Phenotypic and genetic characteristics of 24 cases of early infantile epileptic encephalopathy in East China, including a rare case of biallelic UGDH mutations
Abstract
Background
Early infantile epileptic encephalopathy (EIEE) is a group of highly heterogeneous diseases, both phenotypically and genetically. Usually, it starts early on and manifests as intractable epilepsy, abnormal electroencephalogram, and growth retardation/intellectual impairment. With the advent of next-generation sequencing (NGS), its genetic etiology has attracted increasing clinical attention. This study aimed to investigate the genetic characteristics and clinical phenotypes of patients with EIEE from a central hospital in Eastern China.
Methods
This study retrospectively included the gene variants from 24 EIEE-positive patients admitted between January 2021 and January 2022 to a hospital in Anhui Province, China. The genetic diagnosis was performed in all cases by trio-based whole-exome sequencing (WES). Additionally, Video electroencephalogram (VEEG) and neuroimaging examinations were performed.
Results
A total of 24 children were included. The average age at the first seizure was approximately 5 months. About 42% of children had developmental retardation of varying degrees, 43% had brain structural abnormalities, and 64% had VEEG abnormalities. In addition, other phenotypes, including endocrine metabolism and cardiac structural abnormalities, have been independently reported. In total, fifteen pathogenic gene variants were identified in 24 patients. The main pathogenic genes identified were SCN1A (25%, 6/24), KCNQ2 (8.3%, 2/24), and TBC1D24 (8.3%, 2/24). We also found an extremely rare case of EIEE84 type caused by biallelic UGDH gene variants, predicting that this variant might affect the stability of the protein structure.
Conclusions
SCN1A pathogenic variants are the main factor leading to EIEE, similar to previously published cohort reports. NGS is useful for accurate clinical diagnoses and precise treatment choices. We also reported a rare case of EIEE84 caused by variants in the UGDH gene in a Chinese patient. This study further enriches the known spectrum of pathogenic EIEE genes.
1 INTRODUCTION
Early infantile epileptic encephalopathy (EIEE) is a severe neurological and neurodevelopmental disease that can lead to intractable seizures within weeks to months after birth, including forms of epilepsy, such as infantile spasms and the Ohtahara syndrome (Liu et al., 2021). Currently, the actual EIEE incidence rate is unknown, but it only accounts for a small proportion (~1.2/1000) of all cases of infants with seizures (Gaily et al., 2016). EIEE can lead to developmental delay, intellectual disability, psychomotor development disorder, and even death (Arafat et al., 2017). The genetic factors behind EIEE were initially discovered in 2001 and are mainly associated with single-gene variants, although abnormal chromosomal structures and polygenic regulation can also be causative factors. The genetics of EIEE were identified in approximately 40% of cases (Claes et al., 2001; Essajee et al., 2022; Liu et al., 2021), and since the degree of phenotypic and genetic heterogeneity in EIEE is high, the relationship between clinical phenotypes and genotypes cannot be generalized. Single-gene variants can cause different clinical phenotypes, whereas different variants of various genes can equally lead to similar clinical phenotypes. For example, SCN1A pathogenic variants have been observed in many EIEEs (Kluckova et al., 2020). With the development of new technologies for gene detection such as DNA microarrays and next-generation sequencing (NGS), the discovery rate of new EIEE pathogenic genes increased, and a large number of base variants and copy number variants have already been identified (Chatron et al., 2020; Fry et al., 2021; Hengel et al., 2020). So far, nearly 100 EIEE-related genes have been discovered (www.omim.org), such as genes encoding for neuronal ion channels, neurotransmitter synthesis and release, membrane receptors, transporters, and regulators of cell metabolism (Guerrini & Noebels, 2014).
In this study, we retrospectively summarized the clinical phenotypes and genetic characteristics of 24 positive EIEE cases identified by whole-exome sequencing (WES). In addition, we report a rare case of a biallelic UGDH mutation in China. The purpose of this study was to further elucidate the correlation between the phenotypes and genotypes of regional EIEE.
2 DATA AND METHODS
2.1 Study group
Between January 2017 and August 2020, we reviewed 24 EIEE cases without known causes in the Department of Neurology of Anhui Children's Hospital. The inclusion criteria were as follows: (1) the age at the initial diagnosis of the test cases did not exceed 12 months; (2) cases of central nervous system infection, intrauterine distress, and intracranial hemorrhage. The exclusion criteria included cases with genetic causes confirmed by gene testing. The patients' clinical data were collected, including age, seizure type, video EEG (VEEG), brain magnetic resonance imaging (MRI), and detailed phenotypes. Patients and their parents underwent WES to detect and analyze the likely variants by using appropriate bioinformatics software. The patients' legal guardians signed an informed consent form, and the study was approved by the Medical Ethics Committee of the Anhui Children's Hospital.
2.2 Gene testing
Three milliliters of peripheral EDTA-blood samples were collected from the patients and their parents. After the genomic DNA extraction, high-throughput sequencing was performed using an Illumina NovaSeq 6000 sequencer (Illumina). The average coverage of the target sequences was at least 99%. The data were analyzed and filtered for low-quality readings and then compared with the human genome (hg19/GRch37) after cleaning with the Burrows–Wheeler Alignment. The Genome Analysis Toolkit (GATK) was used to identify single nucleotide and insertion/deletion variants. The variants were analyzed by using a normal population frequency database analysis (ExAC, dbSNP, and 1000G), and the biological effects of the mutations were predicted with SIFT, Provean, and PolyPhen software. The pathogenicity of the single nucleotide variants (SNV) and insertion or deletion mutants was rated based on the guidelines of the American College of Medical Genetics and Genomics (ACMG) and the Association for Molecular Pathology (Richards et al., 2015). Polymerase chain reactions (PCRs) and Sanger sequencing were next performed to confirm the presence of the variants.
2.3 Analysis of the variant UGP protein model
Protein sequences were obtained from the National Center for Biotechnology Information database (https://www.ncbi.nlm.nih.gov/nuccore/NM_003359.4/), and the protein crystal structure (PDB: 3PRJ) was downloaded from the RCSB PDB (https://www1.rcsb.org/structure/3PRJ). The mutant structure was modeled on the wild-type structure using PyMOL 2.2 that eventually displayed the results.
2.4 Literature review of the patients with UGDH variants
We conducted a literature review on patients with neurological abnormalities caused by UGDH variants found on the PubMed database (https://pubmed.ncbi.nlm.nih.gov/) and summarized the phenotype variant data while excluding the articles without molecular diagnostic results and phenotypes.
3 RESULTS
3.1 Phenotypic and genetic characteristics of EIEE
A total of 24 patients were recruited, 13 boys and 11 girls. The average patients' age at the first seizure was approximately 5 months (maximum, 12 months; minimum, 0.5), and the average age at diagnosis was approximately 6 months. Among these patients, 42% (10/24), 43% (9/21), and 64% (14/22) had different degrees of growth retardation, abnormal brain structures, and abnormal VEEG, respectively. Other phenotypes included abnormal endocrine metabolism or cardiac structure. Pathogenic variations in 15 genes were identified in 24 patients, such as SCN1A (n = 6), KCNQ2 (n = 2), TBC1D24 (n = 2), and CACNA1H (n = 2). SCN1A variants were detected in six patients (five boys and one girl), accounting for approximately 25% of the total cohort, similar to the incidence previously reported (Liu et al., 2021; Sun et al., 2021). Five patients were diagnosed with Dravet syndrome (DS) that mainly exhibits focal or generalized seizures, febrile seizures, and other types of seizures with varying degrees of developmental abnormalities and an average onset age of 4.5 months. Moreover, two patients carrying KCNQ2 variants were diagnosed with West syndrome and exhibited metabolic abnormalities, such as hyperlactatemia, without any developmental abnormalities. Two other cases carrying CACNA1H variants were characterized by punctate hair accompanied by language delay or intellectual disability, and their brain structure mainly showed a widening of the temporal extracerebral space. Table 1 summarizes the clinical characteristics and variant information of all patients.
| No. | Sex | Onset age | Gene | Mutation | Epilepsy | Dysontogenesis | Brain imaging | EEG | Other phenotypes |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 5 | GRIN2A | c.2627A > G (p.876I > T) | + | − | NA | NA | |
| 2 | M | 4 | SCN1A | c.5314G>A (p.A1772T) | Accompanied by left-sided, right-sided, or general tonic–clonic seizure, with multiple attacks every day | Mild mental retardation, motor retardation | MRI: white matter signals of the bilateral frontal, parietal, and temporal areas are slightly higher | Diffuse slow wave activity, diffuse and epileptiform small discharge (multiple brain regions) | Diarrhea |
| 3 | F | 5 | ARHGEF9 | c.382G>A (p.G128S) | Focal seizures | − | − | − | − |
| 4 | M | 5.5 | CACNA1H | c.4045G>A (p.A1349T) | Repetitive seizures | Language development delay | CT: bilateral frontal and temporal extracerebral space widened | − | − |
| 5 | M | 5 | TBC1D24 | c.76G>T (p.A1349T)/c.1499C>T (p.A500V) | Repetitive seizures | Language development delay | MRI: T2 signal in the two basal ganglia was slightly higher, and the corpus callosum was thin | − | Patent foramen ovale |
| 6 | M | 4 | SCN1A | c.4391T>C (p.L1464S) | Left limb twitch, status epilepticus | Mild motor and language retardation | MRI: slightly increased left frontotemporal signal | + | − |
| 7 | F | 3 | SCN1A | c.1073C>G (p.P358R) | Repetitive seizures in the setting of fever | Psychomotor retardation | − | + | − |
| 8 | F | 2 | KCNQ2 | c.1639C>T (p.R547W) | Focal seizure | − | MRI: short T1 signal in the posterior longitudinal fissure cistern | + | Hyperlactatemia, lethargy, poor reaction, slight yellowing of the skin, hypertonia |
| 9 | F | 4 | EEF1A2 | c.1196(exo n7)C>A (p.R547W)/c.1195G>A (p.A399T) | Repetitive seizures | Motor retardation | − | + | High free thyroxine, low serum creatinine, hyperlactatemia, hypercalcemia, hyperphosphatemia, low serum iron, hyperpyruvic acidemia, obvious intestinal gas accumulation, and several mesenteric lymph nodes |
| 10 | M | 1.5 | TBC1D24 | c.1525G>A (p.G509R)/c.443A>T (p.E148V) | Myoclonic seizure | − | − | + | Positive for cytomegalovirus IgM antibody, increased blood propionylcarnitine, atrial septal defect, sinus tachycardia, and bilateral sensorineural hearing loss |
| 11 | F | 12 | SLC2A1 | c.29_c.30insG (p.G10Gfs*79) | Focal seizure | − | − | − | − |
| 12 | M | 5 | SCN1A | c.2870G>A (p.W957X) | Focal seizures, tonic seizures, febrile seizures | − | MRI: supratentorial ventricular dilation, slightly high signal intensity on T2WI, and DWI of the dorsal pontine | − | − |
| 13 | F | 4 | KCNQ2 | c.1342C>T (p.R448X) | Focal seizure | Psychomotor retardation | − | − | Hyperlactatemia, decreased immunoglobulin A and G, hyperammonemia, and decreased ceruloplasmin |
| 14 | F | 0.5 | KCNB1 | c.801G>C (p.L267F) | Repetitive seizures | − | − | Extensive multifocal spike–wave, slow spike–wave, multiple spike–wave, and multiple slow spike–wave emission | Fallot's tetrad and patent ductus arteriosus |
| 15 | M | 7 | SCN1A | c.5488_c.5489delCA (p.Q1830Vfs*6) | Status epilepticus and febrile seizures | − | − | + | − |
| 16 | F | 1.9 | STXBP1 | c.1180_c.1183de linsTTT (p.L394Ffs*21) | Repetitive seizures and tonic seizures | − | NA | local epileptic discharge | − |
| 17 | F | 9.5 | PACS2 | loss1 (EXON: 1–25)(all) | Repetitive seizures | Psychomotor retardation | − | + | Special facial features: wide eye distance, flat nose, absence of the upper lip, microcephaly, and hypoproteinemia |
| 18 | M | 4 | GABRB3 | c.913G>A (p.A305T) | Repetitive seizures | − | MRI: High signal intensity on T1WI in the forelimbs of the bilateral internal capsule, widened subarachnoid space in the left temporal pole | + | Hypoalbuminemia |
| 19 | M | 3.8 | SCN2A | c.2607G>A (p.M869I)/ c.991C>T (p.R331X) | Recurrent seizures, tonic seizures | − | − | − | − |
| 20 | M | 12 | KCNA2 | c.1214C>T (p.P405L) | Focal, tonic seizures | Psychomotor and language development retardation | − | − | Leukocytosis and hyperammonemia |
| 21 | M | 12 | CACNA1H | c.2318G>A (p.G773D) | Repetitive convulsions, cluster like, nodding and hugging | Retardation of intelligence, language, and cognition | MRI: Abnormal subcortical signal in the left frontal cingulate cortex; the left temporal pole was wider than the one opposite; symmetric, patchy, slightly high signal near the posterior horn of the bilateral lateral ventricles | NREM sleep: slightly more sharp waves and slow sharp waves in the left frontal pole, frontal region, and anterior temporal region | − |
| 22 | M | 4 | SCN1A | c.2345C>T (p.T782I) | Focal, tonic seizures | Delay of motor development | NA | NA | Hypertonia, multijoint contracture of limbs, scoliosis, dislocation of the hip joint, congenital vertical talus, hypoplasia of the laryngeal cartilage, and anemia |
| 23 | M | 2 | PRICKLE1 | c.1903G>A (p.D635N)/c.113C>T (p.P38L) | Repetitive seizures | − | − | NREM sleep: multifocal slow wave, sharp slow wave, and slow spike–wave mainly occur in the bilateral Rolandic regions | Bronchopneumonia and hepatosplenomegaly |
| 24 | F | 3.2 | UGDH | c.202A>T (p.N68Y)/c.131C>T (p.A44V) | Repetitive seizures | Psychomotor development retardation, intellectual disability | MRI: widened frontal–parietal gyrus | Extensive multifocal spike–wave, slow wave, slow spike–wave, multiple slow spike–wave emission | Right tracheobronchial malformation and patent foramen ovale |
- Abbreviations: CT, computed tomography; DWI, diffusion-weighted imaging; EEG, electroencephalogram; F, female; M, male; MRI, magnetic resonance imaging; NREM, nonrapid eye movement.
3.2 A rare case of UGDH variants
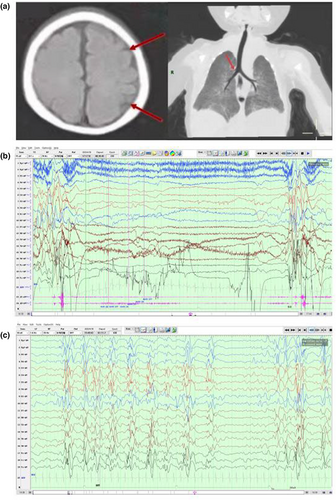
Here, we report a rare case of EIEE 84 (OMIM # 618792) caused by variants in UGDH in China. The patient was a 5-month-old girl baby, G1P1. At 3 months, she had convulsive seizures without induction, and her upper limbs were stretched and swayed upward, accompanied by crying and screaming. The muscular tension of her extremities was low, and the head was unstable. Brain MRI revealed a frontal–parietal gyrus widening, whereas chest computed tomography (CT) showed a right tracheobronchial malformation (Figure 1a). Color Doppler ultrasound showed a patent foramen ovale, and VEEG indicated extensive multifocal spike waves, slow waves, slow spike-waves, and multiple slow spike-wave discharges (Figure 1b,c). A combination of levetiracetam and topiramate was not effective as antiseizure therapy, and despite switching to prednisone with sodium valproate, seizures were still frequent.
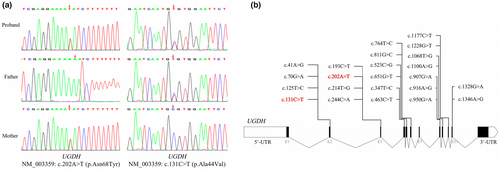
Genetic testing revealed compound heterozygous variants, NM_003359 c.202A>T (p.Asn68Tyr) and c.131C>T (p.Ala44Val) in UGDH. The c.202A>T (p.Asn68Tyr) allele was inherited from the father, whereas c.131C>T (p. Ala44Val) was inherited from the mother, a variant that has been reported by Hengel et al. (2020). These variants were not previously included in the frequency database of the normal population, and since some online software programs may be misleading in making variant predictions (Table 2), we next confirmed the presence of the UGDH variants by Sanger sequencing (Figure 2).
| Gene | UGDH | UGDH |
|---|---|---|
| Transcript | NM_003359 | NM_003359 |
| Variation | c.202A>T (p.Asn68Tyr) | c.131C>T (p.Ala44Val) |
| Provean | Deleterious (−7.95) | Deleterious (−2.56) |
| Polyphen2_HDIV | Probably damaging (1.0) | Possibly damaging (0.871) |
| Polyphen2_HVAR | Probably damaging (0.99) | Benign (0.127) |
| SIFT | Damaging (0.0) | Damaging (0.036) |
| MutationTaster | Disease causing (1) | Disease causing (1) |
| REVEL | Deleterious (0.820) | Deleterious (0.525) |
| 1000 Genomes | Not included | Not included |
| ExAC | Not included | 0.0001 |
| dbSNP | Not included | Not included |
| ACMG rating | Likely pathogenic | Pathogenic |
| Rating evidence | PM1 + PM2 + PM3 + PP3 | PS3 + PM1 + PM2 + PM3 + PP1 + PP5 |
3.3 Analysis of the variant UGDH protein model
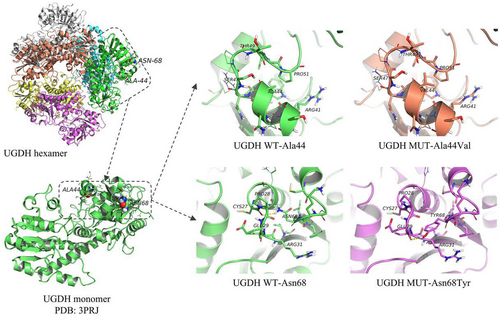
The UGDH protein consists of three domains: a NAD-binding domain (N-terminal, 5–213 amino acid, aa), a central domain (214–323 aa), and a UDP-binding domain (C-terminal, 329–466 aa). UGDH forms dimers through the central domain and interacts with the N- and C-termini of two adjacent dimers to form hexamers for normal functionality (Walsh & Stainier, 2001). The UGDH variants Ala44Val and Asn68Tyr identified in this study are located in the N-terminal domain. In the wild-type structure, Ala44 forms weak hydrogen bonds with Pro51 located in the loop region, whereas the Ala44Val variant may lead to stronger hydrogen bonds with Pro51, thus changing the local structure of the protein. Hengel et al. showed that the variant Ala44Val might affect the protein function by destroying the hexamer structure (Hengel et al., 2020). The other variant (Asn68Tyr) breaks the hydrogen bond with Cys27 and forms a new hydrogen bond with Glu29, which may lead to changes in the local protein structure (Figure 3).
3.4 Literature review
Currently, there are only two published articles on the neuronal phenotype caused by UGDH variants, including 31 patients (12 boys, 19 girls) from 26 unrelated families (Alhamoudi et al., 2020; Hengel et al., 2020). The median age was 7.5 months at onset and 5 years at diagnosis. The incidence of epilepsy was approximately 93.5% (29/31), mainly epileptic spasms, whereas the incidence of intractable epilepsy was approximately 55.2% (16/29). Almost all patients have varying degrees of motor developmental delay and severe intellectual disability (93.5%, 29/31). A total of 22 UGDH variants were reported, with the highest incidence of c.950G>A (p.Arg317Gln) in 12 Saudi Arabian patients. Other hotspot variants include c.131C>T (p.Ala44Val) and c.1328G>A (p.Arg443His), described in detail in the supplementary materials.
4 DISCUSSION
In this article, we reviewed the clinical data and genetic characteristics of 24 patients with EIEE in Eastern China. The patients' main clinical manifestations were epileptic seizures and developmental disorders of varying degrees, such as psychomotor retardation or intellectual disability, as well as EEG and structural brain abnormalities. Occasionally, we also observed metabolic abnormalities, such as hyperlactatemia or hyperammonemia. Fifteen gene variants were identified, including SCN1A (6/24, 25%), CACNA1H (2/24, 8.3%), KCNQ2 (2/24, 8.3%), and TBC1D24 (2/24, 8.3%).
Most genetic epilepsies are related to abnormal ion channel functions, including voltage-gated sodium (NaV) and potassium channels (Kv) (Deng et al., 2014). NaV channels are mostly expressed in the central and peripheral nervous systems, and nine different NaV subtypes have been identified so far (Menezes et al., 2020). A total of seven cases associated with NaV variants were found in this cohort, whereas SCN1A variants were detected in six patients (five males and one female), accounting for approximately 25% of the total cohort, similar to the previously reported incidence (Liu et al., 2021; Sun et al., 2021). A SCN2A variant was identified in another patient. Moreover, Kv channels belong to a diverse superfamily that can inhibit neuronal excitability by regulating the outward potassium current entry (He et al., 2019). We found three cases of abnormal Kv: two KCNQ2 variants and one KCNA2 variant. The KCNQ2 variant is associated with a broad phenotypic spectrum that can lead to benign familial neonatal seizures (BFNS). However, unlike in benign outcomes, such as normal neurodevelopment of self-limited BFNS, KCNQ2 variants can also lead to EIEE7 (OMIM # 613720) with intractable epilepsy characteristics (Weckhuysen et al., 2012). In this study, two patients with KCNQ2 variants showed early seizures, including one with moderate-to-severe psychomotor retardation. The main pathogenic factor in this cohort was the abnormal function of the ion channels, which indicates that most EIEE cases may be related to ion channel gene variants.
In this cohort, we identified a rare case (DEE84, OMIM#618792) of UGDH compound heterozygous variants (Ala44Val and Asn68Tyr). The patient mainly exhibited convulsive seizures with stunting and hypotonia. Furthermore, the effect of the antiepileptic treatment was poor, similar to the phenotypes of previously reported patients (Alhamoudi et al., 2020; Hengel et al., 2020). UGDH is located on chromosome 4q15.1 (Spicer et al., 1998) and encodes for UDP glucose 6-dehydrogenase that catalyzes the continuous oxidation of UDP glucose (UDP-Glc) and converts it into UDP glucuronic acid (UDP-GlcA) (Marcu et al., 1999; Spicer et al., 1998). UDP-GlcA can synthesize and gradually degrade glycosaminoglycans (GAG), which can next produce proteoglycans (PG) through the covalent binding of core proteins or be stored in the extracellular matrix (ECM) as a free polysaccharide (Couchman & Pataki, 2012). GAG and PG play important roles in cellular processes at the early stage of human brain development, such as neuronal migration and differentiation. Therefore, a pathogenic variant of UGDH affects the glycosylation process and consequently causes several conditions, including growth retardation, hypotonia, intellectual disability, and metabolic abnormalities (Freeze et al., 2015). In addition, the patient had patent foramen ovale, which is the first clinical manifestation of DEE84. Although we cannot precisely determine whether it was caused by the UGDH variant, we can conclude based on various animal model experiments that UGDH plays a key role in the process of FGF signaling during development and heart valve formation (García-García & Anderson, 2003; Walsh & Stainier, 2001). Therefore, more reports on DEE84 cases are needed to clarify the correlation between the disease phenotypes and genotypes.
We further analyzed the potential pathogenic mechanisms of the UGDH variants (Ala44Val and Asn68Tyr). In vitro functional experiments have shown that the Ala44Val variant can reduce the thermal stability and destroy the hexamer structure, which affects the catalytic activity of the UGDH protease and reduces the level of UDP-GlcA, thereby leading to neural development retardation and encephalopathy (Hengel et al., 2020; Perenthaler et al., 2020). On the other hand, the novel variant Asn68Tyr breaks the hydrogen bond with Cys27 because of a modified spatial distribution of the aromatic structure, which may lower the local structural stability. In addition, Asn68Tyr is located in the exon-encoded functional domain that has been extensively studied, since neither benign variant (Ser72Pro and Ala82Thr) leads to a significant reduction in the endogenous UGDH levels, thus complementing the evidence of moderate pathogenicity (PM1) of the variant Asn68Tyr (Hengel et al., 2020).
In conclusion, we reviewed the clinical phenotypes and the genetic distribution of 24 patients with EIEE in Eastern China. The research results are similar to previously published cohort study data, which indicates that EIEE has a wide range of genetic and phenotypic heterogeneity, and the disease is mainly related to the abnormal function of ion channels. In addition, the case data of the UGDH variants were also reported in this cohort, enriching the disease and variant spectra of EIEE in the Chinese population. However, this cohort study has some limitations. First, the number of patients in the cohort was small, which may have introduced bias in statistical results. Second, this study did not include long-term follow-up data. Nevertheless, this study improves the clinicians' understanding of the characteristics and genetic causes of EIEE and expands the EIEE subtype spectrum of the entire Chinese population.
AUTHOR CONTRIBUTIONS
Liangliang Jiang and Fang Deng: Designed the study. Liangliang Jiang: Wrote the manuscript. Fang Deng: Revised the article. Liangliang Jiang, Shaohua Bi, Li Lin, and Fan He: Collecting and analyzed data. All authors contributed to the article and approved the submitted version.
ACKNOWLEDGMENTS
We are grateful to these patients and parents for their participation in this study.
FUNDING INFORMATION
This research was sponsored by the Natural Science Foundation of Anhui Province (No. 2108085MH262).
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
This study was approved by the Medical Ethics Committee of Children’s Hospital of Anhui Medical University (Anhui Provincial Children’s Hospital) (EYLL-2020-010).




