Simulation and Comparative Analysis of Different Binding Modes of Non-nucleoside Agonists at the A2A Adenosine Receptor
Corresponding Author
Diego Dal Ben
School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, via S. Agostino 1, 62032 Camerino (MC, Italy
Search for more papers by this authorMichela Buccioni
School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, via S. Agostino 1, 62032 Camerino (MC, Italy
Search for more papers by this authorCatia Lambertucci
School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, via S. Agostino 1, 62032 Camerino (MC, Italy
Search for more papers by this authorGabriella Marucci
School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, via S. Agostino 1, 62032 Camerino (MC, Italy
Search for more papers by this authorClaudia Santinelli
School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, via S. Agostino 1, 62032 Camerino (MC, Italy
Search for more papers by this authorAndrea Spinaci
School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, via S. Agostino 1, 62032 Camerino (MC, Italy
Search for more papers by this authorAjiroghene Thomas
School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, via S. Agostino 1, 62032 Camerino (MC, Italy
Search for more papers by this authorRosaria Volpini
School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, via S. Agostino 1, 62032 Camerino (MC, Italy
Search for more papers by this authorCorresponding Author
Diego Dal Ben
School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, via S. Agostino 1, 62032 Camerino (MC, Italy
Search for more papers by this authorMichela Buccioni
School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, via S. Agostino 1, 62032 Camerino (MC, Italy
Search for more papers by this authorCatia Lambertucci
School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, via S. Agostino 1, 62032 Camerino (MC, Italy
Search for more papers by this authorGabriella Marucci
School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, via S. Agostino 1, 62032 Camerino (MC, Italy
Search for more papers by this authorClaudia Santinelli
School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, via S. Agostino 1, 62032 Camerino (MC, Italy
Search for more papers by this authorAndrea Spinaci
School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, via S. Agostino 1, 62032 Camerino (MC, Italy
Search for more papers by this authorAjiroghene Thomas
School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, via S. Agostino 1, 62032 Camerino (MC, Italy
Search for more papers by this authorRosaria Volpini
School of Pharmacy, Medicinal Chemistry Unit, University of Camerino, via S. Agostino 1, 62032 Camerino (MC, Italy
Search for more papers by this authorGraphical Abstract
Abstract
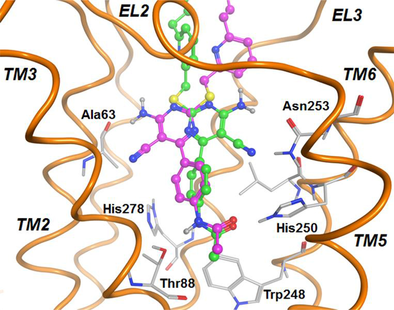
Non-nucleoside agonists of adenosine receptors were analysed at the A2A adenosine receptor to simulate and compare their possible binding modes. The docking studies were performed by using different arrangements of the binding cavity and various docking tools. Mutagenesis results reported in literature were used as reference data for the assessment of the different ligand arrangements observed in this study. The results suggest four possible binding modes, two of which appear compatible with an agonist activity and in agreement with the mutagenesis data. This study provides useful information for the design of new simplified compounds presenting agonist activity at the A2A adenosine receptor.
References
- 1B. B. Fredholm, A. P. IJzerman, K. A. Jacobson, K. N. Klotz, J. Linden, Pharmacol. Rev. 2001, 53( 4), 527–552.
- 2
- 2aS. Schenone, C. Brullo, F. Musumeci, O. Bruno, M. Botta, Curr. Top. Med. Chem. 2010, 10( 9), 878–901;
10.2174/156802610791268729 Google Scholar
- 2bM. de Lera Ruiz, Y. H. Lim, J. Zheng, J. Med. Chem. 2014, 57( 9), 3623–3650;
- 2cC. M. Aherne, E. M. Kewley, H. K. Eltzschig, Biochim. Biophys. Acta 2011, 1808( 5), 1329–1339;
- 2dS. L. Cheong, S. Federico, G. Venkatesan, A. L. Mandel, Y. M. Shao, S. Moro, G. Spalluto, G. Pastorin, Med. Res. Rev. 2013, 33( 2), 235–335.
- 3
- 3aCVT, CV Therapeutics and Astellas Announce FDA Approval for Lexiscan(TM) (regadenoson) Injection in http://www.cvt.com/PressRelease.aspx?releaseID=1128317, 2008;
- 3bK. H. Kirin, Approval for manufacturing and marketing of NOURIAST® tablets 20 mg, a novel antiparkinsonian agent, 2013. http://www.kyowa-kirin.com/news_releases/2013/e20130325_04.html
- 4
- 4aU. Rosentreter, R. Henning, M. Bauser, T. Krämer, A. Vaupel, W. Hübsch, K. Dembowsky, O. Salcher-Schraufstätter, J. P. Stasch, T. Krahn, E. Perzborn, Pat. WO/2001/025210, 2001;
- 4bU. Rosentreter, T. Krämer, M. Shimada, W. Hübsch, N. Diedrichs, T. Krahn, K. Henninger, J. P. Stasch, Pat. WO/2003/008384, 2003.
- 5M. W. Beukers, L. C. Chang, J. K. von Frijtag Drabbe Kunzel, T. Mulder-Krieger, R. F. Spanjersberg, J. Brussee, A. P. IJzerman, J. Med. Chem. 2004, 47( 15), 3707–3709.
- 6
- 6aL. C. Chang, J. K. von Frijtag Drabbe Kunzel, T. Mulder-Krieger, R. F. Spanjersberg, S. F. Roerink, G. van den Hout, M. W. Beukers, J. Brussee, A. P. IJzerman, J. Med. Chem. 2005, 48( 6), 2045–2053;
- 6bJ. R. Lane, E. Klaasse, J. Lin, J. van Bruchem, M. W. Beukers, A. P. IJzerman, Biochem. Pharmacol. 2010, 80( 8), 1180–1189;
- 6cJ. Louvel, D. Guo, M. Agliardi, T. A. Mocking, R. Kars, T. P. Pham, L. Xia, H. de Vries, J. Brussee, L. H. Heitman, A. P. IJzerman, J. Med. Chem. 2014, 57( 8), 3213–3222;
- 6dJ. Louvel, D. Guo, M. Soethoudt, T. A. Mocking, E. B. Lenselink, T. Mulder-Krieger, L. H. Heitman, A. P. IJzerman, Eur. J. Med. Chem. 2015, 101, 681–691;
- 6eJ. R. Lane, C. Klein Herenbrink, G. J. van Westen, J. A. Spoorendonk, C. Hoffmann, A. P. IJzerman, Mol. Pharmacol. 2012, 81( 3), 475–487;
- 6fM. Kato, N. Sato, M. Okada, T. Uno, N. Ito, Y. Takeji, H. Shinohara, M. Fuwa, Pat. WO/2005/105778, 2005;
- 6gN. Sato, Y. Yuki, H. Shinohara, Y. Takeji, K. Ito, D. Michikami, K. Hino, H. Yamazaki, Pat. US2012/0022077, 2012;
- 6hT. Eckle, T. Krahn, A. Grenz, D. Kohler, M. Mittelbronn, C. Ledent, M. A. Jacobson, H. Osswald, L. F. Thompson, K. Unertl, H. K. Eltzschig, Circulation 2007, 115( 12), 1581–1590.
- 7
- 7aG. Lebon, T. Warne, P. C. Edwards, K. Bennett, C. J. Langmead, A. G. Leslie, C. G. Tate, Nature 2011, 474( 7352), 521–525;
- 7bF. Xu, H. Wu, V. Katritch, G. W. Han, K. A. Jacobson, Z. G. Gao, V. Cherezov, R. C. Stevens, Science 2011, 332( 6027), 322–327;
- 7cG. Lebon, P. C. Edwards, A. G. Leslie, C. G. Tate, Mol. Pharmacol. 2015, 87( 6), 907–915.
- 8
- 8aD. Thimm, A. C. Schiedel, F. F. Sherbiny, S. Hinz, K. Hochheiser, D. C. G. Bertarelli, A. Maass, C. E. Müller, Biochemistry 2013, 52( 4), 726–740;
- 8bJ. Li, A. L. Jonsson, T. Beuming, J. C. Shelley, G. A. Voth, J. Am. Chem. Soc. 2013, 135( 23), 8749–8759;
- 8cD. Dal Ben, M. Buccioni, C. Lambertucci, A. Thomas, R. Volpini, In Silico Pharmacology 2013, 1( 1), 24;
- 8dD. Rodriguez, Z. G. Gao, S. M. Moss, K. A. Jacobson, J. Carlsson, J. Chem. Inf. Model. 2015, 55( 3), 550–563.
- 9
- 9aR. Huey, G. M. Morris, A. J. Olson, D. S. Goodsell, J. Comput. Chem. 2007, 28( 6), 1145–1152;
- 9bG. M. Morris, R. Huey, W. Lindstrom, M. F. Sanner, R. K. Belew, D. S. Goodsell, A. J. Olson, J. Comput. Chem. 2009, 30( 16), 2785–2791;
- 9cS. Dallakyan, A. J. Olson, Methods Mol. Biol. 2015, 1263, 243–250.
- 10O. Trott, A. J. Olson, J. Comput. Chem. 2010, 31( 2), 455–461.
- 11J. M. Yang, C. C. Chen, Proteins 2004, 55( 2), 288–304.
- 12Molecular Operating Environment, C.C.G., Inc., 1255 University St., Suite 1600, Montreal, Quebec, Canada, H3B 3X3.
- 13W. Liu, E. Chun, A. A. Thompson, P. Chubukov, F. Xu, V. Katritch, G. W. Han, C. B. Roth, L. H. Heitman, A. P. IJzerman, V. Cherezov, R. C. Stevens, Science 2012, 337( 6091), 232–236.
- 14J. J. Stewart, J. Comput. Aided Mol. Des. 1990, 4( 1), 1–105.
- 15W. D. Cornell, P. Cieplak, C. I. Bayly, I. R. Gould, K. M. Merz, D. M. Ferguson, D. C. Spellmeyer, T. Fox, J. W. Caldwell, P. A. Kollman, J. Am. Chem. Soc. 1995, 117, 5179–5197.
- 16J. A. Ballesteros, H. Weinstein, Methods Neurosci. 1995, 25, 366–428.
- 17D. Sabbadin, A. Ciancetta, G. Deganutti, A. Cuzzolin, S. Moro, MedChemComm 2015, 6( 6), 1081–1085.
- 18K. A. Bennett, B. Tehan, G. Lebon, C. G. Tate, M. Weir, F. H. Marshall, C. J. Langmead, Mol. Pharmacol. 2013, 83( 5), 949–958.
- 19L. H. Heitman, T. Mulder-Krieger, R. F. Spanjersberg, J. K. von Frijtag Drabbe Kunzel, A. Dalpiaz, A. P. IJzerman, Br. J. Pharmacol. 2006, 147( 5), 533–541.
- 20
- 20aD. Dal Ben, M. Buccioni, C. Lambertucci, G. Marucci, A. Thomas, R. Volpini, G. Cristalli, Bioorg. Med. Chem. 2010, 18( 2), 7923–7930;
- 20bF. Varano, D. Catarzi, L. Squarcialupi, M. Betti, F. Vincenzi, A. Ravani, K. Varani, D. Dal Ben, A. Thomas, R. Volpini, V. Colotta, Eur. J. Med. Chem. 2015, 96, 105–121.
- 21
- 21aJ. Kim, Q. Jiang, M. Glashofer, S. Yehle, J. Wess, K. A. Jacobson, Mol. Pharmacol. 1996, 49( 4), 683–691;
- 21bD. Dal Ben, C. Lambertucci, G. Marucci, R. Volpini, G. Cristalli, Curr. Top. Med. Chem. 2010, 10( 10), 993–1018.