Molecular mechanisms for chemoprevention of colorectal cancer by natural dietary compounds
Abstract
Colorectal cancer is one of the major causes of cancer-related mortality in both men and women worldwide. This review focuses on preventing the initiation and promotion of neoplastic growth in colorectal cancer, particularly with natural dietary compounds. Chemoprevention is defined as the use of natural dietary compounds and/or synthetic substances that can delay, prevent, or even reverse the development of adenomas, as well as the progression from adenoma to carcinoma. The molecular mechanisms of their chemopreventive action are associated with the modulation of signaling cascades, gene expressions involved in the regulation of cell proliferation, differentiation, and apoptosis and the suppression of chronic inflammation, metastasis, and angiogenesis. Here, we summarize the currently known targets and signaling pathways whereby natural dietary compounds interfere with the development of colorectal cancer, and thus providing evidence for these substances in colonic cancer chemopreventive action.
1 Introduction
Colorectal cancer (CRC) is one of the major causes of cancer-related mortality in both men and women in most developed world 1. The risk factors of CRC include age, family history, inflammatory bowel diseases (IBD) including ulcerative colitis and Crohn's disease, and environmental and dietary procarcinogens 2. Epidemiological study showed that up to 40% patients with colitis developed colitis-associated CRC 3. In colonic tumorigenesis, the inflammatory cells contribute to the colitis by generating pro-inflammatory cytokines and diversing reactive oxygen species and RNS. Oxidative stress has the potential to affect a large array of carcinogenic pathway, because their targets including DNA, RNA, lipids, and proteins, involved in enhanced malignant transformation and proliferation of initiated cells (Fig. 1). Inflammation also promotes the development of cancer by creating an inflammatory microenvironment during tumor tissue formation. The inflammatory and immuosuppressive cytokines and chemokines secreted from these cells not only promote proliferation, angiogenesis, invasion, and metastasis but also suppress the host's immune system and facilitate tumor growth and development in CRC 4. Many molecules such as LPS/Toll-like receptor 4/nuclear factor-κB (NF-κB) pathway, inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and PGE2 contribute to colitis-associated CRC development in IBD 5.
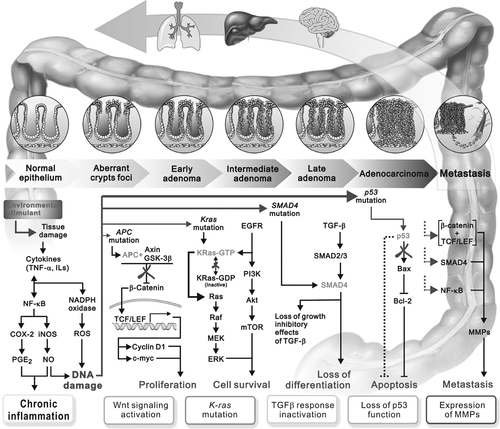
Mechanisms and molecular events that characterize the transition to CRC. Colon carcinogenesis is a complex multistep process, the adenoma–carcinoma sequence, from small benign precursor lesions to metastatic carcinomas. Tissue damage caused by bacteria, carcinogens, environmental insult result in inflammation. During the chronic inflammation, the inflammatory cells are recruited to the damage tissues and induce chromosomal instability and DNA damage in proliferating cells bearing survival advantages and that ultimately contribute to malignant transformation. The initial step in tumorigenesis is that of ACF formation, associated with loss of APC. Early carcinomas acquire accumulation of mutations in additional oncogenes or tumor suppressor genes, such as K-ras, p53, and Smad4, which is downstream of transforming growth factor-β. The Wnt/-catenin and EGFR/PI3K/Akt signaling pathways pathway also play a central role in an early colorectal tumor development. The mechanisms of natural dietary compounds act through the modulation of gene expression involved in the suppression of inflammation, regulation of cell proliferation, differentiation, cell cycle and apoptosis and suppression of angiogenesis and metastasis.
The development of CRC involves various genetic and molecular changes in cell proliferation, cell survival, differentiation, resistance to apoptosis, metastasis, and tumor angiogenesis 1, 2. Loss of adenomatous polyposis coli (APC) function, mutation, and constitute activation of β-catenin and K-ras lead to activation of Wnt/β-catenin/Tcf4 signaling pathway, which subsequently causes the transcription of downstream genes such as cyclin D1, myc, VEGF, and matrix metalloproteinases (MMPs) that are involved in tumorigenesis 6, 7. Moreover, oncogenic mutation of K-ras results in activation of Ras and its downstream effectors, such as Raf/MEK/mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3 kinase (PI3K)/Akt pathways 8. Inactivation of glycogen synthase kinase-3β through phosphorylation by Akt caused stabilization of β-catenin and its nuclear accumulation 9. Epidermal growth factor receptor (EGFR) signaling and transforming growth factor-β pathway also involve in regulating colonocyte growth and differentiation, and are upregulated in hyperproliferative aberrant crypt foci (ACF) as well as contribute to malignant growth of colon cancer 10, 11.
The progression cascades involve the accumulation of mutation genes as well as the alteration of morphological and cellular events 12. Progression of this disease is commonly characterized by histologically distinct steps i.e. colonic crypt hyperplasia, dysplasia, adenoma, adenocarcinoma, and distant metastasis 12. During this progression, formation of ACF in early stage is believed to be a histological biomarker of colonic tumor development 13. ACF also occurs at a higher frequency in colon cancer patients which proposed as a putative preneoplastic lesion 14. Moreover, increased number and multiplicity of ACF are believed to be associated with an increased risk for the development of CRC 13, 14. Inhibition of iNOS, COX-2, 3-hydroxyl-3-methylglutaryl-CoA reductase, retinoid X receptor-α, ER-β, 5-lipoxygenase, β-catenin, signal transducer and activator of transcription-3, or MMPs shows protective effects against colon tumor development in different animal models, suggesting that they are crucial targets for mucosa inflammation and colon tumorigenesis 1.
2 Chemopreventive natural dietary compounds on CRC
During colon tumor progression, specific molecular processes have been targeted for chemopreventic intervention, including chronic inflammation, proliferation and differentiation signaling, apoptosis, cell surface growth factor receptors, angiogenesis, and metastasis (Fig. 1). Despite understanding of the process and mechanism in colonic carcinogenesis, present therapies including surgery, chemotherapy, radiotherapy, and molecular-targeted therapy are still limited for advanced tumors. Hence, a growing amount of scientific attention has been focused on investigating the potential of dietary substances for both prevention and control of colon cancer through chemopreventive strategies 15. Epidemiological and laboratory studies suggest that the consumption of fruits and vegetables is correlated with the decreased risk of colon cancer 16, 17. Many natural dietary compounds in fruits and vegetables have been isolated and their health-promoting properties have been demonstrated. The molecular mechanisms of CRC underlying the chemopreventive effects of selected natural dietary compounds are highlighted below.
3 Chemopreventive mechanisms of flavonoids and flavonolignan on colonic carcinogenesis
Flavonoids are plant secondary metabolites that are ubiquitous in fruits, vegetables, nuts, seeds, and plants (Table 1). Some of them exhibit a broad spectrum of pharmacological properties such as antioxidant, free radical-scavenging, anti-inflammatory, anti-carcinogenic, anti-viral, anti-bacterial, anti-thrombogenic, and anti-atherogenic activities.
| Compound | Structure | Dietary source | Mechanisms | Ref. |
|---|---|---|---|---|
| Quercetin | 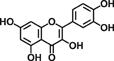 |
 |
Decreases cell growth through disruption of the binding of β-catenin to Tcf-4 | 18 |
| Suppresses the phosphorylation of EGFR and downstream signaling in colon carcinoma cells | 19 | |||
| Induces apoptosis through upregulation of p53, p21, and AMPK signaling | 20 | |||
| Reduces β-catenin/Tcf transcriptional activity, induces G2/M cell-cycle arrest through decreasing gene expression of cyclin D1 and survivin | 21 | |||
| Apigenin | 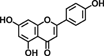 |
 |
Induces apoptosis by enhancing APC protein expression in colon cancer cells | 22 |
| Reduces AOM-induced ACF number and increases apoptosis of luminal surface colonocytes | 23 | |||
| 5-Hydroxy-6,7,8,4′-tetramethoxyflavone | 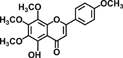 |
 |
Induces G0/G1cell-cycle arrest and apoptosis through modulating p21, CDK-2, CDK-4, and Mcl-1 | 24 |
| EGCG | 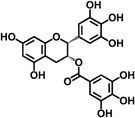 |
 |
Decreases the number of total and large ACF through reducing p-GSK-3β, β-catenin, COX-2, and cyclin D1 level in C57BL/KsJ-db/db mice | 26 |
| Interferes with EGFR signaling in colon cancer cell | 27 | |||
| Inhibits hepatocyte growth factor-induced cell proliferation through suppression of phosphorylation of receptor tyrosine kinase as well as decreases cell invasion | 28 | |||
| Naringenin | 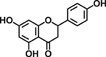 |
 |
Reduces AOM-induced ACF number and increases apoptosis of luminal surface colonocytes | 23 |
| Cyanidin | 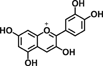 |
 |
Inhibits cell proliferation, iNOS, and COX-2 gene expression in colon cancer cells | 29 |
| Delphinidin | 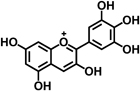 |
 |
Induces apoptosis through activation of caspases cascade, increasing Bax protein and decreasing Bcl-2 protein, and suppression of NF-κB signaling | 30 |
| Genistein |  |
 |
Reduces colonic inflammation and COX-2 gene expression in TNBS-induced chronic colitis rats | 31 |
| Silibinin |  |
 |
Inhibits cell proliferation through cell-cycle arrest via inhibition of cyclin-CDK promoter activity and increases p21 or p27 protein expression | 32 |
| Inhibits AOM-induced ACF formation through decreasing iNOS, COX-2, and cyclin D1 expression in rat | 33 | |||
| Decreases intestinal polyps via suppression of β-catenin/GSK3β signaling, protein expression of cyclin D, c-Myc, and cytokine production in APCmin/+ mice | 34, 35 |
- a GSK-3β, glycogen synthase kinase-3β.
Quercetin is found typically in onion, broccoli, apples, and it has been shown to be a potent antioxidant and anti-carcinogenic agent that decreases cell growth through disruption of the binding of β-catenin to Tcf-4 in SW480 colon cancer cells 18, inhibits EGFR signaling pathway in HT-29 cells 19, and induces apoptosis through upregulation of p53, p21, and AMPK signaling in HT-29 colon cancer cells 20. Moreover, quercetin reduces β-catenin/Tcf transcriptional activity, induces G2/M cell-cycle arrest through decreasing gene expression of cyclin D1 and survivin in SW480 colon cancer cells 21.
Apigenin, present in parsley and celery, evokes its inhibitory effect on carcinogenesis through the induction of apoptosis in human colon cancer cells and reduces azoxymethane (AOM)-induced ACF number in rats 22, 23. 5-Hydroxy-6,7,8,4′-tetramethoxyflavone, in citrus, exerts its chemopreventive activity through the induction of apoptosis and cell-cycle arrest in colon cancer cells 24.
Green tea catechins are the most-studied health-promoting flavonoids in recent years. Epigallocatechin-3-gallate (EGCG) is the most abundant catechin in green tea. Green tea and its constituents have been extensively studied both in vitro and in animal models of carcinogenesis 25. EGCG treatment suppressed AOM-induced colonic premalignant lesions in mice, interfered with EGFR signaling, and inhibited hepatocyte growth factor-induced cell proliferation in human colon cancer cells 26-28. Naringenin, a flavanone present in orange, is believed to contribute to reduce AOM-induced ACF number and increase apoptosis of luminal surface colonocytes 23. Cyanidin, an anthocyanidin present in cherry and strawberry, significantly inhibits cell proliferation and colonic inflammation in human colon cancer cells 29. Delphinidin, an anthocyanidin present in dark fruit, has potential in inhibiting colon cancer growth through induction of apoptosis and cell-cycle arrest in human colon cancer HCT 116 cells 30.
Soybeans and soy foods, which contain phytochemicals including isoflavones, saponins, phytic acid, and phytosterols, exhibit anti-inflammatory and anti-oxidant effects. Genistein, which is an isoflavone, is believed to contribute to reduce colonic inflammation in 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis 31.
Silibinin, isolated from the milk thistle plant Silybum marianum, is the best-documented of the flavonolignans in displaying health-beneficial effects. Silibinin was reported to inhibit cell proliferation and promote cell-cycle arrest in human colon cancer cells 32. More recently, silibinin has been found to inhibit AOM-induced ACF through decreasing iNOS, COX-2, and cyclin D1 expression in rats and suppress β-catenin/GSK3β signaling and cyclin D and c-Myc expression in APCmin/+ mice 33-35.
4 Chemopreventive mechanisms of proanthocyanidin and other polyphenolic compounds
Proanthocyanidins, an important part of the human diet, are synonymous with condensed tannins and are found in fruits, beans, berries, nuts, cocoa, and wine 36. Proanthocyandins inhibited the PI3k/PKB pathway and induced apoptosis in colon cancer cells 37 (Table 2). Curcumin (diferuloylmethane), a dietary pigment from Curcuma longa L., gives the golden-yellow color and unique flavor to curry. Several laboratories have shown that curcumin and/or turmeric have potent anti-inflammatory activity 38. Curcumin has also been reported to inhibit cell invasion by downregulation of COX-2 and MMP-2 expression and inhibit colon cancer cell growth through suppressing EGFR gene expression and modulating Akt/mTOR signaling in CRC cells 39-41. In another study, curcumin has been found to suppress p38 activation, IL-1β production, and MMP-3 expression in the mucosa of children and adults with IBD 42.
| Compound | Structure | Dietary source | Mechanisms | Ref. |
|---|---|---|---|---|
| Proanthocyanidin |  |
 |
Induces apoptosis through downregulation of PI3K and Akt signaling pathway | 37 |
| Curcumin |  |
 |
Inhibits cell invasion by downregulation of COX-2 and MMP-2 expression via inhibiting p65 in colon cancer cells | 39 |
| Inhibits colon cancer cell growth through suppressing gene expression of EGFR and modulating Akt/mTOR signaling | 40, 41 | |||
| Suppression of p38 activation, IL-1β production, and MMP-3 expression in children and adults with active IBD | 42 | |||
| Resveratrol | 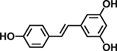 |
 |
Inhibits metastasis via reducing hypoxia-induced factor-1α and MMP-9 expression in colon cancer cells | 43 |
| Suppression of DSS-induced colitis through downregulation of p38, PGES-1, iNOS, and COX-2. | 44 | |||
| Inhibits Wnt signaling and regulation of intracellular β-catenin localization in colon-derived cells | 45 | |||
| Pterostilbene | 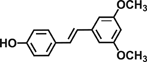 |
 |
Reduces cell proliferation by decreasing c-Myc and cyclin D1 expression, suppresses iNOS and COX-2 expression through downregulating p38 cascade | 46 |
| Inhibits AOM-induced colonic carcinogenesis through downregulation of Wnt/β-catenin, EGFR, PI3K/Akt, NF-κB signaling, and inflammatory gene expression | 47, 48 |
Resveratrol (3,5,4′-trihydroxystilbene) is a compound found mainly in the skin of grapes and red wines. Resveratrol was reported to inhibit metastasis via reducing hypoxia inducible factor-1α and MMP-9 expression in colon cancer cells 43. Dietary supplementation of resveratrol suppressed dextran sulfate sodium (DSS)-induced colitis through downregulation of p38, prostaglandin E Synthase-1, iNOS, and COX-2 in mice 44. Resveratrol also inhibited Wnt signaling and β-catenin localization in colon-derived cells 45. Pterostilbene, a dimethylether analogue of resveratrol, has been demonstrated to have anti-inflammatory action through inhibiting p38 MAPK pathway in colon cells 46. Dietary intake of pterostilbene suppressed ACF formation in the AOM-induced colon carcinogenesis through inhibiting β-catenin/p65 signaling pathway in rats 47. Our recent report has shown that pterostilbene inhibited AOM-induced ACF through downregulation of Wnt/β-catenin, EGFR, PI3K/Akt, and NF-κB signaling pathways in mice 48.
5 Chemopreventive mechanisms of carotenoids
Carotenoids are natural, fat-soluble pigments that provide bright coloration to plants and animals (Table 3). β-Carotene, primarily present in carrots, red palm oil, pumpkin, and leafy green vegetables, exhibited antioxidant and anti-carcinogenic activity. β-Carotene has been demonstrated to reduce high-fat diet induced ACF and COX-2 expression 49. Lycopene, a carotenoid found in tomato, watermelon, papaya, apricot, and orange, exhibited potential antioxidant and anti-carcinogenic activity. Lycopene inhibited cell proliferation of human colon cancer cells via suppression of the Akt signaling pathway and downstream-targeted molecules 50. Lycopene also prevented LPS-induced proinflammatory gene expression by blocking of NF-κB signaling and inhibited cell growth through inactivating Ras signaling pathway 51, 52. Astaxanthin, a major component of carotenoids possesses attractive remedial features, is naturally occurring and found predominantly in shrimp and crayfish. Astaxanthin was reported to inhibit 1,2-dimethylhydrazine-induced rat colon carcinogenesis by modulating the expressions of NF-κB, COX-2, MMPs-2/9, Akt, and ERK-2 signaling pathway in Wistar male rats 53.
| Compound | Structure | Dietary source | Mechanisms | Ref. |
|---|---|---|---|---|
| β-Carotene | 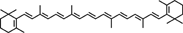 |
 |
Reduces high-fat diet induced ACF number and COX-2 expression | 49 |
| Lycopene | 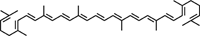 |
 |
Inhibits cell proliferation through suppression of Akt activation, decreases β-catenin protein level, reduces cyclin D1 protein expression, and increases nuclear level of p27 | 50 |
| Prevents LPS-induced proinflammatory gene expression by suppression of IκBα degradation, p65 translocation, and NF-κB transcriptional activity | 51 | |||
| Inhibits cell growth through inactivating Ras signaling | 52 | |||
| Astaxanthin | 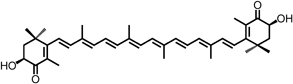 |
 |
Inhibits 1,2-dimethylhydrazine (-induced rat colon carcinogenesis through downregulation of ERK, Akt, NF-κB signalings, and protein expression of COX-2 and MMP-2/-9, as well as increases apoptosis | 53 |
6 Chemopreventive mechanisms of isothiocyanates, 3,3′-diindolylmethane, terpenoids, ω-3 fatty acids, and conjugated linoleic acid
Isothiocyanates, found in cabbage, turnips, broccoli, kale, turnips, cauliflower, and brussels sprouts, have been demonstrated to have a cancer chemopreventive activity 54 (Table 4). Phenethyl isothiocyanate induced cell-cycle arrest by downregulation of cyclins through the activation of p38 MAPK signaling pathway in HT-29 cells 55. Phenethyl isothiocyanate also inhibited cell migration and invasion through downregulation of MMPs gene expression via suppressing protein kinase Cδ, MAPK signaling, and NF-κB binding activity 56. Sulforaphane treatment reduced the number of polyps by inhibiting Akt, ERK signaling, COX-2, and cyclin D1 protein expression in Apc(Min/+) mice and also inhibited cancer cell growth by inducing apoptosis in SW620 cells, which contributes to its anti-inflammatory and anti-carcinogenic activities 57, 58.
| Compound | Structure | Dietary source | Mechanisms | Ref. |
|---|---|---|---|---|
| Phenethyl isothiocyanate | 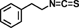 |
 |
Induces cell-cycle arrest by decreasing cyclins (A, D, and E) expression through the activation of p38 MAPK signaling pathway | 55 |
| Benzyl isothiocyanate | 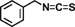 |
Inhibits cell migration and invasion through downregulation of MMPs gene expression via suppressing protein kinase C d, MAPK, signaling and NF-κB levels | 56 | |
| Sulforaphane | 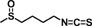 |
Reduces intestinal adenomas through inhibition of Akt, ERK signaling, COX-2, and cyclin D1 protein expression in APCmin/+ mouse | 57 | |
| Induces colon cancer cell apoptosis through p53-dependent proapoptotic signaling | 58 | |||
| DIM | 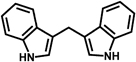 |
 |
Reduces DSS-induced PGE2, nitric oxide and proinflammatory cytokines production in BALB/c miceInduces cell-cycle arrest by inhibiting CDK2 activity and increasing protein levels of p21 and p27 | 59, 60 |
| Borneol | 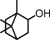 |
 |
Decreases TNBS-induced colitis and proinflammatory cytokines (IL-6 and IL-1β) gene expression in mice | 61 |
| Zerumbone | 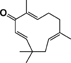 |
 |
Reduces AOM/DSS-induced colonic inflammation, multiplicity in adenocarcinomas, and expression of NF-κB | 67 |
| All-trans-retinoic acid | 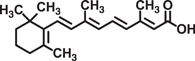 |
Reduces TNBS-induced colitis through decreasing levels of proinflammatory cytokines (TNF-α, IL-1β, and IL-17) in mice | 63 | |
| Retinol | 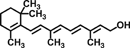 |
 |
Inhibits cell migration and invasion through decreasing gene expression and activities of MMPs | 64 |
| Decreases nuclear level and promotes proteosomal degradation of β-catenin | 66 | |||
| Inhibits cell invasion by suppression of PI3K activity | 65 | |||
| DHA |  |
 |
Induces apoptosis, cell-cycle arrest and decreases colon cancer cell growth independent of p53 status | 68 |
| Suppresses arachidonic acid-induced cell proliferation, PGE2 production, and COX-2 expression | 69 | |||
| Induces proteasome-dependent degradation of β-catenin and decreases its downstream gene MMP-7 and VEGF | 70 | |||
| Induces apoptosis through downregulation of Akt signaling | 71 | |||
| Conjugated linoleic acid | 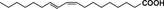 |
 |
Inhibits colon cancer growth through reducing phosphorylation of ERK1/2 and downstream c-myc | 72 |
| Decreases mRNA expression of TNF-α, IL-6, and IL-12 in human colonic epithelial Caco-2 cells | 73 |
- a ERK1/2, extracellular signal-regulated protein kinase.
3,3′-Diindolylmethane (DIM) is a nontoxic cancer-preventive phytochemical isolated from broccoli and cabbage. DIM can reduce DSS-induced PGE2, nitric oxide, and proinflammatory cytokines production in BALB/c mice 59. DIM has also been demonstrated to exert anti-cancer effects in both in vivo and in vitro models. DIM caused cell-cycle arrest by inhibiting cyclin-dependent kinase 2 (CDK2) activity and increasing protein levels of p21 and p27 in HT-29 human colon cancer cells 60.
Terpenoids are a class of secondary metabolites from the common origin of mevalonate and isopentenyl pyrophosphate that are lipophilic in nature 61. Borneol has been reported to suppress TNBS-induced colitis and proinflammatory cytokines gene expression in mice 62. all-trans-Retinoic acid reduced TNBS-induced colitis through decreasing the level of proinflammatory in mice 63. More recently, retinol treatment of colon cancer cells resulted in inhibition of cell migration and invasion 64. Retinol also promoted proteosomal degradation of β-catenin and inhibited cell invasion by suppressing PI3K activity in colon cancer cells 65, 66.
Zerumbone, a tropical ginger sesquiterpene, significantly reduced AOM/DSS-induced colonic inflammation, multiplicity of colonic adenocarcinomas, and NF-κB expression in tumors developed in ICR mice 67.
A multitude of experimental and clinical studies have described potential health benefits for ω-3 PUFA abundant in marine oil. Studies with fish oil, which contains eicosapentaenoic acid and docosahexaenoic acid (DHA) showed that it has anti-inflammatory and anti-cancer properties. DHA treatment inhibits colon cancer growth through inducing apoptosis and cell-cycle arrest 68. DHA also suppressed arachidonic acid-induced cell proliferation, PGE2 production, and COX-2 expression in human colon carcinoma cells 69. Moreover, DHA is believed to induce the degradation of β-catenin and alterations in the expression of T-cell factor-β-catenin target genes, and induce apoptosis by modulating PI3K and p38 MAPK signaling pathways, which might contribute to their chemopreventive effects in human CRC cells 70, 71.
Conjugated linoleic acids (CLA) are a family of PUFA, some isomers occurring naturally in milk, dairy, and beef products. Several studies showed a lower incidence of certain cancers in animals fed CLA-supplemented diets. Treatment with CLA inhibited colon cancer growth through reducing phosphorylation of extracellular signal-regulated protein kinase and downstream c-myc in human Caco-2 cancer cells 72. CLA has also been reported to decrease mRNA expression of tumor necrosis factor α (TNF-α), IL-6 and IL-12 in human Caco-2 cells 73.
7 Chemopreventive mechanisms of sphingolipids
In addition to aforementioned natural dietary compounds, researches on dietary sphingolipids found that they can suppress colon carcinogenesis. Milk sphingolipids were fed to female CF1 mice, which were previously administered 1,2-dimethylhydrazine, and it was found that sphingolipids reduced the number of aberrant colonic crypt foci and aberrant crypts per focus by 70 and 30%, respectively. A longer term study found that sphingolipids had no effect on colon tumor incidence, but up to 31% of the tumors of mice fed sphingolipids were adenomas, whereas all of the tumors of mice fed without sphingolipids were adenocarcinomas 74-76. Different classes of sphingolipids, containing different head groups (sphingomyelin, glycosphingolipids, and ganglioside), showed similar effects 76. Symolon et al. 77 also showed that dietary soy sphingolipids suppressed tumorigenesis and gene expression in 1,2-dimethylhydrazine-treated CF1 mice and ApcMin/+ mice. In their study, the number of aberrant colonic crypt foci could be reduced by 38 and 52% at 0.025 and 0.1% of soy sphingolipids in the diet w/w, respectively, and cell proliferation in the upper half of the crypts could be reduced by 50 and 56% at the same concentrations of soy sphingolipids in the diets. Adenomas in the APCmin/+ mice were decreased by 22 and 37% when the mice fed with 0.025 and 0.1% w/w soy sphingolipids diets. For gene expression confirmation, they found that soy sphingolipids decreased two transcription factors (hypoxia-induced factor 1α and transcription factor 4) of mRNA expression which are associated with cancer.
A significant part of orally administered sphingolipids was found in the small intestine and colon of mice 78. The enzymes (sphingomyelinase, glucosylceramidase, and ceramidase) that are normally found in the small intestine digest these compounds. The sphingolipids that are not digested and adsorbed into the blood stream through the small intestine are transported to the colon, where they are hydrolyzed, mainly by colonic microflora. The released bioactive molecules, ceramides, and the sphingoid bases have biological functions involved in regulating cell growth, induction of cell differentiation, and apoptosis. More studies are underway to determine if sphingolipids can be used as chemopreventive agents.
8 Concluding remarks
CRC is a leading cause of cancer-related morbidity and mortality worldwide. Recent evidence indicated that increasing of fruits, vegetable, or fiber is unlikely to prevent a large proportion of CRCs 79. The nature of in vivo animal experiments may be responsible for the inconsistencies between human and animal studies whereby larger amounts of dietary compounds are provided to animals in a closely controlled setting where CRC is artificially induced. Furthermore, genetic variability in humans can account for various effects in human cohorts. Therefore, various chemopreventive strategies have been investigated. Multistage tumorigenesis in this organ with its sequence of accompanying chronic inflammation, genetic, molecular signaling, and metabolic pathways alterations, provides an attractive model for the evaluation of chemopreventive agents. Besides dietary compounds, lifestyle factors including avoidance of smoking and heavy alcohol use, prevention of weight gain, and maintenance of a reasonable level of physical activity are also correlated with lower risks of CRC 79. The ultimate goal of chemoprevention by natural dietary compounds is the reduction of CRC incidence by intervening development pathways in tumor cells which promote growth and metastases of CRC. In this review, the implementation of chemoprevention of CRC by various natural dietary compounds represents an inexpensive, readily applicable approach to control and reduce CRC incidence.
Acknowledgements
The authors have declared no conflict of interest.




