MYH2 myopathy, a new case expands the clinical and pathological spectrum of the recessive form
Roberta Telese and Serena Pagliarani have equally contributed to this work.
Abstract
Background
Hereditary myosin myopathies are a group of rare muscle disorders, caused by mutations in genes encoding for skeletal myosin heavy chains (MyHCs). MyHCIIa is encoded by MYH2 and is expressed in fast type 2A and 2B muscle fibers. MYH2 mutations are responsible for an autosomal dominant (AD) progressive myopathy, characterized by the presence of rimmed vacuoles and by a reduction in the number and size of type 2A fibers, and a recessive early onset myopathy characterized by complete loss of type 2A fibers. Recently, a patient with a homozygous mutation but presenting a dominant phenotype has been reported.
Methods
The patient was examined thoroughly and two muscle biopsies were performed through the years. NGS followed by confirmation in Sanger sequencing was used to identify the genetic cause.
Results
We describe the second case presenting with late-onset ophthalmoparesis, ptosis, diffuse muscle weakness, and histopathological features typical for AD forms but with a recessive MYH2 genotype.
Conclusion
This report contributes to expand the clinical and genetic spectrum of MYH2 myopathies and to increase the awareness of these very rare diseases.
1 INTRODUCTION
Hereditary myosin myopathies are a group of muscle disorders with variable age of onset and heterogeneous clinical features, caused by mutations in the skeletal muscle myosin heavy chain (MyHC) genes which are organized in a multigene cluster on chromosome 17 (Oldfors, 2007). In adult human skeletal muscle, there are three major MyHC isoforms: MyHC I (slow/ß-cardiac MyHC) encoded by MYH7 (OMIM *160760) and expressed in slow type 1 muscle fibers and in heart ventricles; MyHC IIa, encoded by MYH2 (OMIM *160740) and expressed in fast type 2A and 2B muscle fibers, and MyHC IIx, encoded by MYH1 (OMIM *160730) and expressed only in fast type 2B muscle fibers (Oldfors, 2007). MYH7 mutations have been associated with either hypertrophic or dilated cardiomyopathy, pure skeletal myopathies, and a combination of myopathy and cardiomyopathy (Oldfors, 2007). So far, MYH1 has not been associated to any genetic disease.
Mutations in MYH2 have been associated to myopathies with both dominant and recessive autosomal transmission (OMIM #605637). The autosomal dominant (AD) MyHC IIa myopathy is characterized by congenital joint contractures that resolve with time, late-onset and progressive proximal limb muscle weakness, ophthalmoplegia, and ptosis. The reduction in the number and size of type 2A fibers together with rimmed vacuoles are the main features of muscle biopsy (Cabrera-Serrano et al., 2015; D’Amico et al., 2013; Martinsson et al., 2000). The autosomal recessive (AR) MyHC IIa myopathy is characterized by mild, non-progressive, early onset muscle weakness, mild facial involvement, ophthalmoplegia and, occasionally, ptosis. Muscle biopsy shows a complete loss of 2A fibers along with unspecific myopathic changes such as fiber size variability, internalized nuclei, and interstitial fatty infiltration (Hernandez-Laın, Esteban-Perez, Domınguez -Gonzalez, & Cantero Montenegro, 2017; Lossos et al., 2005, 2013; Tajsharghi et al., 2010, 2014; Tsabari, Daum, Kerem, Fellig, & Dor, 2017; Willis et al., 2016).
Recently, a patient with homozygous recessive MYH2 mutation and phenotypic features consistent with the AD form was described (Findlay, Harms, Pestronk, & Weihl, 2018), opening the route to a possible new genotype-phenotype correlation.
The case we describe here shows overlapping features with the abovementioned case report (Findlay et al., 2018), thus contributing to widen the clinical and genetic spectrum of MYH2 myopathies, in particular of the AR form. It also broadens the knowledge of these very rare muscle diseases of which only few cases have been reported so far, globally scattered (Cabrera-Serrano et al., 2015; D’Amico et al., 2013; Hernandez-Laın et al., 2017; Lossos et al., 2005, 2013; Martinsson et al., 2000; Tajsharghi et al., 2010, 2014; Tsabari et al., 2017; Willis et al., 2016).
2 METHODS
2.1 Ethical compliance
The study has been approved by the local ethic committee.
2.2 Muscle biopsies
Biopsy specimens from biceps brachialis were frozen in isopentane cooled with liquid nitrogen. Routine histological and histochemical analyses were performed as standard (Dubowitz & Sewry, 2007; Prelle et al., 1998) as well as ultrastructural analyses (Ripolone et al., 2015).
Myosin heavy chains immunohistochemical analysis was performed using the following commercial monoclonal antibodies: Lyophilized Mouse monoclonal antibody MyHC (slow) (Novocastra; dilution 1:50); Anti-MYH1 antibody [MY-32] (Abcam, www.abcam.com; dilution 1:100) and Anti-Myosin 2, clone 5B11.1 (Merck Millipore; dilution 1:300).
2.3 Genetics
Next-generation sequencing targeted gene panel was used to investigate the coding regions of 58 genes responsible of non-syndromic muscle disorders. Variant annotation was performed as previously described (Savarese et al., 2016). In silico analyses of polymorphic human variations included consultation of the following databases: dbSNP (http://www.ncbi.nlm.nih.gov/projects/SNP/), EVS6500 (evs.gs.washington.edu), ClinVar (http://www.ncbi.nlm.nih.gov/clinvar/), ExAC Browser (exac.-broadinstitute.org/), and 1000G dataset (www.1000genomes.org). The damaging effects of the single nucleotide variants were evaluated in silico using Mutation Taster (www.mutationtaster.org), PolyPhen-2 (genetics.bwh.harvard.edu/pph2/), SIFT (http://sift.jcvi.org), and PANTHER (http://www.pantherdb.org/). Full dataset of gene variants generated in this study can be found in the ClinVar genetic repository (https://www.ncbi.nlm.nih.gov/clinvar/; submission code SUB5328174).
mRNA was extracted from skeletal muscle (brachial biceps) from patient and control specimens using ReliaPrepTM RNA Tissue Miniprep System (Promega) and was reverse transcribed (1.5 µg of total mRNA) using Ready-To-Go™ You-Prime First-Strand Beads (GE Healthcare) following the manufacturer's instructions.
We analyzed the proportion of the three major MyHC isoforms using the method described by Tajsharghi and colleagues (Tajsharghi et al., 2002). Briefly, we used a couple of primer that amplify simultaneously one portion of the cDNA of MyHCI (518 bp), MyHCIIa (527 bp), and MyHCIIx (521 bp). The forward primer was FAM labeled and PCR amplification was performed as described (Tajsharghi et al., 2002). PCR fragments were separated on a 3130 Genetic Analyzer ABIPRISM (Applied Biosystem, www.thermofisher.com) and analyzed using GeneMapper 5 (Applied Biosystem, www.thermofisher.com).
Then, the cDNA was amplified and sequenced to verify the presence of both mutated alleles using the following primers: For-GACAAAAACTCCTGGTGCCATGG and Rev-CGGCTTCAGCCTGAACTTGG for the amplification of exons 19–22; For-GGAGATAAAAGCCAAGAACGC and Rev-GTTCAAGAGATGCCTCTGCTTC for the amplification of exons 30–33 (ref. seq.: NM_017534; PCR conditions are available on request).
3 RESULTS
3.1 Case report
Written informed consent, approved by Ospedale Maggiore Policlinico, Milano, was obtained from the patient and his relatives.
The patient is a 43-year-old man born from non-consanguineous, healthy parents. He has two healthy children, aged 3 and 7 years. He had a normal psychomotor development and no joint contractures at birth. He could play sports during adolescence though with mild exercise intolerance.
He started complaining about fatigability at the age of 17 when he noticed marked difficulty in playing sports and running due to lower limb muscular weakness (ileopsoas, tibialis anterior, and gastrocnemious: Medical Reasearch Council [MRC] 4+ bilaterally) and worsening clumsiness. Serum creatine kinase (CK) was normal. Electromyography (EMG) revealed complex repetitive discharges and sporadic neuromyotonic discharges. At the age of 20, the patient presented slight ptosis and general reduction in muscle bulk that was more marked in the lower limbs. Chest X-ray examination did not show scoliosis. Other neuromuscular diseases that could present with complex repetitive discharges and sporadic neuromyotonic discharges at the EMG have been excluded. Miastenia gravis was excluded due to negative EMG repetitive nerve stimulation and absence of specific serological antibody. CIDP was excluded on the basis of international criteria (Van den Bergh et al., 2010).
Thereafter, the patient developed a progressive weakness of orbicularis and oral muscles, resulting in ophthalmoparesis and mild diplopia, clearly evident at the age of 25 years. Swallowing problems were never referred. Over the years, he developed moderate scapular winging and waddling gait. In his 30 s, the patient began to complain about difficulty in climbing stairs; on medical examination, muscle weakness was present in both proximal and distal muscles (iliopsoas: MRC 4; tibialis anterior and gastrocnemius: MRC 3). CK levels increased up to 1,500 U/L and EMG presented a diffuse myopathic pattern. Complete ophtalmoplegia was present at that time. Cardio-pulmonary examination was normal. No malignancy has ever been detected during the whole follow-up.
3.2 Histopathological analysis and muscle MRI
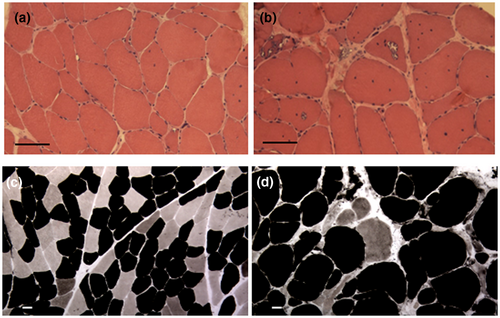
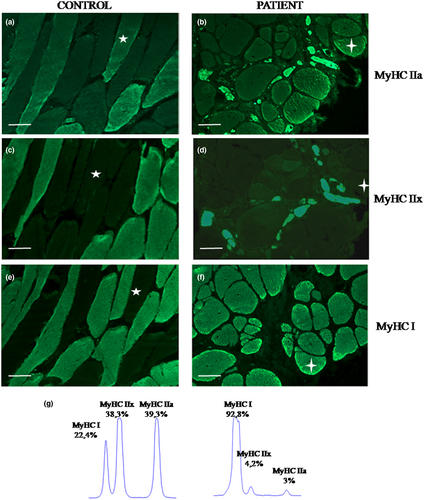
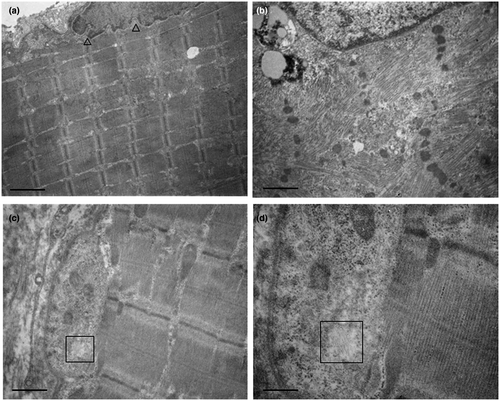
The patient underwent two biopsies of the biceps brachii muscle. The first one, performed when he was 17 years old, showed scattered mildly hypotrophic fibers and many internal nuclei (Figure 1a). The second one, performed at the age of 39 years, showed rimmed vacuoles, severe fiber size variability, scattered necrotic fibers, fatty infiltration, and few 2B fibers (Figure 1b,d). ATPase staining and immunofluorescence for myosin isoforms showed marked predominance of type 1 fibers and few type 2 fibers. The latter were type 2B fibers, co-expressing both MyHCIIa and MyHCIIx (Figures 2b,d,f, and 1d). No type 2A fibers have been detected. Ultrastructural examination of the first biopsy showed scattered nuclei indentations (Figure 3a); the second biopsy showed rimmed vacuoles, chaotic myofibrillar organization, and myofilament nuclear inclusions (Figure 3b–d).
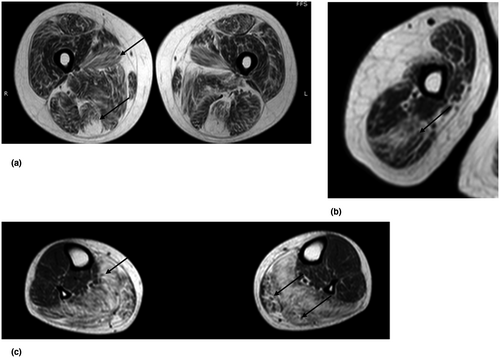
A muscle MRI was performed at the time of the second biopsy when the patient was 39. Axial TSE T1 and STIR T2 sequence targeted to pelvic girdle, lower limbs, and right arm were obtained. The study showed diffuse fibro-adipose substitution that was particularly overt in lumbar paraspinal muscles and in the gluteus maximus. In thighs, fibro-adipose replacement was prominent in the medial and posterior compartments (mostly in adductor longus and semitendinosus), while quadriceps were relatively spared. In calves, soleus and gastrocnemius were deeply substituted by fibrous tissue. Triceps brachii was the most involved muscle in the arm. No inflammation was detected in any of the examined muscles (Figure 4).
3.3 Genetic analysis
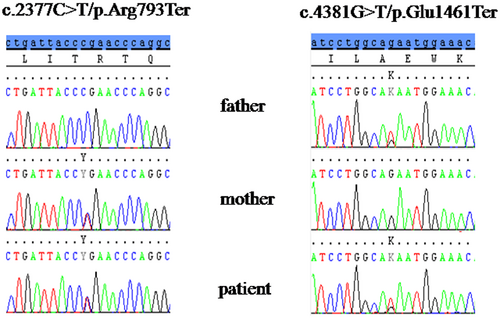
Genetic analysis for oculopharingeal muscular dystrophy (PABPN1), hereditary inclusion body myopathy (GNE), facio-scapulo-humeral dystrophy (D4Z4 fragment length screening), for late-onset Pompe disease (GAA), myotonic dystrophy type 1 and 2 (DMPK and ZNF9) resulted normal. NGS targeted gene panel was used to investigate the coding regions of 58 genes responsible for non-syndromic muscle disorders and led to the identification of two novel truncating mutations in MYH2 (ref. seq.: NM_017534) in compound heterozygosity: c.2377C>T/p.Arg793Ter in exon 21 and c.4381G>T/p.Glu1461Ter in exon 32. Mutations were confirmed by Sanger sequencing in the patient and his parents: the mother was heterozygous for c.2377C>T and the father was heterozygous for c.4381G>T (Figure 5). Clinical examination of both parents was normal: they did not show any kind of muscle involvement.
We analyzed the relative expression of MyHCI, MyHCIIa, and MyHCIIx transcripts in a muscle sample from the second brachial biceps biopsy as previously described (Tajsharghi et al., 2002). In the patient MyHCI mRNA was predominant (92.8%), whereas MyHCIIa and MyHCIIx mRNAs represented the 3% and the 4.2%, respectively (mean of three independent PCRs; cDNA was from two independent reverse transcriptions; Figure 2g). In the control the distribution of the three mRNAs was different: 22.4% of MyHCI mRNA, 38.3% of MyHCIIx Mrna, and 39.3% of MyHCIIa transcript (sample: brachial biceps). Sequencing of MyHCIIa cDNA was performed to evaluate the presence of nonsense-mediated mRNA decay (NMD) and revealed the presence of both mutated alleles.
4 DISCUSSION
Myosin heavy chain IIa myopathies are very rare diseases and exact data on prevalence and incidence are not well known: to date, 21 AD (Cabrera-Serrano et al., 2015; D’Amico et al., 2013; Martinsson et al., 2000) and 34 AR (Hernandez-Laın et al., 2017; Lossos et al., 2005, 2013; Tajsharghi et al., 2010, 2014; Tsabari et al., 2017; Willis et al., 2016) cases have been reported. Clinical and morphological phenotypes are continuously updated by new reports.
Recently, a case was reported by Findlay and colleagues (Findlay et al., 2018) describing a patient with homozygous p.Arg246His mutation in the MYH2 gene that affects the myosin motor domain and shows rimmed vacuoles and marked myopathic alterations at muscle biopsy. Residual MyHCIIa expression was demonstrated in hybrid fibers. The clinical phenotype was characterized by ophthalmoplegia and late-onset progressive muscle weakness.
Similar clinical and pathological findings were present also in our patient (see Table 1). Disease onset in adulthood, progressive muscle weakness, elevated CK levels, ophthalmoplegia, mild facial weakness, and histological features recall those described by Findlay and colleagues. Moreover, the absence of congenital contractures and the pattern of diffuse weakness are characteristics more similar to those described for AR patients. The first EMG revealed complex repetitive discharges and sporadic neuromyotonic discharges, no longer present in the second EMG: neuromyotonic discharges have never been described in myosin diseases and are characteristics of other neuromuscular diseases excluded in our patient; these findings were probably aspecific. Although muscle biopsy showed rimmed vacuoles, dystrophic changes, and fibro-fatty substitution that are typical of AD cases, we detected also the absence of type 2A fibers as in biopsies of AR patients so far described. However, our patient has two truncating mutations that led to the synthesis of a residual mutated protein that determines a histopathological picture similar to that of AD patients, as described up to now.
| Characteristics | Proband | Dominant phenotype | Recessive phenotype |
|---|---|---|---|
| Onset | Adulthood | Adulthood | Childhood |
| Congenital contractures | Absent | Present | Absent |
| Scoliosis | Absent | Infrequent | Frequent |
| Weakness |
Distal and proximal Legs > Arms |
Proximal > distal Legs > arms |
Distal and proximal |
| Facial weakness | Mild | Infrequent | Present |
| Ophthalmoplegia | Present | Present | Present |
| CK level (U/L) | 10x (1,500 U/L) | 2–10x | Normal to 4x |
| Disease progression | Progressive | Progressive | Non progressive |
| Biopsy | Dystrophic changes, severe fibro-fatty substitution, rimmed vacuoles. Absence of 2A fibers with residual MyHCIIa. Nuclear inclusion filaments. | Dystrophic changes, severe fibro-fatty substitution, rimmed vacuoles. Reduced number and size of type 2A fibers. Nuclear inclusion of 15–21 filaments. | Myopathic changes with fatty infiltration. Type 1 predominance or uniformity with reduced or absent MyHCIIa and 2A fibers. Disruption of the myofibrillar network. |
- Abbreviations: AD, autosomal dominant; AR, autosomal recessive; CK, creatine kinase.
Our patient carried two new heterozygous truncating mutations: p.Arg793Ter, which is located in the myosin motor domain and interrupts the protein 1148 amino acids before the natural stop codon, and p.Glu1461Ter, which interrupts the coiled coil rod domain 480 amino acids before the stop codon. Surprisingly, despite double truncating mutations, our patient expressed minor amounts of MYH2 mRNA (Figure 2g) that led to the synthesis of residual myosin IIa in type 2B fibers, as shown by immunofluorescence (Figure 2b).
The NMD pathway is able to select and degrade mRNAs harboring premature termination codons (PTCs), so it was possible that both mutated alleles were degraded by NMD, but this is in contrast with the results of immunofluorescence assay. At first, we hypothesized that the allele carrying p.Arg793Ter was degraded by NMD and that the residual protein shown by the immunofluorescence was produced by the other mutated allele, which although mutated was able to produce a reduced amount of truncated and non-functional, or partially functional, protein that can be detected by the MyHCIIa antibody. Sequencing of skeletal muscle mRNA ruled out this hypothesis, showing the presence of both MYH2 mutated alleles, although in smaller amounts than the control (patient: 3% vs. control: 39%), as shown in Figure 2g. The low amount of both mutated MyHCIIa mRNAs in presence of two truncating mutation is probably due to an incomplete efficiency of NMD: some mRNAs escape degradation and are translated. Indeed, a different NMD efficiency is documented due to the distance between PTC and exon-junction complex, the number of exon-junction complexes downstream the PTC, and the exon sequence downstream PTC; furthermore, the NMD efficiency is variable in different tissues and in different cells (Hoek et al., 2019; Linde, Boelz, Neu-Yilik, Kulozik, & Kerem, 2007; Zetoune et al., 2008).
Tajsharghi and colleagues first hypothesized the pathogenic role of the mutated protein in AD patients, arguing that clinical symptoms are linked to the amount of protein present in muscle during development and growth: that is, joint contractures, typically present at birth, are due to the high expression of MyHCIIa in fetus, whereas the further downregulation of the protein in childhood explains the resolution of contractures (Tajsharghi et al., 2002). MyHCIIa expression increases in adulthood, particularly in extraocular muscles, explaining the adult-onset of ocular palsy and weakness of proximal muscles (Cabrera-Serrano et al., 2015; D’Amico et al., 2013). Rimmed vacuoles are typical of the AD form and selectively occur in muscle fibers expressing high levels of MyHCIIa (Tajsharghi et al., 2002).
Our case and the previous one by Findlay demonstrate that AR patients can show a clinical phenotype partially overlapping with AD patients (progressive myopathy, ptosis, severe ophthalmoparesis) and a muscle histopathology similar to that described in these patients (rimmed vacuoles and disruption of myofibrils organization in the sarcomere). This is probably due to the combination of the lack of WT MyHCIIa expression and the residual production of mutated proteins.
We had the outstanding opportunity to follow-up this patient for over two decades both from the clinical and the morphological point of view, recording a unique disease history. At the clinical examination the chronic progression was evident and was also confirmed by the two muscle biopsies: electronic microscopy performed in both samples showed how the slow accumulation of abnormal myosin protein had progressively subverted the sarcomeric arrangement up to a complete myofibrillar disorganization.
The lack of a specific morphological pattern and the absence of congenital joint contractures made this patient's early diagnosis difficult. Clinical-pathological reports are of pivotal importance to collect information that can guide and help physicians and researchers to broaden their knowledge of these rare diseases and to investigate in depth the pathogenetic and pathological mechanism.
In conclusion, the phenotype of MyHCIIa myopathy, that so far seemed exist in two well-established and rather different forms, could be more similar to a continuum of symptoms between two extremes that nowadays are classified as the AD and AR forms. This report wishes to reinforce physicians and geneticist attention on this disease whose presentation could vary from classical patterns.
ACKNOWLEDGMENTS
The Authors wish to acknowledge the Associazione Centro Dino Ferrari, the Biobank of skeletal muscle, peripheral nerve, DNA, and cell cultures, member of the Telethon Network of Genetic Biobanks and the Eurobiobank Network. Authors also thank Mr. Germano Mosconi (1932-2012) for his unique support.
CONFLICT OF INTEREST
Authors declare absence of any conflict of interest.
AUTHORS’ CONTRIBUTION
RT, SP, LP contributed to the conception and writing of the work; AL and LP contributed to the clinical evaluation of the patient; SP, DC, and FMS contributed to genetic analysis; PC, GF, and NG contributed to pathological processing of muscle biopsy; GC and CC contributed to imaging studies; GPC and MS contributed to the final revision of the work. All Authors have approved the submitted version and have agreed both to be personally accountable for the author's own contributions.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.