune derangement, elevated serum transaminase, and extensive necrotic of the liver tissue.[2] The levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bile acid (TB) decreased after methylprednisolone, probiotics, prebiotics, and synbiotic treatment (Figure 1A). In the NC group, the livers were bright red with smooth surfaces. The livers of AIH mice were blood red, granular and small in size. In contrast, the livers of the MP, Pre, Pro, and Syn groups were similar to those of normal mice (Figure 1B). As shown in Figure 1C,D, extensive necrotic, a large amount of F4/80+ macrophage cell infiltration were found in the liver tissues of the mice in the AIH group, which indicated liver damage. Supplementing MP, Pre, Pro, or Syn could decrease the severity of liver injury and F4/80+ macrophage cell infiltration via liver histopathological assays (Figure 1C,D). Injection of Con A rapidly increased the level of caspase-3 and Bax in liver tissue.[21] As showed in Figure 1E,F, proteins expression levels of caspase-3 and Bax were decreased in MP, Pre, Pro, and Syn groups.
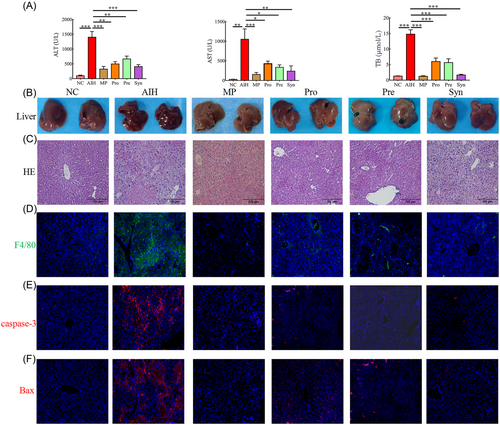
Notably, these results suggested that compared to the probiotics and prebiotics, the synbiotic had the strongest protective effect on the liver injury induced by Con A in mice.
2.2 Probiotics, Prebiotics, and Synbiotic Restored Immune Imbalance in the AIH Mice
Previous studies revealed that T cells were the cardinal cells involved in immune imbalance of AIH.[22] Here, we compared the spleen sizes of the mice in each group (Figure S2A, Supporting Information). The spleens of the mice in the NC group were bright red without swelling, while the spleens of the mice in the AIH group showed noticeable swelling and increased volume. Compared to the AIH group, the Pre, Pro, and Syn groups displayed lower degrees of swelling (Figure S2A, Supporting Information). In order to verify whether Pre, Pro, and Syn play an important role in modulating T cell activation in AIH, splenocytes from different groups were harvested and detected by flow cytometry. Under the influence of probiotics, prebiotics, and synbiotic, the percentages of Th17 (CD4+ IL-17+) cells in the spleens were decreased and the Tregs (CD4+ FOXP3+) was increased (Figure 2A). Besides, our data indicated that Con A could significantly reduce the number of peripheral blood white blood cells and lymphocytes (Figure S2B, Supporting Information). Nevertheless, the number of peripheral blood white blood cells were increased by synbiotic and lymphocytes were increased by probiotics and synbiotic in the AIH mice.
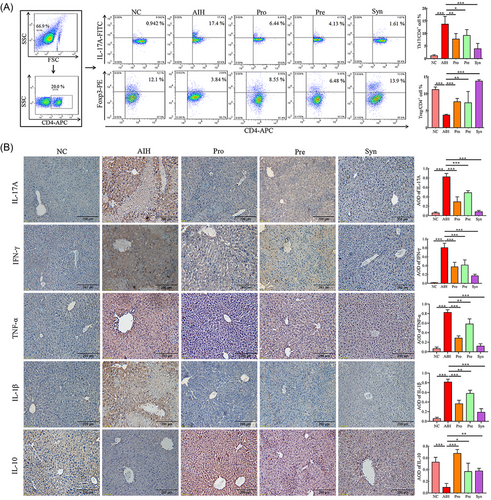
AIH mice can produce higher level of pro-inflammatory cytokines in liver, including interleukin 1β (IL-1β), interleukin 17A (IL-17A), interferon-γ (IFN-γ), tumor necrosis factor α (TNF-α).[23] Here, in our study, protein expression of these cytokines was measured in the AIH mice feeding with or without probiotics, prebiotics, and synbiotic supplementation by immumohistochemical staining. Then, it was found that proinflammatory IL-1β, IL-17A, IFN-γ, TNF-α proteins expression were higher and antiinflammatory interleukin 10 (IL-10) protein expression levels was low in hepatic tissues of AIH mice compared with normal mice (Figure 2B). Notably, the protein expression pattern of these cytokines was altered by probiotics, prebiotics, and synbiotic treatment, resulting in the protein expression level being close to normal mice (Figure 2B).
In brief, these results indicated that the probiotics, prebiotics, or synbiotic were able to restore immune imbalance and inhibit inflammation of AIH in mice and showed the synbiotic with the strongest effect on immune regulation.
2.3 Probiotics, Prebiotics, and Synbiotic Restored the Function of the Intestinal Barrier in the AIH Mice
Considering intestinal dysbiosis in AIH may affect gut permeability and subsequently lead to release of bacterial LPS into the circulation,[7] whether Probiotics, prebiotics, and synbiotic modulates gut integrity was proved. The length and density of the ileum villi is indicative of the digestive and absorptive functions. The shape of normal mouse ileum villi has straight finger-like protrusions arranged neatly and densely. Figure 3A showed that in the AIH group, the ileum villi base was broken, the arrangement was loose and disordered, and the number of goblet cells that secrete mucus was reduced. Under the administration of the probiotics, prebiotics, or synbiotic, the ileum structure was recovered and the number of goblet cells was increased. Interestingly, the ileal villi in the Syn group were longer than those in the NC group (Figure 3A). Abundance of tight junction proteins ZO-1 and occludin may reflect the permeability of the intestinal barrier. The expression of ZO-1 and occludin were elevated in the ileum tissues of the mice in the Pre, Pro, and Syn groups (Figure 3B,C). Protecting the intestinal barrier may inhibit bacterial components LPS translocation to the circulation. LPS levels were increased in the serum of AIH mice, and decreased in the Pro, Pre, and Syn groups (Figure 3D).
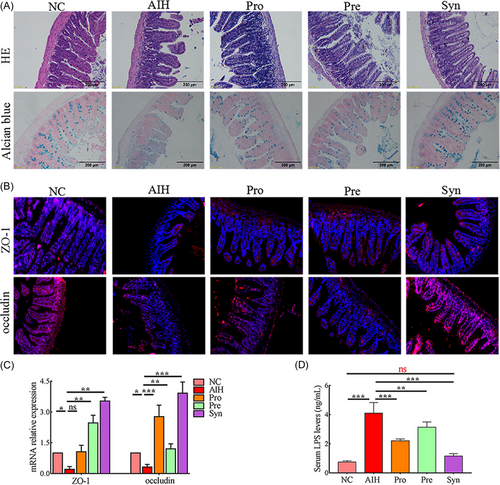
These data suggested that the probiotics, prebiotics, and synbiotic had a protective effect on the intestinal barrier, especially synbiotic.
2.4 Probiotics, Prebiotics, and Synbiotic Altered the Composition of the Gut Microbiota in the AIH Mice
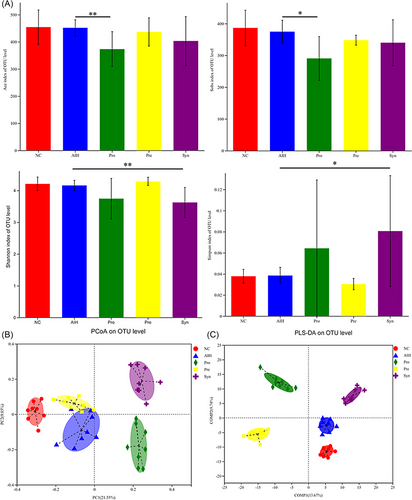
To characterize the microbial populations in the feces of the mice, we measured bacterial populations by 16S rRNA gene sequencing. The α-diversity of the gut microbiota was calculated by the Ace index, Chao index, Shannon index, and Simpson index, while the species richness and diversity significantly did not decrease in the AIH group (Figure 4A). In contrast, our results showed that supplementation with probiotics (Pro) and synbiotic (Syn) decreased gut microbial α diversity, which may be a decrease in the number of potentially harmful bacteria. The β-diversity of the gut microbiota was calculated by principal coordinate analysis (PCOA) and partial least squares discrimination analysis (PLS-DA). It was observed that the microflora aggregation of mice fed probiotics, prebiotics, and synbiotic was significantly stayed away from the mice of AIH (Figure 4B). PLS-DA indicated that probiotics, prebiotics, and synbiotic had strong effect on changing the composition of bacterial community in AIH mice (Figure 4C). Collectively, these findings demonstrate that the richness, diversity and composition of intestinal flora can be shifted by oral administration of probiotics, prebiotics, and synbiotic in AIH mice.
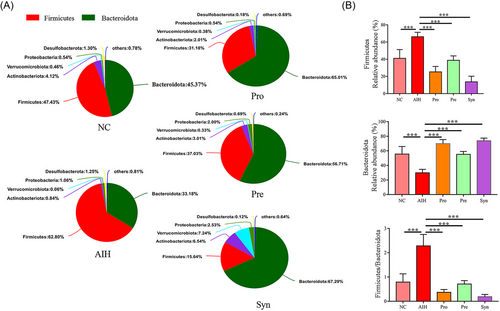
Under these circumstances, upon performing taxonomic classification analysis, we found that Firmicutes and Bacteroidetes dominated the intestinal microbiota at the phylum level in all samples (Figure 5A). Under the administration of probiotics, prebiotics, and synbiotic the relative abundance of Firmicutes (p < 0.001) and Firmicutes/Bacteroidetes ratio (p < 0.001) was significantly decreased and the Bacteroidetes (p < 0.001) increased (Figure 5B).
In the meanwhile, the data shown that a total of eight genera were obviously affected by the probiotics, prebiotics, or synbiotic compared with AIH group in the top 14 genera (Figure 6). It was observed that some genera (norank_f__Muribaculaceae, Bifidobacterium, Akkermansia) significantly increased, whereas others (Lachnospiraceae_NK4A136_group, unclassified_f__ Lachnospiraceae, norank_f__Lachnospiraceae, Alloprevotella, and Escherichia-Shigella) decreased drastically in the Syn grouop (Figure 6). Besides, probiotics supplement remarkably increased Alloprevotella and decreased Lachnospiraceae_NK4A136_group to a great extent. Surprisingly, under the same situation prebiotics only increased Alloprevotella.
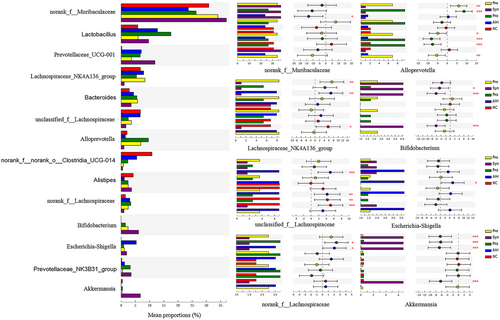
Taken together, these results suggested that the changes of bacteria composition by synbiotic were more significant at the phylum or genera level (Figures 5 and 6).
2.5 Probiotics, Prebiotics, and Synbiotic Altered Gut Microbiota Biomarker in the AIH Mice
Here, we found that the bacterial community structure with notable differences among the NC, the AIH, the Pro, the Pre, and the Syn group, which was further analyzed by adopting the linear discriminant analysis (LDA) effect-size method (LEfSe) to explore the gut microbiota biomarker associated (Figure 7). Firmicutes, Clostridia, Lachnospiraceae, Lachnospirales, Lachnospiraceae_NK4A136_group, unclassified_f__Lachnospiraceae, Enterobacteriaceae, Enterobacterales and Escherichia-Shigellam played critical roles and could be taken as a biomarker in the AIH group (LDA score >4.0). Prevotellaceae and Alloprevotella functioned importantly and could be used as a biomarker in the Pro group. Oscillospirales, Oscillospiraceae, Bacteroides, Bacteroidaceae, Streptococcus, and Streptococcaceae played a crucial part and could be employed as a biomarker in the pre group. Bacteroidia, Prevotellaceae_UCG-001, Muribaculaceae, Akkermansia, Akkermansiaceae, Verrucomicrobiales, Verrucomicrobiota, and Verrucomicrobiae were vital and could be regarded as a biomarker in the Syn group.
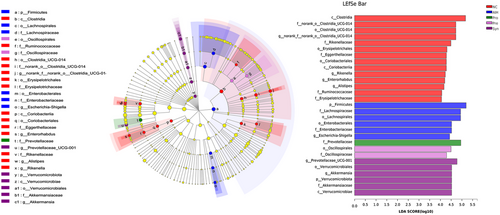
It is important to note that the number of taxa with differential abundance in the Syn group was more than that in the other groups, which showed that synbiotic may have stronger liver protection by increasing of some specific microbiota.
2.6 Probiotics, Prebiotics, and Synbiotic Relieved Liver Inflammatory Injury by Inhibiting the TLR4/NF-κB Signaling Pathway
We found that the LPS levels were increased in the serum of AIH mice (Figure 3D). TLR4 is a sensory receptor for LPS and has a very important role in the up-regulation of inflammation and the release of inflammatory cytokines.[24] Considering that, the effects of probiotics, prebiotics, and synbiotic on LPS level and TLR4 and NF-κB protein expression in hepatic tissues were identified. Probiotics, prebiotics, and synbiotic treatment decreased the LPS levels, and the Syn group decreased most obviously. In the AIH group, LPS translocation led to the activation of TLR4 and the NF-κB signaling pathway (Figure 8D). The probiotics, prebiotics, and synbiotic reduced the expression of TLR4, NF-κB, and P-IκB/IκB by inhibiting LPS translocation. The three microecological modulator obviously blocked the activation of the TLR4/NF-κB signaling pathway, and therefore the production of pro-inflammatory cytokines (IL-1β, IL-17A, IFN-γ, TNF-α) were also inhibited (Figure 2B).
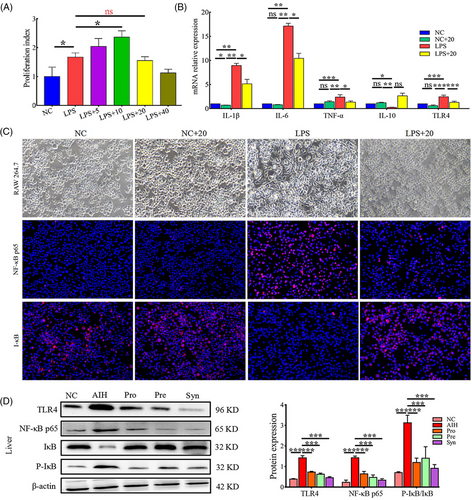
Prebiotic is a “substrate that is selectively utilized by host microorganisms conferring a health benefit” to stimulate the increased abundance of Bifidobacterium and Lactobacillus probiotics in the intestinal flora. Thus, we speculated that whether probiotics, prebiotics, or synbiotic, probiotics cell components (i.e., postbiotics) make a certain contribution to alleviate AIH inflammation. In order to determine the potential substances of probiotics, prebiotics and synbiotic, the effects of postbiotics on TLR4/NF-κB signaling pathway in RAW264.7 cells were determined by immunofluorescence under LPS-stimulated conditions. We first investigated whether postbiotics could exert a similar antiproliferative effect on macrophages. We found that LPS stimulation of macrophages significantly increased proliferation. And proliferation of LPS-stimulated macrophages was not affected by postbiotics pretreatment at a dose of 20 µg mL−1 (Figure 8A). However, pretreatment with 40 µg mL−1 postbiotics strongly decreased the proliferation rate of LPS-stimulated macrophages. Postbiotics at 40 µg mL−1 maybe induce cytotoxicity, which did not occur at 5 and 20 µg mL−1 (Figure 8A). The mRNA expressions of IL-1β, IL-6, TNF-α, and TLR4 were significantly decreased in the LPS+20 group compared to that in the LPS group (Figure 8B). The mRNA expressions of IL-10 did not increase significantly in the LPS+20 group. As shown in Figure 8C, the expression of NF-κB p65 was significantly increased in LPS-stimulated group compared to that in the NC group. In the case of exposure to 20 µg mL−1 postbiotics, the expressions of NF-κB p65 was significantly inhibited. The expressions of IκB were significantly decreased in the LPS group, which can be increased by exposure to 20 µg mL−1 postbiotics (Figure 8C).
Together, the results of in vivo and vitro indicated that probiotics, prebiotics, and synbiotic relieved liver inflammatory injury by inhibiting the TLR4/NF-κB signaling.
2.7 Gut Microbiota-Associated Host Parameters and Effects of Probiotics, Prebiotics, and Synbiotic on the Gut Microbiota Function
From heatmap correlation analysis, it is seen that in the 14 top genera, 13 genera were notably associated with blood parameters, immune cells, degree of LPS translocation, genes expression of tight junction proteins, proteins expression of TLR4/NF-κB signaling pathway (Figure 9A). norank_f__Oscillospiraceae, norank_f__Lachnospiraceae, Prevotellaceae_UCG-001, and Lactobacillus were obviously and positively correlated with ALT, AST, TB, proinflammatory IL-1β, IL-17A, IFN-γ, and TNF-α proteins expression, the percentages of Th17 cells and TLR4/NF-κB-related proteins expression, but noticeably and negatively correlated with IL-10 proteins expression, the percentages of Treg cells and number of peripheral blood white blood cells (WBC). For ZO-1 and occludin mRNA relative expression, Akkermansia, Bifidobacterium, and norank_f__Muribaculaceae were obviously and positive correlation, while norank_f__norank_o__Clostridia_UCG-014 and unclassified_f__Lachnospiraceae were noticeably and negatively. Altogether, the correlation analysis showed that these bacterial may alleviate AIH and the 13 bacterial play crucial roles in AIH development.
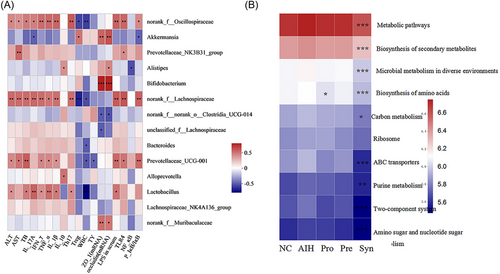
At the same time, we employed to PICRUSt to predict the potential function of bacteria in all groups, which was analyzed in the context of the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (Figure 9B). The results indicated that the synbiotic dramatically affected the nine functions compared with AIH mice in the top 10 functions, which metabolic pathways, biosynthesis of secondary metabolites, microbial metabolism in diverse environments, biosynthesis of amino acids, carbon metabolism, ABC transporters, purine metabolism, two-component system, and amino sugar and nucleotide sugar metabolism were significantly inhibited. Additionally, biosynthesis of amino acids was affected by probiotics. And surprisingly, in the top 10 functions, prebiotics did not play an obvious role. Concluding, synbiotic treatment improved gut microbiota functions involving metabolism.
3 Discussion
Autoimmune hepatitis (AIH) was a severe inflammatory liver disease of unknown etiology. It has been reported that increased intestinal pathogenic bacteria facilitate Con A-mediated liver injury.[25] In the current study, the data obtained confirm that intestinal flora took part in ConA-induced hepatocytes damage. In the meanwhile, the treatment of AIH with the probiotics, prebiotics, and synbiotic regulated gut microbiota and immune response, maintained intestinal barrier integrity, blocked lipopolysaccharide (LPS) translocation, and inhibited the activation of the TLR4/NF-κB pathway, which resulted in less production of inflammatory factors and facilitated the remission of AIH.
Con A induced mouse AIH can successfully mimic human AIH.[26] In vitro studies have found that Con A was directly toxic for hepatocytes to induce hepatocytes apoptosis.[27] The pathological features of AIH are high ALT, AST, and TB in serum and massive necrosis in liver tissue.[28] The present study demonstrated that the probiotics, prebiotics, or synbiotic have the effect of restoring liver function by downregulating ALT, AST, and TB, and reducing liver necrosis by reduce hepatocyte apoptosis in AIH mice. Glucocorticoid are considered the standard treatment strategy for AIH.[3] In this study, methylprednisolone (MP) group was employed as a positive control to evaluate the treatment effect of probiotics, prebiotics, or synbiotic. In fact, our findings indicated that probiotics, prebiotics, or synbiotic were able to alleviate the pathological damage of AIH in mice and showed the same even improved effects as MP. Specifically, it should not be ignored that glucocorticoid can cause a certain degree of hepatic cell edema and other side effects. Con A not only activates T cells, but with the excessive activation of T cells comes the production of various cytokines and chemokines and the activation of other immune cells.[29] The chemokines such as MCP-1, MIP-1A, MIP-1B attract macrophages to the liver to accelerate disease progression. Activated Kupffer cells and monocytes macrophages can produce pro-inflammatory cytokines TNF-α, IL-1β, IL-6, and so on, which are involved in the pathogenesis of liver injury.[30] It was found that Lactobacillus had the potential to reduce the infiltration of F4/80 or CD68 macrophages in mouse liver.[31, 32] Bifidobacterium and Lactobacillus administration resulted in improvements in hepatic damage and inhibited the production of liver inflammatory factors IL-18, IFN-γ, TNF-α, IL-1β, IL-4 in mice on a high-fat diet or exposed to alcohol.[33, 34] In the present study, there was a significant amount of infiltration of macrophages and extensive necrotic in the liver of AIH mice, and probiotics, prebiotics, or synbiotic treatment could significantly reduce macrophages infiltration and the production of proinflammatory cytokine IL-17A, IFN-γ, TNF-α, and IL-1β.
Most scholars emphasize the importance of CD4+ T cell in the development of AIH disease. IL-17A is mainly produced by the pro-inflammatory Th17 cells, which are closely associated with the pathogenesis of autoimmune disorders, such as AIH,[35] systemic lupus erythematosus,[36] and multiple sclerosis.[37] Pathogenic Th17 cells have been functionally defined according to their capacity to induce severe disease in models of autoimmunity. Tregs, the main regulators of the immune system, restrict the inflammatory and suppress the autoimmune response via production of anti-inflammatory factors such as IL-10 and TGF-β.[38] The inability of Tregs to efficiently suppress IL-17A production by Th17 cells may be crucial to the pathogenesis of AIH.[39] In this study, the proportion of Th17 cells were significantly decreased and the proportion of Tregs were significantly increased in the probiotics, prebiotics, or synbiotic groups. With the recovery of the immune balance, the hyperfunction of the spleen was weakened and the volume was decreased with the number of leukocyte and lymphocytes increased. The results indicated that the probiotics, prebiotics, and synbiotic alleviated AIH in mice by immunomodulatory.
The elevated LPS level in AIH patients is mainly due to the impaired intestinal barrier and dysbiosis of the gut microbiota.[40] Normally, LPS transfer to extraintestinal organs can be prevented by an intact intestinal barrier. In the current study, increased serum LPS concentration indicated disruption of the intestinal barrier in AIH mice. Moreover, the structure of the ileum was destroyed, the number of goblet cells that secrete mucus was reduced, and the expression of tight junction proteins ZO-1 and occludin proteins was decreased. Other than that, it is found that synbiotic had the strongest effect on intestinal barrier improvement, probably because norank_f__Muribaculaceae, Bifidobacterium, and Akkermansia significantly increases and unclassified_f__Lachnospiraceae decreases drastically in the Syn group. Norank_f__Muribaculaceae, Bifidobacterium, and Akkermansia were found to have an important role in promoting gut barrier integrity through producing short chain fatty acids to upregulate tight junction proteins.[41, 42]
Furthermore, our data indicate that norank_f__Oscillospiraceae, norank_f__Lachnospiraceae, Prevotellaceae_UCG-001, and Lactobacillus were obviously and positively correlated with ALT, AST, TB, IL-1β, IL-17A, IFN-γ, TNF-α and the percentage of Th17 cells, and negatively correlated with IL-10, percentage of Treg cells, and the number of peripheral blood white blood cells (WBC). Norank_f__Lachnospiraceae decreased drastically in the Syn group. While the effect of probiotics and prebiotics on these four bacteria is not obvious. Liu et al. also found that down-regulating the abundance of norank_f__Lachnospiraceae can relieve the imbalance of bacterial and liver injury.[43] In this case, our experiments provided unequivocal evidence for protective role of synbiotic in AIH and may be an alternative or adjunct therapy for AIH treatment.
On the whole, synbiotic is more effective than probiotics or prebiotics alone in restoring liver function, immunomodulatory, suppressing inflammation, and repairing intestinal barriers. Synbiotic produce synergy between the prebiotics and the probiotics to restore the balance of intestinal flora. In this paper, there was an astonishing finding: synbiotic promoted probiotics and inhibited the growth of potentially harmful bacteria. Probiotics can directly improve the species and abundance of intestinal flora.[16] Prebiotics can significantly increase the abundance of colonic bacteria such as Lactobacillus and Bifidobacterium,[44] and concentration of short-chain fatty acids (SCFA).[45] And it has been demonstrated that SCFA, particularly propionic and butyric acids, have a protective effect on the liver.[46] One aim of combining probiotics with prebiotics was to efficiently deliver the probiotics to colon and reduce its damages when passing through upper gastrointestinal tract.[47] Additionally, prebiotics provide a suitable environment for probiotics to reproduce during the process of probiotics altering the intestinal flora.[8] The combination of probiotics and prebiotics is easier to improve and maintain a healthy balance of intestinal microecology for a long time. Hence, synbiotic is more effective than probiotics or prebiotics alone in AIH mice.
Previous study has shown that many therapeutic agents can alleviate liver injury through interfering with the TLR4 signaling pathway.[48] TLR4, as an important receptor of LPS, when combining with LPS results in activation of NF-κB pathway and promoting overproduction of pro-inflammatory mediators.[49] The study has confirmed that compared with AIH mice, serum LPS concentration was significantly decreased in the mice of the synbiotic group. In addition, the result showed that probiotic, prebioyics or synbiotic reduced the expression of TLR4, NF-κB, and P-IκB/IκB by inhibiting LPS translocation. TLRs can be found on multiple hepatocytes, especially on Kupffer cells.[50] Our results indicate that a large amount of F4/80+ macrophage infiltration in the liver tissues in AIH mice which may promotes liver inflammation and damage. Therefore, we applied LPS to ignite inflammation in RAW 264.7 macrophages to mimic an inflammatory microenvironment and explore the anti-inflammatory activity of postbiotics of Bifidobacterium and Lactobacillus, and the underlying mechanisms. In most situations, it is difficult to precisely determine roles of probiotics or prebiotics because in vivo metabolic studies are not available. Nevertheless, it should be considered that probiotics cell components (i.e., postbiotics) make a certain contribution to alleviate AIH inflammation. Since gut bacteria proliferate and split at a very fast rate, postbiotics is widespread in the intestinal tract. In the current study, the postbiotics could block the activation of the TLR4/NF-κB signaling pathway and the expressions of TNF-α, IL-6, and IL-1β. Together, it can be inferred that the anti-inflammatory effect of synbiotic in liver might be partly due to postbiotics, which block the activation of the TLR4/NF-κB signaling pathway.
These results manifest that the impact of supplementation with probiotics, prebiotics, or synbiotic may be at least partly due to the increase in the population of beneficial species and decrease harmful bacteria. Bacteroidetes and Firmicutes are the main phyla in the gut microbiota, accounting for 90% of gut microbiota.[51] Studies have found that the elevated abundance of Bacteroidetes contributed greatly to the recovery of liver function.[52] The potentially harmful bacteria Firmicutes can easily result in fatty livers and hepatitis.[53] As expected, synbiotic could significantly elevated the relative abundance of Bacteroidetes, while remarkably suppress the relative abundance of Firmicute. In the current study, the predicted bacterial KEGG functional profiles by PICRUST exhibited gut microbiota functions involving metabolism. According to our results, compared with probiotics and prebiotics, the treatment with synbiotic could more significantly change the composition of intestinal bacteria. Therefore, the function of the bacteria would be changed.
In summary, synbiotic had best effects on AIH through modulation of gut microbiota and immune imbalance. So far, we have proved that these microecological modulators can alleviate AIH in mice, and more research is needed to explore whether this synbiotic can antagonize or even reverse AIH induced liver fibrosis. In addition, preclinical studies are needed to explore the efficacy of this synbiotic in AIH patients.
4 Conclusion
Compared with probiotics or prebiotics, synbiotic had best effects on AIH by reshaping the perturbed gut microbiota, maintaining intestinal barrier integrity, regulating immune response, alleviating inflammation and hepatocellular death. Synbiotic obviously facilitated AIH remission, and the possible mechanism is shown in Figure 10. These findings suggest that the combined use of probiotics and prebiotics are effective in improving probiotic and prebiotic efficacy and can be an important tool for preventing and adjuvant treating patients for AIH.
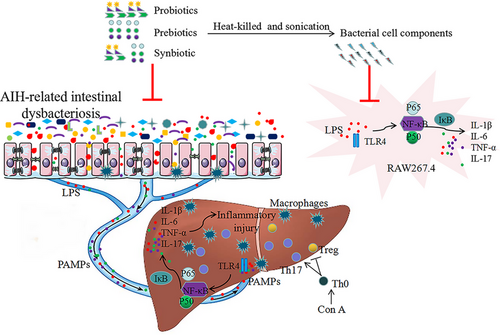
5 Experimental Section
AIH Mouse Model Establishment and Treatment
Male C57BL/6 mice (aged 5 weeks; 19–22 g) were obtained from the Laboratory Animal Center of Shanxi Medical University (Shanxi, China). The mice were housed in a specific pathogen-free (SPF) facility at a constant room temperature. All mice were randomly divided into six groups including the normal control group (NC, n = 8), AIH group (AIH, n = 8), probiotics group (Pro, n = 8), prebiotics group (Pre, n = 8), synbiotic group (Syn, n = 8), methylprednisolone group (MP, n = 8). The mice in the NC and AIH groups were gavaged daily with 0.2 mL phosphate buffer solution (PBS). The mice in the Pro group were fed 3 × 109 CFU day−1 probiotics every day. The mice in the Pre group were fed 2 g kg−1 day−1 prebiotics every day. The mice in the Syn group were fed 3 × 109 CFU day−1 probiotics and 2 g kg−1 day−1 prebiotics every day. Treatment was continued for 7 days. The mice in the AIH, Pro, Pre, and Syn groups were immunized with 0.1 mL concanavalin A (Con A, 25 mg kg−1, Sigma, USA) through intravenous injection via tail veins to induce AIH (Figure S1, Supporting Information). The mice in the NC group were injected with 0.1 mL PBS. Mice in the MP group were received methylprednisolone (MP, 3.12 mg kg−1, Solarbio, China) by intragastric administration once 0.5 h after the Con A injection.[54] Mice were sacrificed 18 h after the Con A challenge. Blood, tissue (spleens, livers, ileums), and cecal content were collected. The study was permitted by the ethical committee of Shanxi Medical University (Permit Number: SYDL2021001).
In the present study, Bifidobacterium and Lactobacillus lacking in AIH patients to AIH mice were fed. In order to better simulate the intestinal microecological transplantation, the study selected 15 different strains of probiotics: Bifidobacterium lactis HN019, Bifidobacterium bifidum Bb 06, Bifidobacterium animalis BB-12, Bifidobacterium lactis Bi-07, Bifidobacterium longum R 175, Bifidobacterium longum fidobacterium B 94, Lactobacillus rhamnosus GG, Lactobacillus casei LC 11, Lactobacillus helveticus R 52, Lactobacillus paracasei Lpc-37, Lactobacillus plantarum R 1012, Lactobacillus reuteri HA 188, Lactobacillus rhamnosus R 11, Lactobacillus acidophilus NCFM, and Streptococcus thermophilus St21. The 15 strains of probiotics in this study belong to the “List of Strains Available for Food” issued by the General Office of the Ministry of Health of China in April 2010.[55] Three different prebiotics: galactooligosaccharides (GOS), fructooligosaccharide (FOS), inulin were selected. Adults took 10 g day−1 to be the optimal and well-tolerated dose of prebiotics which led to a significant increase in colonic Bifidobacteria in healthy volunteers consuming their usual diet,[56] so a single mouse was given 2 g kg−1 day−1 as determined by formula conversion.[57]
Preparation of Postbiotics
Postbiotics defined as inanimate microorganisms and/or their components that conferred a health benefit on the host. Postbiotics were prepared from heat-killed the 15 different strains of probiotics. Probiotics freeze-dried powder was resuspended in PBS (10 mg mL−1). The cells were killed for 15 min at 100 °C. Heat-killed the probiotics was broken by sonication. Suspension of disrupted cells was centrifuged for 10 min at 800 × g and 4 °C to separate the supernatant and precipitate. The supernatant was centrifuged for 30 min at 10,400 × g and 4 °C to obtain the precipitate and dry. The precipitate were resuspended in PBS (5, 10, 20, 40 µg mL−1).
Cell Cultures
The mouse macrophage cell line RAW264.7 (ATCC) was cultured in a Dulbecco modified Eagle medium (DMEM) (Gibco, USA) in 10% fetal bovine serum (FBS) (Gibco, USA) in a humidified incubator containing 5% CO2 at 37 °C. RAW264.7 cells were cultured with or without bacterial components of probiotics or postbiotics (5, 10, 20, 40 µg mL−1) in culture medium for 4 h. Subsequently, LPS was added at a final concentration of 1 µg mL−1. After LPS addition, RAW264.7 cells were further incubated for 16 h. Cell proliferation assay was performed on macrophages cultured in the 96-well plate. Cell proliferation rate was measured using the Cell Counting Kit-8 (BOSTER, China).
White Blood Cell and Lymphocyte, Serum Aminotransferase, Total Bile Acid, and LPS Measurements
After mice were sacrificed, their blood serum was centrifuged. Serum levels of ALT, AST, and TB were determined using the automated chemistry analyzer (BioMajesty, Japan) from TaiYuan Hospital of Traditional Chinese Medicine. EDTA-K2 anticoagulant blood of mice was collected, and white blood cell (WBC) and lymphocyte (LY) were detected by automatic blood cell analyzer. The levels of LPS in the serum were analyzed with ELISA kits (Cloud-Clone Corp., China) according to the manufacturer's instructions.
Histopathological Staining
Liver and ileal tissues were fixed in 4% buffered paraformaldehyde for 48 h and then embedded in paraffin. The sections (5 µm) were mounted on slides, deparaffinized in xylene, rehydrated in decreasing concentrations of ethanol, and subjected to hematoxylin and eosin (H&E) staining. Ileal tissues sections were stained with Alcian blue.
Immunohistochemical (IHC) and Immunofluorescence (IF) Staining
The sections (5 µm) were deparaffinized in xylene and rehydrated in decreasing concentrations of ethanol. The sections were submerged into Tris-ethylenediaminetetraacetic acid antigenic retrieval buffer and heated for 5 min at 121 °C by autoclave. The sections were then treated with 3% hydrogen peroxide in methanol, blocked with 5% bovine serum albumin, and incubated with antibodies against F4/80 (CST, USA), Caspase-3 (CST, USA), Bax (CST, USA), ZO-1 (Abcam, UK), occludin (Abcam, UK), IL-17A (Bioss, China), IFN-γ (Bioss, China), IL-1β (Bioss, China), TNF-α (Bioss, China), and IL-10 (Bioss, China) overnight at 4 °C. Subsequently, the sections with antibodies against F4/80, Caspase-3, Bax, ZO-1, and occludin were incubated with FITC-conjugated or AlexaFluor 555-conjugated secondary antibodies (Bioss, China) at room temperature in the dark for 30 min and nuclear staining was achieved by DAPI (Bioss, China). The sections with antibodies against IL-17A, IFN-γ, TNF-α, IL-1β, and IL-10 were incubated with biotin-conjugated secondary antibodies (BOSTER, China) at room temperature for 60 min and stained with DAB (BOSTER, China) and nuclear staining was achieved by hematoxylin. An Olympus upright microscope was used to observe the staining of the sections, and Image-J software was used to calculate the average optical density (AOD). Macrophages were cultured on glass coverslips in a 6 well plate and fixed with 4% paraformaldehyde. Fixed cells were permeabilized with 0.5% Triton X-100 in PBS for 15 min and blocked with 5% bovine serum albumin then incubated with antibodies against NF-κB P65 (Abcam, UK), IκB (Abcam, UK), further incubated with Alexa Fluor 555-conjugated secondary antibodies (Bioss, China). DAPI was lastly applied to dye the nucleus. An Olympus inverted fluorescence microscope was used to observe the staining of the sections.
Flow Cytometry
The spleen was placed on a 200-mesh sieve moistened with PBS, and a 1 mL syringe was used to draw the pre-cooled PBS solution into the spleen and swell the membrane for collection of the spleen cell suspension. After adding erythrocyte lysate, the suspension was centrifuged and the supernatant was discarded. The pellet was washed with PBS, and 1 mL of culture solution containing 10% fetal bovine serum (FBS) was added to the precipitated cells. The cells were counted and the cell concentration was adjusted to 1.0 × 107 mL-1. To detect Th17 and Treg cells, 1 mL of single cells were seeded per well in 6-well plates; they were then incubated with ionomycin (500 ng mL−1), PMA (5 ng mL−1), and Brefeldin A (1 µg mL−1) for 5 h and harvested. The harvested cells were stained with APC-conjugated anti-mouse CD4 at room temperature in the dark for 30 min and incubated with CytoFix/Cyto Perm buffer (BD Biosciences, USA) at room temperature for 15 min. Finally, they were stained with FITC-IL-17A mAb (BD Biosciences, USA) and PE-Foxp3 mAb (BD Biosciences, USA), incubated at room temperature in the dark for 30 min, and analyzed by FACS. Data were analyzed using FlowJo software (BD Biosciences, USA).
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was isolated from ileum tissue and macrophages using Trizol reagent according to the manufacturer's instructions. cDNA was synthesized from an equal amount of total RNA according to the manufacturer's instructions. The products were mixed with SYBR Green PCR mix and subjected to real-time PCR analysis using a BIOER LineGene 9600 Plus Fluorescent Quantitative Detection System (FQD-96A, BIOER, China). The used primers (provided by Sangon Biotech, Shanghai, China) were listed in Table S1, Supporting Information.
Extraction of Fecal DNA and High-Throughput Sequencing
DNA in the cecal content was extracted with the DNA extraction kit, and the quality of DNA extraction was determined by 0.8% agarose gel electrophoresis and quantified by a UV spectrophotometer. The 16S rRNA hypervariable region (V3–V4) was amplified by PCR using the extracted DNA as a template. The primer sequences were ACTGCATCCGCAGCGTCGA and CCTGTACGGTCTTGCATAT. The PCR products were detected with 2% agarose gel electrophoresis and purified with AXYGEN gel extraction kit. The preliminary quantification results of electrophoresis were obtained by fluorescing the PCR amplified products using the Quant-iT PicoGreen dsDNA assay kit. According to the quantitative results, the samples were mixed to the corresponding proportions. For Illumina MiSeq sequencing, a TruSeq Nano DNA LT Library Prep Kit from Illumina was used to first prepare a sequencing library. The MiSeq sequencer was used for 2 × 300 bp double-end sequencing, and the corresponding reagent was MiSeq Reagent Kit V3 (600 cycles).
Western Blot Analysis
Liver tissue (0.5 g) was lysed on ice with 500 µL of RIPA lysis buffer for 30 min in the presence of protease and phosphatase inhibitors and then sonicated for 1 min at 60 Hz. After centrifugation at 12,000 rpm for 15 min at 4 °C, the supernatant was harvested. Protein concentrations were determined with the BCA protein assay kit (BOSTER, China) and the protein was thermally denatured at 100 °C for 10 min. The protein was then isolated by sodium dodecyl sulfate polyacrylamide gel and transferred to a nitrocellulose membrane. After blocking the membranes with skimmed milk powder, the membranes were incubated overnight at 4 °C with rabbit anti-mouse primary antibodies including TLR4 (Abcam, UK), NF-κB P65 (Abcam, UK), IκB (Abcam, UK), p- IκB (Abcam, UK) and β-actin (CST, USA). After washing with TBST, the membranes were incubated with goat anti-rabbit secondary antibody (CST, USA) at 37 °C for 1 h followed by incubation with a chemiluminescent substrate for visual detection using the Tanon imaging system. Image-J software was used to calculate the IODs.
Bioinformatic Analysis
A 97% identity cut-off was used to cluster the operational taxonomic units (OTUs). The study removed OTUs with a relative abundance <0.005% to decrease the influence of low abundance, spurious OTUs. The species annotation was performed using the SILVA database. The alpha diversity was analyzed by QIIME (Ver-sion 1.7.0). The similarity among the microbial communities in different samples was determined by PCOA and PLS-DA based on Bray-curtis dissimilarity using Vegan v2.5-3 package. For differential bacteria, phylum level and genus level were determined using the one-way analysis of variance (ANOVA) in GraphPad Prism 7 (GraphPad Software). To select potential microbial biomarkers, statistical analysis using SPSS 17.0 software (IBM Corp.; p < 0.05) was combined with LEfSe (linear discriminant analysis effect size, linear discriminant analysis [LDA] score > 4.00). Apart from that, Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) based on OTUs was employed to predict the abundances of functional categories using Kyoto Encyclopedia of Genes and Genomes (KEGG). Pearson's correlation analyses were used to assess correlations between blood parameters, immune cells, LPS translocation degree, genes expression of tight junction proteins, proteins expression of TLR4/NF-κB signaling pathway.
Statistical Analysis
The data were presented as the mean ± SD of five or more independent experiments. Normal distribution was confirmed by the Shapiro-Wilk test. One-way analysis of variance (ANOVA), LSD, or Tamhane test were used to compare the statistical differences among multiple groups. A value of p < 0.05 was considered significantly different. SPSS 22.0 were used for statistical analysis.
Acknowledgements
This project has received funding from Shanxi Provincial Natural Fund Project (Probiotics to relieve autoimmune hepatitis and its mechanism, 201901D111194) and Shanxi scholarship Council of China (Probiotics alleviate the cytokine storm by inhibiting the TLR4-dependent NF-κB signaling pathway, 2020–079). The authors thank Zhongke Yikang for providing probiotics and prebiotics. They are grateful to Laboratory of Tai Yuan Hospital of Traditional Chinese Medicine for testing the concentration of transaminase.
Conflict of Interest
The authors declare no conflict of interests.
Author Contributions
Q.Q.L. and H.Y. contributed equally to this work. W.P.F. and Q.Q.L. designed the research. Q.Q.L., H.Y., X.K., H.H.T., Y.B.K., L.L., X.D.Y., H.X.L., P.R., X.Y.K., M.W.T. conducted the research. W.P.F., Q.Q.L., and H.Y. analyzed data. Q.Q.L. wrote the manuscript draft. W.P.F., Y.B.K., and L.L. revised the manuscript draft. M.W.T. provided technical and material support. W.P.F. had primary responsibility for the final content. All authors read and approved the final manuscript. Q.Q.L. and H.Y. share first authorship.
Open Research
Data Availability Statement
The data that support the findings of this study are openly available in the BioSample database at http://www.ncbi.nlm.nih.gov/bioproject/925295, reference number [PRJNA925295].




