Diagnostic utility of integrated analysis of exome and transcriptome: Successful diagnosis of Au-Kline syndrome in a patient with submucous cleft palate, scaphocephaly, and intellectual disabilities
Abstract
Background
A weakness of exome analysis lies in inability to characterize aberrant splicing other than those involving consensus donor-acceptor sequence. To overcome this limitation, we developed a novel analytic method SAVNet that combines transcriptome and exome analysis which enabled the successful detection of carriers of splicing variants in the disease-causing genes of autosomal recessive disorders within a normal cohort. However, the clinical utility of the SAVNet analysis in delineating splicing defects in patients without a diagnosis has yet to be documented.
Method
We performed SAVNet analysis using the integrated analysis of exome and transcriptome analysis from the peripheral blood of the patient. The patient is an undiagnosed Japanese female patient with submucous cleft palate, scaphocephaly and intellectual disability with no words at 8 years of age. Dysmorphic features included a long face, a short palpebral fissure, thick lips with an open month, premaxillary hypoplasia, a depressed nasal bridge, and satyr ears.
Result
A SAVNet analysis showed that a heterozygous intronic variant located at the −10 position of exon 5 of the HNRNPK gene on chromosome 9 created a new splice acceptor sequence “ag” and led to the incorporation of 9 intronic nucleotides into the coding sequence. The mutant protein would have three extra amino acid residues, Leu-Leu-Gln, inserted within the critical KH domain. The patient was diagnosed as having recently delineated Au–Kline syndrome, which is characterized by cleft palate, craniosynostosis, and intellectual disability.
Conclusion
The successful molecular diagnosis of the presently reported patient illustrates the diagnostic utility of the SAVNet analysis as an innovative way of implementing an integrated exome-transcriptome analysis in clinical settings.
1 INTRODUCTION
Exome analysis is a powerful diagnostic tool that has revolutionized clinical molecular diagnostics. Nevertheless, the diagnostic sensitivity of exome analysis is far from being complete. An inherent weakness of exome analysis lies in its inability to characterize aberrant splicing other than the detection of mutations at the consensus donor/acceptor dinucleotides (ie, gt/ag). The use of whole transcriptome data for the sake of mutation detection was once considered to be promising, but successful examples have been largely confined to scenarios, in which the clinical diagnosis is relatively secure. Conversely, the clinical utility of a whole transcriptome analysis has yet to be established for situations, in which no provisional clinical diagnosis is available.
The utility of transcriptome analyses can be improved if RNA-seq data are analyzed in conjunction with the exome data, as exemplified in the recent success of the SAVNet method (Splicing-Associated Variants NET), which was developed for use in the field of cancer genomics (Shiraishi et al., 2018). We applied this method to screen for carriers of germline splicing variants in disease-causing genes of autosomal recessive disorders among a phenotypically normal cohort from the 1000 Genome Project (Yamada, Suzuki, Shiraishi, & Kosaki, 2019). Forty aberrant splicing events including exon skipping, the creation of a new splice site, and the use of a cryptic splice site in response to the disruption of the authentic site were detected in 1916 genes among 31 of the 179 subjects from the 1000 Genomes Project. The detection rate for provisionally truncating pathogenic variants was 20% higher than that for a conventional exome analysis. However, the clinical utility of the SAVNet analysis in delineating the molecular pathology of undiagnosed patients has yet to be published.
Here, we report a patient with syndromic clefting accompanied by intellectual disability and craniosynostosis who was successfully diagnosed using a SAVNet analysis. This diagnosis illustrates the diagnostic utility of integrated exome-transcriptome analyses in clinical settings.
2 PATIENT REPORT
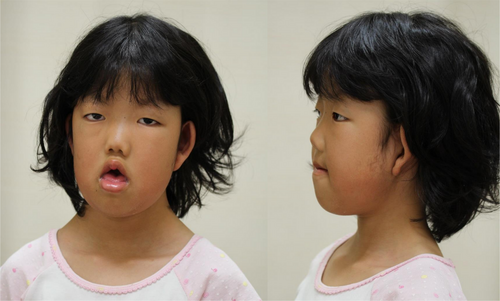
The patient was a second-born child of healthy and non-consanguineous Japanese parents with no significant family medical history. She was born at 41 and 1/7 weeks of gestation with no trouble during her perinatal period. Her birth weight was 2,948 g (−0.9 SD), her length was 48.0 cm (−1.1 SD), and her OFC was 34.0 cm (+0.24 SD). She underwent a submucous cleft palate repair at the age of 8 months and was found to have long QT syndrome at that time. At the age of 1 year and 10 months, she underwent a cranioplasty for scaphocephaly. She gained head control at 9 months, walked alone at the age of 2 years, and was incapable of excreting independently at the age of 8 years. She exhibited severe intellectual disability; at the age of 8 years, her speech development was equivalent to that of a 2-year old. Dysmorphic features included a long face, a short palpebral fissure, thick lips with an open month, premaxillary hypoplasia, a depressed nasal bridge, and satyr ears. (Figure 1).
3 MOLECULAR STUDIES
Informed consent from the parents and approval from the local institutional review board were obtained for the molecular studies. After written informed consent was obtained, peripheral blood samples were obtained from the patient and her parents.
An exome analysis was performed as previously reported (Takenouchi et al., 2015). In short, mapping of the sequence reads to the human reference genome (GRCh37) was performed according to the Burrows-Wheeler Aligner (BWA) (Li & Durbin, 2009) and the Genome Analysis Tool Kit (GATK) (McKenna et al., 2010) best-practice guidelines, as packaged in the integrated analysis suite variant tools (San Lucas, Wang, Scheet, & Peng, 2012). The called variants were annotated with SnpEff (Cingolani et al., 2012). No plausible candidate causative genes were suggested through the extensive filtering and annotation of 13,643 rare genomic variants with a minor allele frequency of less than 0.03 according to the ToMMo Japanese normal population database (Cingolani et al., 2012) (Fang et al., 2016).
Subsequently, a transcriptome analysis was performed using total RNA extracted from peripheral blood. The Globin-Zero Gold rRNA Removal Kit [Illumina] was used to remove rRNA according to the manufacturer's protocol. Paired-end RNA-Seq libraries were prepared using the Illumina TruSeq Standard mRNA Sample Prep kit [Illumina]. All the samples were sequenced on an Illumina HiSeq 2500 platform (100-nt read length). In total, we obtained 26 million paired end reads per sample.
The 13,643 rare genomic variants obtained from the exome analysis were fed into the SAVNet analysis (Ref, https://github.com/friend1ws/SAVNet) as the initial list of genome variants that were possibly associated with splicing alterations for each gene (Yamada et al., 2019). Two candidate variants from two genes, HNRNPK (OMIM #600712) chr9(GRCh37):g.86591976A>C and CAPN10 (OMIM #605286) chr2(GRCh37):g.241530465C>T, were obtained. The reverse strand of chromosome 9 surrounding the single nucleotide substitution chr9(GRCh37):g.86591976A>C defines the exon-intron boundary of HNRNPK (Figure 2). The nucleotide substitution corresponds to a change from “t” to “g” at the −10 position of exon 5 of HNRNPK. This base change created a new splice acceptor site “ag” and led to the incorporation of 9 intronic nucleotides into the coding sequence. The mutant protein would have three extra amino acid residues, Leu-Leu-Gln, inserted (p.52Lys_56AsninsLeuLeuGln). The three amino acid insertion occurred within the KH domain, which is one of three critical KH domains of the protein. The haploinsufficiency of HNRNPK has very recently been shown to be associated with syndromic cleft palate associated with craniosynostosis and intellectual disability and is now referred to as Au–Kline syndrome. Only 10 variants have been reported so far (Au et al., 2015) (Lange et al., 2016) (Miyake et al., 2017) (Dentici et al., 2018) (Au et al., 2018).
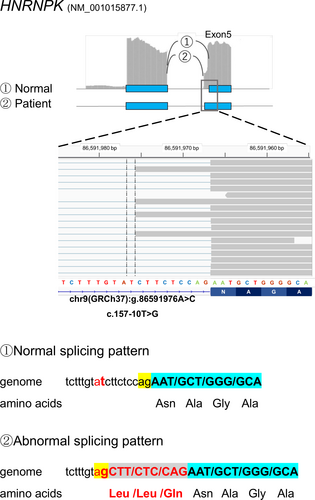
Concurrently, exome analysis of the patients yielded 56 de novo mutation variants including two variants, GPR50 and TMEM150A. Neither of the two genes were plausible candidates. The HNRNPK variant, chr9(GRCh37):g.86591976A>C detected by SAVNet analysis occurred de novo. Because of its intronic position at −10, exome analysis alone would not have predicted the HNRNPK as the prime candidate even though the probability score of the HNRNPK variant in inducing abnormal splicing was calculated to be positive by the Splice AI prediction program (Jaganathan et al., 2019). Meanwhile, the CAPN10 variant (NM_023085.3 c.470+37C>T) mentioned above was disregarded as a candidate gene because the variant was derived from the unaffected father. We concluded that the three amino acid insertion in HNRNPK was the cause of the patient's phenotype.
4 DISCUSSION
Here, we reported a patient with craniosynostosis, cleft palate, and intellectual disabilities whose diagnosis had remained unknown after a clinical evaluation and conventional exome analysis. A subsequent transcriptome analysis of mRNA derived from peripheral blood and an integrated analysis of the exome and transcriptome data using the SAVNet analysis detected two rare variants that were shown to be associated with abnormal splicing. One of the two variants resided in an intron of the HNRNPK gene, which is the causative gene for Au–Kline syndrome (AKS, OMIM # 616580). Since the specific combination of clinical features in the currently reported patient (ie, cleft palate, craniosynostosis, and intellectual disability) matched those of subjects with truncating variants of HNRNPK, it is very likely that the insertion of the three amino acid residues Leu-Leu-Gln diminished the function of the HNRNPK protein.
We have evaluated the RNA-seq data by another recently developed software OUTRIDER for mutation analysis from RNA-seq data (Brechtmann et al., 2018). The OUTRIDER software detects abnormal expression level due to aberrant splicing. We did not detect HNRNPK as expression outlier (data not shown). Normal expression level of HNRNPK can be accounted for by the observation the HNRNPK, chr9:g.86591976A>C variant led to in-frame change because in-frame change would not invoke nonsense-mediated mRNA decay.
HNRNPK is a component of an intronic splicing enhancer complex that activates the splicing of multiple genes including those associated with neurogenesis (Cao, Razanau, Feng, Lobo, & Xie, 2012). Hence, the splicing of multiple genes other than HNRNPK could have been affected. It is possible that the specific combination of dysmorphic features (ie, cleft palate and craniosynostosis) may have been caused by the aberrant splicing of downstream genes affecting palatogenesis and cranial suture formation.
In summary, we demonstrated the clinical utility of the SAVNet analysis in delineating the molecular pathology of an undiagnosed patient with syndromic cleft palate.
ACKNOWLEDGMENTS
We thank Ms. Chika Kanoe, Ms. Yumi Obayashi, and Ms. Keiko Tsukue for their technical assistance in the preparation of this article. This work was supported by Initiative on Rare and Undiagnosed Diseases (grant number JP17ek0109151) from the Japan Agency for Medical Research and Development, and by JSPS KAKENHI, Grant-in-Aid for Early-Career Scientists (grant number JP19K17342).
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION
The conception or design of the work: Mamiko Yamada, Kenjiro Kosaki. Acquisition of data: Mamiko Yamada, Chihiro Abe-Hatano, Kenji Kurosawa. Analysis of data: Mamiko Yamada, Yuichi Shiraishi, Hisato Suzuki, Tomoko Uehara, Kenjiro Kosaki. Interpretation of data for the work: Mamiko Yamada, Hisato Suzuki, Tomoko Uehara. Drafting the work: Mamiko Yamada. Revising it critically for important intellectual content: Kenjiro Kosaki, Toshiki Takenouchi.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Database of Pathogenic Variants at https://dpv.cmg.med.keio.ac.jp/dpv-pub/top, reference number DPVS: 13,135.2.