Dairy nutrients and their effect on inflammatory profile in molecular studies
Abstract
Dairy products contain milk fat, proteins, minerals, vitamin D, and other bioactive nutrients that have the potential to contribute to the association observed between increased dairy intake and a decreased risk of inflammation. The objective of this paper is to review the role of dairy bioactive molecules including dairy fat, proteins, micronutrients, and vitamins on inflammation markers in adipose, macrophage, and vascular tissues, which play a key role in the regulation of inflammation. A review was conducted to identify current scientific literature on dairy nutrients and inflammation in cell studies published until November 2014. The majority of saturated fatty acids (FAs) activate proinflammatory markers. Therefore, other dairy FAs or components may offset these harmful effects. Protein and amino acid composition of dairy products may have anti-inflammatory action. Magnesium may have beneficial effects on inflammatory profile; on the contrary, studies on vitamin D demonstrate conflicting results. In conclusion, numerous studies assessed the effects of individual or mixtures of FAs on inflammatory markers; yet, there is far less research on the effects of other dairy bioactive nutrients. The exact bioactive molecule or combination of these molecules in dairy products, which underlies the inverse association between dairy intake and inflammation remains to be elucidated.
Abbreviations
-
- 1,25(OH)2D3
-
- 1α,25-dihydroxyvitamin D3
-
- ACE
-
- angiotensin I converting enzyme
-
- CLA
-
- conjugated linoleic acid
-
- COX-2
-
- cyclooxygenase-2
-
- CVDs
-
- cardiovascular diseases
-
- FA
-
- fatty acid
-
- ICAM-1
-
- intercellular adhesion molecule 1
-
- LA
-
- linoleic acid
-
- LaA
-
- lauric acid
-
- MA
-
- myristic acid
-
- MCP-1
-
- monocyte chemoattractant protein-1
-
- NF-κB
-
- nuclear factor-kappa B
-
- NO
-
- nitric oxide
-
- OA
-
- oleic acid
-
- PA
-
- palmitic acid
-
- SA
-
- stearic acid
-
- SFA
-
- saturated FA
-
- T2DM
-
- type 2 diabetes mellitus
-
- TLR
-
- Toll-like receptor
-
- TNF-A
-
- tumor necrosis factor alpha
-
- VCAM-1
-
- vascular cell adhesion molecule 1
1 Introduction
Low-grade systemic inflammation is considered as a key etiologic factor in the development and progression of the metabolic syndrome, type 2 diabetes mellitus (T2DM), and cardiovascular diseases (CVDs). Circulating mediators of inflammation actively contribute to vascular and atheromatous change during atherosclerosis, and many of these inflammatory proteins are secreted directly from adipocytes and adipose tissue derived macrophages, including tumor necrosis factor alpha (TNF-A), IL-1beta, IL-6, IL-8, and monocyte chemoattractant protein-1 (MCP-1), together with anti-inflammatory factors such as IL-10. Macrophages play a central role in atherogenesis through the accumulation of cholesterol as well as the production of inflammatory mediators and cytokines. In the same way, endothelial cells are both active participants in and regulators of inflammatory processes. Taken together, adipose and vascular tissues play major roles in the regulation of low-grade systemic inflammatory, and by so directly contribute to the pathogenesis of chronic diseases.
A cross-sectional study suggests that consumption of dairy products is inversely associated with low-grade systemic inflammation 1. Clinical trials have examined the role of dairy products on inflammatory biomarkers. Stancliffe et al. 2 have recently reported that adequate dairy consumption (3.5 servings of dairy per day) suppress inflammatory markers including TNF-A, IL-6, and MCP-1 in overweight or obese subjects. Further, consumption of a dairy product naturally rich in cis-9, trans-11 conjugated linoleic acid (CLA) for 10 weeks showed a reduction in IL-6, IL-8, and TNF-A in ten healthy subjects 3. Similarly, other studies have demonstrated that dairy-supplemented diets resulted in lower inflammatory markers (IL-6, TNF-A, MCP-1) in overweight and obese subjects 4, 5. Additionally, a systematic review suggested beneficial or neutral impact of dairy products on inflammation 6. Further, it has been proposed that dairy components may be involved in the downregulation of regulator genes encoding for proinflammatory cytokines. An animal study demonstrated that specific dairy components modulate expression pattern and pathways involved in energy metabolism and inflammation in both adipose and muscle tissues 7. Moreover, the human peripheral blood mononuclear cell transcriptome appeared to be modulated by specific nutrients present in milk and yogurt 8.
Yet, the mechanisms underlying the observed inverse association between specific dairy consumption and inflammation remain unclear. Bovine milk is a nutrient-rich food stuff as it contains fatty acids (FAs), proteins, carbohydrates, and micronutrients. Beneficial effects of dairy products are often attributed to their mineral content; nonetheless, specific milk FA such as short-chain and medium-chain saturated FA (SFA) and dairy trans-FA could regulate adipose tissue genes and cytokine excretion 9. Additionally, milk proteins, including caseins and whey proteins, may improve inflammatory and oxidative stress markers 10. Finally, these milk macronutrients may have a synergic action with other milk micronutrients such as calcium, magnesium, and potassium 11. Additionally, in some countries cow's milk is fortified with vitamin D and thus represents the main source of vitamin D in diet. Therefore, the aim of this paper is to review in vitro mechanistic studies describing the effects of specific dairy components on inflammatory profiles in physiologically relevant tissues.
A literature search was conducted in PubMed to identify original articles of dairy nutrients on inflammation using the main following terms: dairy fat or milk fat or milk FA or dairy protein or milk protein or casein or whey or calcium or magnesium or vitamin, combined with inflammation or inflammatory markers. Cell studies that incubated one or several dairy nutrients in human or animal adipocytes, macrophages or endothelial cells, and accessed inflammatory gene expression and/or inflammatory marker release were included in the review. No restriction was imposed on publication date or publication status for inclusion in the review. Cell studies using cells collected after a clinical intervention were excluded from the review. The search was also conducted in Embase to look for possibly relevant articles that may not have been recorded in PubMed.
2 Dairy fat
Milk contains ∼ 34 g/L of fat, more than half being SFAs (70% of total FA) and 30% unsaturated FAs (Fig. 1). Evidence from epidemiological studies suggests that SFA intake is detrimental to CVD risk, while MUFA and PUFA intake is protective 12. Low-fat dairy products, which are an essential part of the blood lowering DASH (Dietary Approaches to Stop Hypertension) diet, have been associated with a decreased risk of CVD and T2DM 13, 14. Yet, epidemiological studies do not report an association between the consumption of high-fat dairy products and increased risk of CVD or T2DM 15.

2.1 Saturated FAs
Numerous in vitro studies have assessed the proinflammatory effects of SFA, particularly long-chain SFA such as palmitic (PA, 16:0) and stearic (SA, 18:0) acids. These studies are described in more details in Tables 1-3. In adipose tissue, PA activates the nuclear factor-kappa B (NF-κB), a key transcriptional activator of the inflammatory cascade 16-18. Specifically, adipocytes treated with PA exhibited an increase in TNF-A production together with a decrease in IL-10 production 16 and adiponectin gene expression 19. Adiponectin is the most abundant peptide secreted by adipocytes, being a key component in the interrelationship between adiposity, insulin resistance, and inflammation. In another study, PA also induced the expression of TNF-A, but the increase in mRNA abundance was not reflected by a greater protein concentration in the media 17. In addition, PA and SA increased both mRNA and cytokine level of IL-6 and MCP-1 in adipocytes 18, 20, 21. PA and SA were also found to activate silencing Toll-like receptor (TLR) 4, which plays a role in the activation of the innate immune system 22 and increase cell apoptosis 23. Stimulation of TLR-4 activates proinflammatory pathways and induces cytokine expression in a variety of cell types. On the contrary, another study found that PA had no effects on TNF-A, IL-6, and MCP-1 excretion or on silencing TLR-2 and TLR-4 activation in adipocytes 24. Taken together, the majority of in vitro studies have shown that PA and SA induce a proinflammatory profile in adipocytes. Similar results with these long-chain SFAs are also demonstrated in other tissues 22, 25-33. Specifically, PA- and SA-activated TLR-4 receptor 22, 34 and increased TNF-A, IL-6, IL-8, and IL-1B mRNA expression and secretion in monocyte 34-37 and macrophage 28 cell models. In endothelial cells, PA increased nitric oxide (NO, a free radical), E-selectin (a cell adhesion molecule activated by cytokines), IL-6, IL-8, and MCP-1 concentrations 25, 38. The addition of SA on endothelial cells modified inflammatory markers, including increasing IL-6 while decreasing MCP-1 levels 25. These paradoxical situations where SFA alone activate inflammation in vitro, whereas dairy products, including products higher in fat, do not influence CVD risk in human studies, suggest that the deleterious effects of PA and SA in milk are offset by other dairy components.
| Reference | FA studied | Treatment | Cell model | Results |
|---|---|---|---|---|
| Ahn et al. 48 | LA, CLA isomers | 100 μM | 3T3-L1 | LA: ↑ TNF-A, IL-6, CRP expression |
| 24 h | 10t12c-CLA: ↑ TNF-A and CRP expression | |||
| 9c11t-CLA: ↔ TNF-A, IL-6, and CRP expression | ||||
| 9c11t-CLA: ↑ adiponectin secretion | ||||
| Ajuwon and Spurlock 17 | LaA, PA | 0–500 μM | 3T3-L1 | PA: ↑ TNF-A and IL-6 expression |
| 24 h | PA: ↑ IL-6 excretion | |||
| PA: ↔ TNF-A excretion | ||||
| LaA: ↔ IL-6 excretion | ||||
| Bradley et al. 16 | PA, OA | 50 and 500 μM24 or 48 h | 3T3-L1 | PA: ↑ TNF-A and ↓ IL-10 expression and protein excretion |
| OA: ↔ TNF-A and IL-10 production | ||||
| Bueno et al. 19 | LaA, PA, OA, LA | 250 μM | 3T3-L1 | PA and LA: ↓ adiponectin gene expression |
| 48 h | ||||
| Chung et al. 59 | CLA isomers | 30 μM | Human adipocytes | 10t12c-CLA: ↑ IL-6, IL-8, and TNF-A |
| 0–3– 6–12–24 h | excretion | |||
| Cullberg et al. 41 | OA, PA | 500 μM | 3T3-L1 | OA, PA ↔ MCP-1-induced expression and |
| 24 h | secretion | |||
| De Boer et al. 20 | PA | 125 μM | 3T3-L1 + RAW264.7 | PA: ↑ MCP-1 expression and excretion |
| 12 h | PA ↑ IL-6 excretion | |||
| Dordevic et al. 21 | MA, PA, OA | 0.1–0.25–0.5 mM2 or 4 h | 3T3-L1 preadipocytes and mature adipocytes | 0.5 mM of MA, PA, and OA ↑ MCP-1 and IL-6 expression more in preadipocytes than in mature adipocytes |
| All FA: ↔ TNF-A expression | ||||
| Granados et al. 42 | OA | 0–500 μM | 3T3-L1 | OA ↓ resistin and ↑ adiponectin gene |
| 24 h | expression | |||
| Guo et al. 23 | PA, OA, LA | 0–500 μM | 3T3-L1 | PA: ↑ cell apoptosis |
| 24 h | OA and LA: ↓ effect of PA | |||
| Han et al. 18 | LaA, MA, PA, SA, OA, LA | 250 μM with either 5 or 25 mM glucose | 3T3-L1 | LaA, MA, PA ↑ MCP-1 expression and ROS production at both glucose concentrations |
| 7 days | All FA ↑ MCP-1 expression at 25 mM glucose except AA, EPA, and DHA | |||
| Moloney et al. 57 | 9c11t-CLA, LA | 50 μM | 3T3-L1 | CLA: ↓ induced TNF-A expression and |
| 7 days | production compared to LA | |||
| Murumalla et al. 24 | LaA, PA, OA | 40–100 μM | Human adipocytes | LaA, PA: ↔ TNF-A, IL-6, and MCP-1 excretion |
| 6–12 h | OA: ↓ TNF-A, IL-6, and MCP-1 excretion | |||
| All FA: ↔ TLR-2 and TLR-4 activation | ||||
| Ohira et al. 39 | Butyrate | 0–1 mM | 3T3-L1 + RAW264.7 | Butyrate ↓ IL-6, TNF-A, and MCP-1 |
| 24 h | production | |||
| Shaw et al. 106 | PA, SA, OA | 250 μM | 3T3-L1 | PA ↑ TLR pathway |
| 48 h | PA, SA ↑ CCL5 expression | |||
| OA ↓ TLR pathway and CCL5 expression | ||||
| Shi et al. 22 | PA, SA | 400 μM | 3T3-L1 | PA and SA activate TLR-4 |
| 12 h | ||||
| Zhai et al. 60 | CLA isomers | 75.4 μM | 3T3-L1 | 10t12c-CLA ↑ TNF-A expression |
| 8 days |
- CCL5, chemokine (C-C motif) ligand 5; ROS, reactive oxygen species; CRP, C-reactive protein.
| Reference | FA studied | Treatment | Cell model | Results |
|---|---|---|---|---|
| Bunn et al. 36 | PA, SA | 0–500 μM ± | THP-1 | PA: >125 μM ↑ IL-6 and TNF-A |
| insulin 5 ng/mL, | Human | expression | ||
| 30 min 12 or 24 h | monocytes | PA: 500 μM ↑ IL-6 and TNF-A expression and excretion | ||
| SA: 500 μM ↑ IL-6 expression and excretion | ||||
| SA: 500 μM ↑ TNF-A expression | ||||
| PA + insulin: ↑ IL-6 expression and excretion | ||||
| Cheng et al. 107 | CLA mix | 20–200 μM | RAW 264.7 | CLA ↓ NF-κB activation |
| 12 or 18 h | CLA ↓ NO and PGE2 production | |||
| CLA ↓ iNOS and COX-2 expression | ||||
| Cullberg et al. 41 | OA, PA | 500 μM | 3T3-L1 | OA, PA ↔ MCP-1-induced expression |
| 24 h | and secretion | |||
| Dasu and Jialal 35 | PA, SA, OA and FA mix | 0–500 μM ± glucose 5–20 mM | THP-1 | PA, SA, and FA mixture: ↑ IL-1β and MCP-1 excretion in a dose-dependent manner |
| 24 h | ||||
| Erridge and Samani 108 | LaA, MA, PA, SA | 200 μM | RAW 264.7 | ↔ TLR activation |
| 4 h | ||||
| Håversen et al. 28 | LaA, MA, PA, SA, | 50 or 100 μM | THP-1 | PA and SA: ↑ TNF-A, IL-8, IL-1β |
| LA | 0.5–27 h | expression and secretion | ||
| LaA, MA, and LA: ↔ proinflammatory cytokine excretion | ||||
| Huang et al. 34 | LaA, PA | 0–500 μM | RAW 264.7 | PA and LaA activate TLR and NF-κB |
| 18 h | THP-1 | PA and LaA ↑ TNF excretion (RAW 264.7) | ||
| PA ↑ IL-8 excretion (THP-1) | ||||
| L'homme et al. 37 | PA, SA, OA, LA | 200 μM | THP-1 | PA and SA ↑ IL-1β secretion by NLRP3 |
| 8 h | PBMC | inflammasome | ||
| OA and LA ↔ IL-1β secretion and prevent the activation of the NLRP3 inflammasome | ||||
| Laine et al. 27 | LaA, PA, OA, LA | 50–150 μM | U937 human | LaA and PA: ↑ IP-10 gene expression |
| 4–72 h | macrophages | |||
| Lee et al. 26 | LaA | 50–100 μM | RAW 264.7 | LaA activate TLRs |
| 4 h | ||||
| Lee et al. 58 | CLA isomers | 200 μM | RAW 264.7 | 9c11t-CLA ↔ IL-1β, TNF-A, and IL-6 |
| 24 h | LPS-induced expression | |||
| 10t12c-CLA ↓ IL-1β and IL-6 LPS-induced expression | ||||
| McClelland et al. 52 | 9c11t-CLA, OA, LA | 50 μM | THP-1 | CLA: ↓ MCP-1 and COX-2 expression |
| 6 or 18 h | ||||
| Rybicka et al. 53 | CLA, LA | 30 μM | THP-1 | CLA: ↓ SOD2 expression |
| 48 h | ||||
| Shi et al. 22 | PA, SA | 400 μM | Mouse | PA and SA activate TLR-4 |
| 12 h | macrophages | |||
| Zhao et al. 45 | LA, ALA, PA | 0–100 μM | THP-1 | LA, ALA: ↓ IL-6, IL-1β, and TNF-A |
| 24 h | LPS-induced expression compared to PA |
- iNOS, inducible nitric oxide synthase; IP-10, interferon gamma-induced protein 10; NLRP3, NOD-like receptor P3; NO, nitric oxide; PBMC, peripheral blood mononuclear cells; PGE2, prostaglandin E2; ROS, reactive oxygen species; SOD, superoxide dismutase;
| Reference | FA studied | Treatment | Cell model | Results |
|---|---|---|---|---|
| Artwohl et al. 63 | PA, SA, OA, LA, | FA: 100–300 μM | HSMC | SA, OA, LA, ALA ↑ cell apoptosis |
| ALA | FA-mix: 300–900 μM24–48 h | (300 μM) in a chain-length and double bound manner | ||
| All FA ↑ cell apoptosis (48 h) | ||||
| FA-mix ↑ cell apoptosis (600–900 μM) | ||||
| Ciapaite et al. 30 | PA, OA | 100–500 μM | HUVEC | PA ↓ cell proliferation |
| 48 or 72 h | OA ↑ cell proliferation | |||
| PA and OA activate caspase-3 activity | ||||
| PA and OA activate NF-κB (72 h) | ||||
| Eder et al. 109 | CLA isomers | 50 μM | HAEC | CLA ↓ NO production |
| 24 h | ||||
| Harvey et al. 29 | Butyrate, caproic | 50–100 μM | HAEC | MA, PA, SA ↓ cell growth in a chain |
| acid, caprylic | 24 h | length dependent manner | ||
| acid, capric, LaA, | Butyrate-stimulated cell growth | |||
| MA, PA, SA | SA ↑ ICAM-1 expression and activate NF-κB pathway | |||
| Krogmann et al. 31 | PA, SA, OA, LA | 500 μM | HCAEC | PA and SA ↑ gene expression of |
| 20 h | several cytokines | |||
| PA activate NF-κB | ||||
| ↔ OA and SA | ||||
| Lamers et al. 32 | PA, OA | 100 μM | HVSMC | OA + CM ↑ cell proliferation, |
| 18 h | activated NF-κB, and ↓ | |||
| ± Adipocyte- | adiponectine gene expression | |||
| conditioned | PA + CM ↑ ROS, ↑ IL-6, and ↓ | |||
| medium (CM) | adiponectine gene expression | |||
| OA and PA ↑ CCL5 | ||||
| Livingstone et al. 38 | PA, SA, OA, tPA, tVA, LA, ALA FA mixtures | FA mixtures: 400 μM Individual FA: 20–150 μM | HAEC | PA ↑ NO and E-selectin concentrations tPA and tVA ↓ NO concentration compared to PA |
| 24 h | FA mixtures ↔ markers of endothelial function | |||
| Quan et al. 33 | PA | 100–400 μM | HVSMC | PA ↑ IL-8 expression and production |
| 3–24 h | via NF-κB/TLR-4 pathway | |||
| Reissig et al. 54 | LA, PA | 10 μM | HCAEC | LA ↓ ICAM-1, VCAM-1, and E-selectin |
| 2 days | expression compared to PA | |||
| Schleser et al. 50 | CLA isomers | 5–50 μM | HAEC | ↔ ICAM-1, VCAM-1, E-selctin, |
| LA | 20 h | MCP-1-induced expression | ||
| ↔ U937 monocyte adhesion | ||||
| Shaw et al. 110 | PA, OA, LA | 10–25–100 μM | HUVEC | LA, OA ↑ MCP-1 expression |
| 6 or 24 h | ||||
| Soto-Vaca et al. 25 | Butyrate, LaA, MA, | 200 μM | HCAEC and | MA and PA ↑ IL-8 (HCAEC) |
| PA, SA, OA, LA, | 8 h (HAEC) | HCASM | LaA ↓ IL-8, and MCP-1 (HCASM) | |
| ALA, CLA, tPA, | 20 h (HCASM) | MA ↓ MCP-1 (HCASM) | ||
| tVA | PA ↑ IL-6, IL-8, and MCP-1 (HCASM) | |||
| SA ↑ IL-6 and ↓ MCP-1 (HCASM) | ||||
| LA ↓ IL-6 and MCP-1 (HCASM) | ||||
| tVA ↑ IL-6 (HCASM) | ||||
| CLA ↓ IL-6 (HCASM) | ||||
| Stachowska et al. 111 | CLA isomers | 100 μM | HUVEC | 9c11t-CLA ↓ ICAM-1 and VCAM-1 expression |
| LA | 0.5–12–24 h | 10t12c-CLA ↓ VCAM-1 expression | ||
| Staiger et al. 44 | PA, OA, LA | 100–500 μM | HCAEC and | PA and OA ↑ IL-6 mRNA in HCAEC |
| 20 h | HCASM | and HCASM cells | ||
| Toborek et al. 112 | OA, LA, ALA | 60–90 μM | HUVEC | LA activates NF-κB |
| 3 h | LA ↑ TNF, MCP-1, ICAM-1, and VCAM-1 expression | |||
| Zapolska-Downar et al. | Butyrate | 10 mM | HUVEC | Butyrate ↓ IL-1- or TNF-stimulated |
| 40 | 24 h | VCAM-1 and ICAM-1 expression | ||
| Zhang et al. 46 | ALA | 10–200 μM | HUVEC | 10–50–100 μM ALA ↓ apoptosis |
| 18 h + glucose 33 mM | induced by high glucose | |||
| for 72 h | 200 μM ALA ↑ apoptosis induced by high glucose | |||
| ALA attenuated the high glucose induced diminution of eNOS activity and NO production | ||||
| Zhang et al. 47 | ALA | 10–200 μM | HUVEC | 50 μM ALA ↓ ICAM-1 and P-selectin |
| 18 h + glucose 28 mM for 72 h | expression induced by high glucose |
- CCL5, chemokine (C-C motif) ligand 5; eNOS, endothelial nitric oxide synthase; HAEC, human aortic endothelial cells; HCAEC, human coronary artery endothelial cells; HCASM, human coronary arterial smooth muscle; HSMC, human smooth muscle cells; HUVEC, human umbilical vein endothelial cells; HVSMC, human vascular smooth muscle cells; ROS, reactive oxygen species; SOD, superoxide dismutase; MA, myristic acid; tVA, trans-vaccenic acid; tPA, trans-palmitoleic acid.
In particular, milk fat contains short-chain SFA (C4:0 to C8:0, 3–7% of total FA) and medium-chain SFA (C10:0 to C14:0, 11% of total FA). Few molecular studies assessing the effect of short-chain SFA on inflammatory parameters are available in literature and described in more details in Tables 1-3. One study showed that the presence of short-chain FAs (butyric acid (4:0)) decreased production of IL-6, TNF-A, and MCP-1 in the medium, using a coculture of 3T3-L1 mouse preadipocytes and RAW264.7 macrophages 39. In endothelial cells, butyric acid reduced IL-1 or TNF-stimulated expression of vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1) and stimulated cellular growth 29, 40. The upregulation of VCAM-1 and ICAM-1 in endothelial cells occurs as a result of increased gene transcription in response to cytokines. Medium-chain FA could also play a role in inflammation. In particular, lauric acid (LaA, 12:0) did not increase mRNA levels of IL-6 and TNF-A as well as did not activate the NF-κB pathway in 3T3-L1 adipocytes 17. In human primary adipocytes, LaA had no effect on IL-6, TNF-A, and MCP-1 levels and did not activate the TLR-2 and TLR-4 in cells 24. On the contrary, Yeop et al. 18 reported that lauric and myristic acids (MA, 14:0) increased MCP-1 gene expression in 3T3-L1 adipose cells. Overall, short- and medium-chain FA may improve or at least have no effects on inflammatory profile. More cell studies are needed to assess the effect of short- and medium-chain SFA.
2.2 Unsaturated FAs
Milk fat also contains a large amount of MUFAs (24–35% of total FA). Particularly, oleic acid (OA, 18:1 n-9), which is the second major FA in milk fat could be at the origin of the beneficial effect of dairy products on inflammation. OA had been largely studied in cell studies as described in more details in Tables 1-3. For example, OA had no effect on TNF-A, MCP-1, and IL-10 excretion in 3T3-L1 adipocytes 16, 41. In another study, OA has been shown to decrease excretion of TNF-A, IL-6, and MCP-1 in human adipocytes 24. OA also decreased resistin gene expression and increased adiponectin gene expression 42. In macrophages, OA had a neutral effect on inflammatory marker release 27, 41, 43 and could prevent the activation of the NLRP3 inflammasome 37. Those data suggest an anti-inflammatory role of OA in adipocytes and macrophages. In endothelial cells, OA had no effect on IL-6, IL-8, and MCP-1 levels 25 but a study reported an increased mRNA level of IL-6 after incubation with OA 44. Overall, these data suggest an anti-inflammatory role of OA.
Milk fat also contains small amounts of PUFAs (2.3% of total FA), mainly linoleic acid (LA, 18:2 n-6). PUFAs are classified into omega-3 and omega-6 groups. Alpha-LA (ALA, 18:3 n-3), an omega-3 PUFA precursor of docosahexaenoic acid (22:6 n-3), has been shown to have anti-inflammatory properties in cell studies 45-47. On the contrary, LA (18:2 n-6) is an omega-6 PUFA, which is known to promote arachidonic acid mediated proinflammatory eicosanoids. However, LA does not have clear effects on inflammatory profiles in cell studies. Adipocytes incubated with LA increase TNF-A and IL-6 expression 48 as well as a decreased adiponectin gene expression 19, whereas endothelial cells 44, 38, 49, 50 and macrophages 27, 51-53 incubated with LA did not change inflammation levels. LA may also have a beneficial effect on inflammation by inhibiting the effect of PA 23, 45, 54. Taken together, omega-3 and omega-6 PUFAs may modify inflammatory response.
2.3 Trans-FAs
Dairy fat include about 2.7% of trans-FAs 55, mainly trans-vaccenic acid (trans-11 18:1), trans-palmitoleic acid (trans-9 16:1) and CLAs. Trans fat occurs naturally in dairy and has been thought to differentially affect the risk of CVD compared to industrial trans-FAs 56. One study observed lower concentrations of E-selectin following trans-palmitoleic acid incubation, with trans-vaccenic acid appearing neutral in endothelial cells 38. Nonetheless, no differences were noted in gene expression; thus, further work is needed to support any protective relationship. CLA belong to a class of dienoic derivatives of LA, which are found primarily in beef and dairy products. The cis-9, trans-11 (rumenic acid) and trans-10, cis-12 isomers are the most abundant in milk. It is thought that the effects of CLA on inflammation are isomer dependent. There is also evidence for anti-inflammatory actions of cis-9, trans-11 CLA including TNF-A and IL-6 expressions as well adiponectin secretion in 3T3-L1 adipocytes 48, 57 or macrophage cell cultures 58. Oppositely, the trans-10, cis-12-CLA induces IL-6 production 59 and TNF-A expression 48, 60 in adipocytes in vitro. Mixtures of CLA are also known to reduce plasma adiponectin levels 61. Comparable results on inflammatory (MCP-1 and cyclooxygenase-2 (COX-2)) gene expressions were seen in THP-1 macrophage cell studies 52, 53. Thus, some CLA isomers may modify the anti-inflammatory profile of dairy products.
In sum, these results suggest that individual dietary FA can modulate differently cytokine gene expression and production. However, milk fat is characterized by a wide variety of FAs compared to other fat sources such as vegetable oils. Few cell studies assessed the effect of trans-FA and short- and medium-chain SFA, which are specific dairy FAs. Coconut oil, which is good source of medium-chain SFA, mainly LaA, may have beneficial health effects by rising HDL-C levels 62. Yet, cell studies from this review show that LaA tends to a neutral effect on inflammatory markers. Artwohl et al. suggested that individual FAs or FA mixtures may have an effect on inflammation in a dose, chain-length, and double bound manner 63. Nevertheless, in vitro studies using physiological FA mixtures and doses are limited. One recent study incubated endothelial cells with FA mixtures or individual FAs. The authors concluded that FA mixtures of dairy lipids did not substantially affect markers of endothelial function, while individual FAs did 38. Yet, there is a need for more research to mimic the physiological situation of exposing cells to dairy FA mixtures, rather than single FAs to determine effects on inflammation profiles in physiologically relevant tissues.
3 Dairy proteins
Milk proteins can be divided into caseins and whey proteins groups that differ with their amino acid profile and properties (Fig. 2). Studies describing the effects of dairy proteins are described in more details in Table 4. First, caseins make up 80% of the proteins in bovine milk. Casein-derived tripeptides isoleucine–proline–proline and valine–proline–proline have been shown to possess hypotensive effects in human studies 64. Further, in vitro studies have shown that peptides and hydrolysate fractions from caseins influence endothelial cell function via NO production 65, or angiotensin I converting enzyme (ACE) inhibiting activity 66. It is well known in the literature that impaired NO production causes inflammation. Further, the valine–proline–proline tripeptide moderates monocyte adhesion to inflamed endothelial cells 67. Second, whey proteins make up 20% of milk protein. It consists primarily of alpha-lactalbumin and beta-lactoglobulin (70% of whey proteins). Whey protein-derived peptides have ACE inhibitor activity and antioxidant properties 68. Alpha-lactalbumin has been shown to supress the production of proinflammatory cytokines such as IL-6 and TNF-A from THP-1 cells 69. Yet, Lin and Kuo 70 determined that commercial alpha-lactalbumin may have proinflammatory effects on macrophages due to endotoxin contamination; thus caution is needed when interpreting results. Lactoferrin, a glycoprotein present in whey proteins in milk, also downregulates the cytokine production of TNF-A, IL-1beta, IL-6, and IL-8 in a human monocytic cell line 71, 72. The mechanism suggested involves the interference of lactoferrin with NF-κB activation 71. Further, glycomacropeptide is present at 10–15% in milk whey as a result of the action of the rennet enzyme during the cheesemaking process. Glycomacropeptide may exert proinflammatory effect on TNF-A, IL-1beta, and IL-8 by stimulating NF-κB pathways in human monocytes cells 73. In sum, dairy proteins may have a potential role in modifying inflammation parameters.
| Reference | Protein/peptide/amino acid studied | Treatment | Cell model | Results |
|---|---|---|---|---|
| Blümer et al. 74 | Amino acid mix | Each amino acid concentration is four times higher than in fasted rats ± insulin 100 nM | 3T3-L1 | Amino acid + insulin: ↑ adiponectin secretion |
| 24 h | ||||
| Garcia-Macedo et al. 81 | Glycine | Cells grown and differentiated with 10 mM | 3T3-L1 | Glycine:↓ IL-6, resistin, and TNF-A expression |
| glycine | Glycine:↑ adiponectin and PPAR-gamma expression | |||
| Sun and Zemel 83 | Leucine ± calcitriol (10 μM) | 2.5 mM | 3T3-L1 | ↑ Adiponectin production |
| 48 h | ||||
| Enomoto et al. 69 | α-LA, glycated and | 10 or 100 μg/mL | THP-1 | Phosphorylated and glycated α-LA ↑ |
| phosphorylated α-LA by dry heating | 24 h | suppressive effect of α-LA on TNF-A and IL-6 production induced by LPS | ||
| Hasegawa et al. 79 | Alanine, cysteine, histidine, | 0.2–2–20 mM | THP-1 | Cysteine, histidine, and glycine: ↓ |
| glycine | 2 h | NF-κB TNF-induced activation | ||
| Cysteine, histidine: ↓ ICAM-1 expression and IL-8 production | ||||
| Håversen et al. 71 | Lactoferrin | 50–500 μg | THP-1 | Lactoferrin ↓ TNF, IL-1β, IL-6, and |
| 18 h | IL-8 gene expression and secretion | |||
| Lin and Kuo 70 | α-LA | 1–50 μg/mL | RAW 264.7 | ↑ NO and PGE2 production |
| 18 h | ↑ COX-2 and iNOS expression | |||
| Manna and Jain 78 | Cysteine | 100–500–1000 μM + high | Human U937 | Cysteine: ↓ ROS production |
| glucose 20 h | monocytic cells | ↓ MCP-1, IL-8, TNF, IL-1β, and IP-10 production | ||
| Mattsby-Baltzer et al. 72 | Human and bovine | 50 μg/mL | THP-1 | ↓ IL-6 LPS-stimulated response in a |
| lactoferrin + peptide lactoferricin B | 4–24 h | time-dependent manner | ||
| Requena et al. 73 | Glycomacropeptide | 1 mg/mL | THP-1 | ↑ TNF-A, IL-1β, and IL-8 secretion in |
| 24 h | a dose-dependent manner | |||
| Spittler et al. 82 | Glycine | Glycine 0–10 mM, 40 h | Human | ↓ LPS-induced TNF-A production |
| 0–72 h | monocytes | ↑ IL-10 expression | ||
| Aihara et al. 67 | Tripeptide VPP | 1 mM | HUVEC/THP-1 | ↓ PMA-induced adhesion of THP-1 |
| 24 h | cells to HUVEC cells | |||
| Hasegawa et al. 80 | Alanine, cysteine, histidine, | 0.2–2–20 mM | HCAEC | Cysteine, histidine, and glycine: ↓ |
| glycine | 2 h | NF-κB TNF-induced activation | ||
| Cysteine, histidine, and glycine: ↓ IL-6 production | ||||
| Kanikarla-Marie and Jain 77 | Cysteine | 500 μM 2 h + ketones or high glucose 24 h | HUVEC/THP-1 | ↓ Adhesion of THP-1 cells to HUVEC cells |
| ↓ Induced ROS production | ||||
| ↓ Induced ICAM-1 expression | ||||
| Ringseis et al. 65 | Peptides and hydrolysates | Peptides: 1 nM to 1 mM | HAEC | ↑ NO production |
| from caseins and soy | Hydrolysates: 0–2.5 mg/mL | |||
| 24 h | ||||
| Rousseau-Ralliard et al. 66 | α-Casein hydrolysates | 0.01–10 000 mg/L | HUVEC | ↓ ACE activity in a dose-dependent |
| 2 h | manner |
- α-LA, alpha-lactalbumin; HAEC, human aortic endothelial cells; HCAEC, human coronary artery endothelial cells; HUVEC, human umbilical vein endothelial cells; iNOS, inducible nitric oxide synthase; PGE2, prostaglandin E2; PMA, phorbol 12-myristate 13-acetate; PPAR, peroxisome proliferator-activated receptor; ROS, reactive oxygen species; VPP, valine–proline–proline.
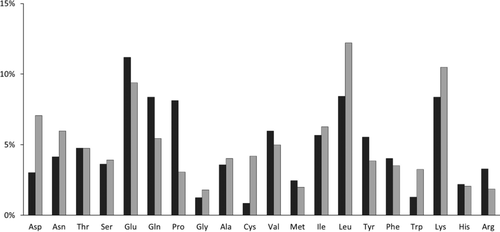
Further, milk proteins are a good quality nutritional protein source as they have a high digestibility and a high content of essentials amino acids (Fig. 2) such as leucine, lysine, valine, isoleucine, and threonine. A study demonstrated that various mixtures of amino acids together with insulin can increase adiponectin secretion in adipocytes 74. Caseins contain high proportions of glutamate, glutamine, and proline amino acid residues. Glutamine has been shown to reduce ICAM-1 expression in endothelial cells activated by preeclamptic plasma 75. Arginine, a precursor of NO that can be found in caseins, has been used to revert endothelial dysfunction in in vitro and in vivo studies. On the other hand, whey proteins contain higher proportions of cysteine, lysine, glycine, and branched chained amino acids leucine and isoleucine compared to casein 76 (Fig. 2). Cysteine can inhibit NF-κB activation and therefore decrease MCP-1, IL-8, IL-1B, IL-6, and ICAM expression and production in monocytes and endothelial cells 77, 80. Studies have shown the anti-inflammatory properties of glycine; specifically decreases in IL-6 and TNF gene expression together with increase in adiponectin and IL-10 gene expression in adipose tissue and monocytes 81, 82. Leucine has been shown to be able to modulate adiponectin production in adipocytes cells 83. Overall, the unique amino acid composition of dairy products can modulate cytokine gene expression and production; yet, studies on inflammatory parameters using milk-derived amino acid are limited. Further, the intake of low-fat dairy products in clinical studies could represent a relevant model to study physiological effects of dairy proteins on inflammatory markers.
4 Minerals
Dairy products are a source of key micronutrients such as calcium, phosphorus, magnesium, zinc, and selenium. Calcium is the macroelement naturally present in higher fractions in dairy products, approximately 1200 mg/L. Further, dairy products are also recognized as a source of phosphorus, 950 mg/L. Magnesium can also be found in dairy products, 120 mg/L. Milk is a good source of microelements including zinc 3–4 mg/L and selenium 30 μg/L. Human studies demonstrated that dietary calcium or magnesium intakes is modestly and inversely associated with some but not all markers of systematic inflammation and endothelial dysfunction 84, 89.
A cell study showed that magnesium sulfate inhibits endothelial cell activation, as measured by levels of IL-8 and ICAM-1 expression, via NF-κB pathway 90. Two others cell studies found a beneficial effect of magnesium sulfate on vascular function in endothelial cells 91, 92. However, no supplementary studies have been done to demonstrate the effects of other dairy micronutrients on inflammatory parameters in the cellular models considered in this review. Thus, more research is needed to understand the effects of the micronutrients contained in dairy on metabolic risk factors.
5 Vitamins
The concentration of fat-soluble vitamins, specifically vitamin A and vitamin D, in dairy products depends on milk fat content. In some countries, skim milk is fortified with vitamins A and D to improve its nutritional quality. Milk is generally considered an important source of vitamin A for growth, development, immunity, and eye health. However, no cellular studies have been conducted to our knowledge on vitamin A and inflammation. Further, fortified cow milk is considered as an excellent source of vitamin D. The active form of vitamin D, 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3) or calcitriol is known to be associated with calcium and phosphorus homeostasis and maintenance of skeletal structure. Vitamin D receptors are present in most tissues, including the endothelium, vascular smooth muscle, and adipocytes. Directly or indirectly, 1,25(OH)2D3 has a role in the regulation of many genes, such as those involved in anti-inflammatory and insulin sensing effects (reviewed in 93, 94). The anti-inflammatory mechanisms may also be modulated by the regulation of intracellular calcium levels 95.
Cell studies describing the effects of vitamin D are described in more details in Table 5. Studies have shown decreases in the expression and protein levels of inflammatory markers such as IL-6, MCP-1, and IL-1beta, after 1,25(OH)2D3 treatment in human adipocytes and 3T3-L1 adipocytes 96-98. Similarly, 1,25(OH)2D3 treatment decreased the expression of the TNF-A-mediated proinflammatory markers in 3T3-L1, adipocyte-macrophage coculture, and human adipocytes 96, 97. Oppositely, 1,25(OH)2D3 also decreased adiponectin secretion in adipocytes 97. Results from another study also demonstrated that 1,25(OH)2D3 increases inflammatory cytokine expression and protein release, including TNF-A, IL-6, and MCP-1 in adipocytes as well as TNF-A and IL-6 in macrophages 99. Yet, the suppression of 1,25(OH)2D3 by dietary calcium reduced inflammatory cytokine expression and oxidative stress in adipose tissue 99. Other researchers also demonstrated that higher concentration of 1,25(OH)2D3 could enhance macrophage-derived chemokine expression in THP-1 and human primary monocytes 100. However, moderate doses of 1,25(OH)2D3 treatment suppresses TNF-A and interferon gamma-induced protein 10 (IP-10) expression in THP-1 and human primary monocytes 100. In cultured human umbilical vein endothelial cells undergoing oxidative stress, pretreatment with 1,25(OH)2D3 was able to reduce the apoptosis-related gene expression 101. Similarly, a study demonstrated that pretreatment with 1,25(OH)2D3 inhibited the TNF-A-induced NF-κB activation in human coronary artery endothelial cells 102. Specifically, pretreatment with 1,25(OH)2D3 inhibited TNF-A-induced VCAM-1 expression and IL-8 production 103. Further, another study showed that the increase in protein levels of ICAM-1 and VCAM-1 induced by TNF-A was decreased after incubation of the cells with 1,25(OH)2D3 104.
| Reference | Milk component | Treatment | Cell model | Results |
|---|---|---|---|---|
| Gao et al. 98 | Vitamin D3 | 10–100 nM | Human | ↓ MCP-1, IL-6, and IL-8 secretion |
| 24 h | preadipocytes | ↓ THP-1 migration | ||
| ↓ TNF, IL-1β, and MCP-1 gene expression and protein release | ||||
| Kudo et al. 103 | Vitamin D | 1–100 nM | HCAEC | ↓ TNF-induced VCAM-1 expression and IL-8 excretion |
| Kuo et al. 100 | Vitamin D | 1–100 nM | THP-1 | ↓ TNF excretion |
| ↔ IL-8 excretion | ||||
| Lorente-Cebria et al. 97 | Vitamin D | 100 nM | Human adipocytes | ↓ MCP-1 mRNA and protein levels |
| 24 h | Induced with TNF-A: ↓ adiponectin mRNA and protein levels | |||
| Marcotorchino et al. 96 | Vitamin D | 1–100 nM | Adipocytes | ↓ IL-6, MCP-1, and IL-1β mRNA and |
| 24 h | Adipocyte- | protein levels | ||
| macrophage | ↓ TNF expression | |||
| coculture | Similar effects in the coculture model | |||
| Martinesi et al. 104 | Vitamin D3 | 10–100 nM | HUVEC | ↔ Viability and proliferation |
| 24 h | ↓ ICAM-1 and VCAM-1 TNF-induced secretion | |||
| Sun and Zemel 99 | Calcitriol | 10 nM | Human adipocytes | ↑ MCP-1 and IL-6 expression in 3T3-L1 |
| 48 h | 3T3-L1 | ↑ TNF-A and IL-6 expression in RAW.264 | ||
| RAW.264 | Effects blocked by calcium-channel antagonist | |||
| Suzuki et al. 102 | Vitamin D3 | 1–100 nM | HCAEC | ↓ NF-κB TNF-induced activation and |
| 30 min | E-selectin expression | |||
| Uberti et al. 101 | Vitamin D | 1 nM | HUVEC | ↓ Apoptosis-related gene expression |
| 15 min | ↑ NO production | |||
| + H2O2-induced stress |
- HCAEC, human coronary arterial endothelial cells; HUVEC, human umbilical vein endothelial cells.
Overall, the improvement of proinflammatory status under 1,25(OH)2D3 effect suggests that low-grade inflammation could be linked to vitamin D insufficiency. However, higher doses of vitamin D may have opposite effects on inflammatory parameters. These conflicting results from studies may explain the difficulties in determining the precise role and dose of 1,25(OH)2D3 for the optimal benefits on inflammation.
6 Conclusions
Figure 3 summarizes the dairy nutrients and their effects on inflammation. Taken together, the mechanisms underlying the inverse association between dairy product intake and inflammation remain to be elucidated. Earlier in vivo mechanistic studies have investigated the positive and specific effects of these molecules individually. Numerous studies have demonstrated the effects of various FA on inflammation markers; however, few studies have shown effects of other bioactive components such as dairy proteins and micronutrients contained in dairy products. Yet, as described in this review, all of these components may contribute to the observed epidemiological association between increased dairy product consumption and a decreased risk of developing a low-grade inflammation state. The beneficial effects on gene expression of dairy bioactive molecules can come from their additive or synergistic effects. For example, Kim et al. 11 recently demonstrated a possible synergism between alpha-linolenic acid, CLA, and calcium in inhibiting adipocyte differentiation. More studies are needed to evaluate the effects of selected nutrients contained in dairy on cytokines levels and gene expression involved in the pathogenesis of the metabolic syndrome, T2DM, and CVDs may help reveal their mechanisms of action.
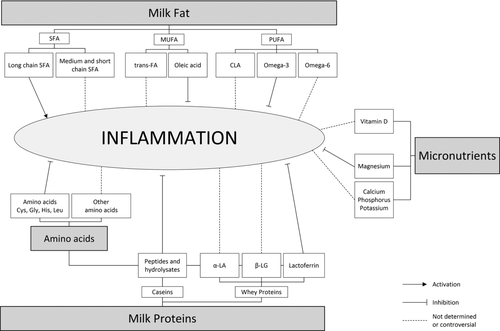
Acknowledgment
M.S.D.S conducted the review of the literature. She was also involved in the analysis and interpretation of data, revising the article, and final approval of the version to be published. I.R. was involved in the literature search, analysis, and interpretation of data. Moreover, she wrote the manuscript and decided its main contents. M.S.D.S. received bursary from the Centre de recherche en Endocrinology moléculaire et oncologique et génomique humaine (CREMOGH) and I.R. received a Junior 1 Research Fellowship from the Fonds de recherche du Québec-Santé (FRQ-S).
The authors have declared no conflict of interest.




