Chinese collaborative study of survival analysis in 980 patients with AL amyloidosis
Hokhim Yau, Liye Zhong and Sheng Li contributed equally to this work.
Abstract
Background
The prognosis of patients with light-chain amyloidosis (AL) has improved markedly in the past decade in China; whether the current staging systems are suitable to predict the overall survival (OS) of the patients remains undetermined.
Methods
Based on 980 biopsy-proved AL patients with 5-year follow-up from China Registration Network for Light-chain Amyloidosis, we evaluated the efficacy of existing staging systems and developed a new stratification model. This involved analyzing parameters such as N-terminal pro-brain natriuretic peptide (NT-proBNP) thresholds and estimated glomerular filtration rate (eGFR).
Results
We found that 30% patients were classified as stage I, 25% as stage II, 26% as stage III, and 19% as stage IV disease using the Mayo 2012 staging system, with varying median OS values. However, the observed median OS values were notably higher than previously reported. By incorporating NT-proBNP thresholds and eGFR values, we developed a four-stage score system. With this new model, 41.6% patients were reclassified to stage I, 34.3% to stage II, 17.8% to stage III, and 6.3% to stage IV disease, with adjusted median OS values.
Conclusion
The new stratification model exhibited improved consistency compared to traditional staging systems. It effectively identified patients with the best and worst prognoses, even among those receiving comprehensive treatment. Specifically, NT-proBNP levels exceeding 9000 pg/mL combined with an eGFR less than 60 mL/min/1.73 m2 proved superior in prognosticating patient outcomes. Overall, the median OS of AL amyloidosis patients in China has significantly improved, underscoring the need for tailored prognostic models in clinical practice.
Abbreviations
-
- AL
-
- systemic light chain amyloidosis
-
- ASCT
-
- autologous stem cell transplantation
-
- AUC
-
- area under the curve
-
- CI
-
- confidence interval
-
- CRENAL
-
- China Registration Network for Light-chain Amyloidosis
-
- cTNI
-
- cardiac troponin I
-
- cTNT
-
- cardiac troponin T
-
- dFLC
-
- difference of free light-chain
-
- eGFR
-
- estimated glomerular filtration rate
-
- NR
-
- not reached
-
- NRI
-
- Net reclassification improvement
-
- NT-proBNP
-
- N-terminal pro-brain natriuretic peptide
-
- OS
-
- overall survival
1 INTRODUCTION
Systemic light chain (AL) amyloidosis is a multisystem disease caused by the deposition of misfolded immunoglobulin light chains produced by clonal plasma cells or B cells [1]. Despite historically poor outcomes, the prognosis of AL amyloidosis patients has been markedly improved in the past decade due to earlier diagnosis [2], better risk stratification, improvement in supportive care [3, 4], and availability of novel treatments [5, 6]. The prognosis of AL amyloidosis patients is highly dependent on the extent and severity of organ involvement. However, among patients with similar clinical presentations, outcomes can vary dramatically. The development of a risk classification system is crucial for accurately assessing patient prognosis and may help in selecting the optimal treatment approach. In clinical practice, National Comprehensive Cancer Network (NCCN) guidelines for AL amyloidosis prognosis staging were proposed by the Mayo Clinic in 2012. The detection of biological indicators used to predict survival of patients with AL amyloidosis is mainly based on the use of N-terminal pro-brain natriuretic peptide (NT-proBNP), cardiac troponin T (cTNT), and difference of free light-chain (dFLC) [7]. The number of diagnosed AL patients has increased drastically in the last 5 decades according to the China Registration Network for Light-chain Amyloidosis (CRENAL), which collaborated with 29 main medical centers all around China. However, the alterations in median overall survival (OS) across different stages have not been thoroughly investigated. In the current study, we reported that the median OS of Chinese patients was significantly longer than previous reports [7]. The patients who received bortezomib-based treatments showed markedly increased median OS compared with those without. NT-proBNP of more than 9000 pg/mL combined with an estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m2 predicts the poorest prognosis of AL patients, even for those receiving a new regime.
2 METHODS
2.1 Study design
One thousand two hundred and sixty-nine diagnosed patients with AL amyloidosis from January 2000 to the 30th of September, 2020 were retrospectively assessed using the database of CRENAL (https://www.boshicloud.com/) in the current study. Figure S1 shows the cases from 29 centers distributed around China. All the data of patients at the time of initial diagnosis and their treatment and prognosis were recorded. The study was performed according to the principles of the Declaration of Helsinki (as revised in 2013) and approved by the Medical Ethics Committee of Guangdong General Hospital, Guangdong Academy of Medical Sciences (No. GDREC2019844H). All participants provided written informed consent.
2.2 Population
All patients were diagnosed with AL amyloidosis by tissue biopsy. The deposits were characteristic of the AL type according to immunohistochemistry, immunofluorescence, or laser microdissection with mass spectrometry-based proteomic analysis [8]. The endpoint of the study was patient survival, which was defined as the time from establishment of pathological diagnosis to death/last follow-up. The definition of organ involvement was based on the noninvasive consensus diagnostic criteria according to the 2005 ISA criteria [9]. The exclusion criteria were: follow-up time <3 months (death within 3 months was not excluded), lack of survival endpoint, and data such as cTNT, cardiac troponin I (cTNI), NT-proBNP, dFLC, and eGFR were not recorded.
2.3 Statistical analysis
The baseline characteristic categorical variables were expressed as a percentage of the total; continuous variables were expressed as median and the 10th–90th percentile range. Cox proportional hazard analysis was used to determine the factors affecting OS. The definition of OS was the time from diagnosis until death or the time until when the patient was still alive at the last follow-up. The X-tile 3.6.1 software [10] was used to determine the best cut-off value of p < 0.05 in Cox multiple regressions. Logistic regression was applied to calculate the optimal critical value for the prediction probability of all death events. The receiver operating characteristic (ROC) analysis was applied to determine the correlation between the predicted probability and the death event, and the area under the curve (AUC) and confidence interval (CI) were determined to identify the combination with the highest goodness of fit. The ROC curve was applied to compare the AUC and 95% CI, and the p value was compared based on the DeLong test. Net reclassification improvement (NRI) was used to assess the global performance of the new score system and Mayo score systems. All analyses were based on the R software (version 4.0.2) and GraphPad Prism (version 8.3.0).
3 RESULTS
3.1 Patients' characteristic
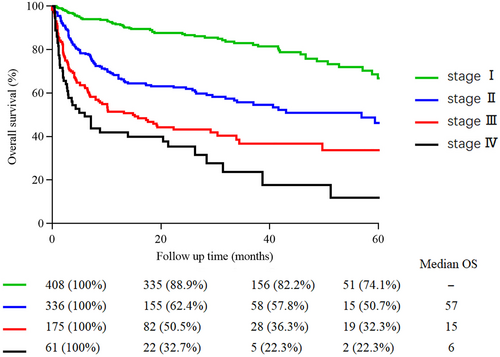
Nine hundred and eighty cases were finally included for analysis, 137 (10.8%) cases lacking a survival endpoint, and 152 (11.8%) cases with a follow-up period of <3 months or severely incomplete data were excluded. The baseline characteristics are shown in Table 1. The estimated median follow-up period for the entire cohort was 52 months (95% CI: 48–56 months). At the last follow-up time, 645 patients (65.8%) were alive, and the median follow-up period was 42 months 731 (74.6%) patients presented with renal involvement and 649 (66.2%) with cardiac involvement. Patients received a bortezomib-based regimen in 473 (48.3%) patients and a non-bortezomib regimen in 507 (51.7%) patients. Non-bortezomib regimens included autologous stem cell transplantation (ASCT), melphalan-, thalidomide-, lenalidomide-based regimens, and others. As depicted in Table 2, the median OS of patients at Mayo 2012 stage IV received a bortezomib-based regimen was 26 months, and the non-bortezomib regimen was 6.8 months. Those data suggest that the median OS of AL patients at different stages has largely improved after comprehensive treatment.
| Characteristic | No. of patientsa (%) | Median (95% CI) | Rangeb |
|---|---|---|---|
| Age (yr) | 980 (100) | 59 (58–60) | 46–70 |
| Sex, female (%) | 980 (100) | 335 (36.6) | – |
| SBP (mmHg) | 980 (100) | 109 (106–110) | 90–134 |
| DBP (mmHg) | 980 (100) | 70 (69–70) | 56–84 |
| cTnI (ng/mL) | 851 (86.8) | 0.05 (0.04–0.06) | <0.01–0.36 |
| cTnT (ng/mL) | 153 (15.6) | 0.08 (0.06–0.10) | 0.01–13.52 |
| NT-proBNP (pg/mL) | 980 (100) | 2212 (1892–2652) | 110–12300 |
| LV ejection fraction (%) | 896 (91.4) | 62 (61–63) | 43–73 |
| Urine protein (g/24 h) | 888 (90.6) | 2.8 (2.4–3.2) | 0.14–9.71 |
| Creatinine (μmol/L) | 980 (100) | 80 (78–83) | 53–181 |
| eGFR (mL/min/1.73 m2) | 980 (100) | 85.0 (82.8–87.0) | 32.3–108.0 |
| Serum albumin (g/L) | 980 (100) | 30 (30–32) | 19–42 |
| Total bilirubin (μmol/L) | 978 (99.8) | 10.4 (10.0–11.0) | 4.8–26.7 |
| Alkaline phosphatase (U/L) | 910 (92.9) | 84 (81–86) | 54–227 |
| dFLC (mg/L) | 851 (86.8) | 140.8 (127.0–169.1) | 19.7–893.2 |
| κ/λ (%) | 851 (86.8) | 0.16 (0.14–0.21) | 0.02–16.98 |
| Bone marrow plasma cells (%) | 864 (88.2) | 4.0 (3.5–4.5) | 0.5–9.5 |
| No. of organs involved | 686 (70) | 2 (2–3) | 1–4 |
| Treatment regimen | |||
| Bortezomib-based regimen | 473 (48.3) | – | – |
| Melphalan-based regimen | 107 (10.9) | – | – |
| Thalidomide-based regimen | 75 (7.7) | – | – |
| Lenalidomide-based regimen | 28 (2.9) | – | – |
| ASCT | 45 (4.6) | – | – |
| Others | 156 (15.9) | – | – |
| No treatment | 96 (9.7) | – | – |
- Abbreviations: AL, light chain; ASCT, autologous stem cell transplantation; CI, confidence interval; cTnI, cardiac troponin I; cTnT, cardiac troponin T; DBP, diastolic blood pressure; dFLC, difference of free light chain; eGFR, estimated glomerular filtration rate; LV, left ventricular; No., number; NT-proBNP, N-terminal pro-brain natriuretic peptide; SBP, systolic blood pressure; κ/λ, kappa/lambda ratio.
- a Patients with data available.
- b 10th to 90th percentiles.
| Prognostic model | Criteria | Stage | Median overall survival in months | |||
|---|---|---|---|---|---|---|
| Reported | Study in CRENAL | Bortezomib-based regimen | Non-bortezomib regimen | |||
| Mayo 2012 stage | cTnT<0.025 ng/mL | Stage I | 94.1 (95% CI: 64–154) | NR | NR | NR |
| NT-pro-BNP<1800 pg/mL | Stage II | 40.3 (95% CI: 24–59) | 79 (95% CI: 55–91) | NR | 79 (95% CI: 33–126) | |
| dFLC<180 mg/dL | Stage III | 14 (95% CI: 11–18) | 34 (95% CI: 24–44) | NR | 28 (95% CI: 11–45) | |
| Stage IV | 5.8 (95% CI: 5–7) | 9 (95% CI: 3–14) | 26 (95% CI: 4–49) | 6.8 (95% CI: 0–13) | ||
- Abbreviations: CI, confidence interval; cTnI, cardiac troponin I; cTnT, cardiac troponin T; dFLC, difference of free light chain; mo, months; NR, not reached; NT-proBNP, N-terminal pro–B-type natriuretic peptide.
3.2 Overall survival of patients with or without organ involvement
Kaplan–Meier curves for OS among the CRENAL cohort (n = 980) patients based on organs involved are shown in Figure 1a. Our data showed that the median OS of patients without renal or cardiac involvement was not reached. The median OS of patients with renal involvement only proved by the kidney biopsy was 72.9 and 74.5 months for those by clinical diagnostic criteria (p = 0.673). Patients with cardiac involvement only or more showed the worst prognosis, with a median OS of 31.8 months. Our data provided strong evidence that OS of AL patients markedly correlated with heart and kidney involvement, and cardiac involvement was of utmost importance in determining the prognosis.
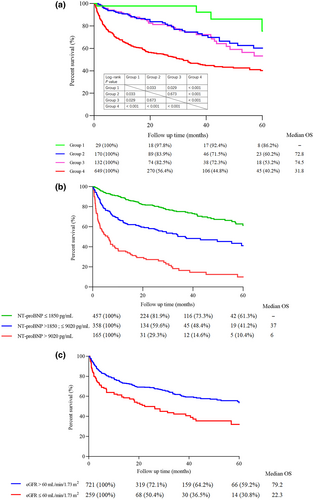
OS of AL amyloidosis patients of the CRENAL cohort. (a) OS of AL amyloidosis patients in groups of different organs involvement. Group 1: Without kidney or heart involvement. Group 2: With kidney involvement proved by renal biopsy and without heart involvement. Group 3: With kidney involvement proved by the clinical diagnostic criteria and without heart involvement. Group 4: With cardiorenal involvement or with other organs involvement. The number of patients at risk (survival proportions) at each time point is shown below the x-axis. MS for median survival time is shown on the bottom right of the x-axis. Survival curves were compared using the log-rank test. (b) OS stratified by presenting NT-proBNP (i.e., significantly poorest OS for patients with NT-proBNP >9020 pg/mL). (c) OS stratified by eGFR. AL, light chain; CRENAL, China Registration Network for light-chain Amyloidosis; eGFR, estimated glomerular filtration rate; MS, median survival time; NT-proBNP, N-terminal pro-brain natriuretic peptide; OS, overall survival.
3.3 Prognostic variables
The risk factors of OS were screened by univariate and multivariate analysis (Table 3). Using the maximal chi-square statistics approach, all independent risk factors were transferred into two cut-off values as simple single-factor models shown in Figures S2 and S3. Table S1 shows the correlation between the cut-off value and risk ratio of different prognostic factors. All single-factor models were measured with ROC analysis (Figure S4). NT-proBNP demonstrated the highest AUC of 0.740, which is significantly different from cTNI 0.713 (p = 0.019). AUC of dFLC, total bilirubin, systolic blood pressure, bone marrow plasma cells, eGFR, alkaline phosphatase, and age were 0.650, 0.620, 0.613, 0.611, 0.604, 0.598, and 0.558, respectively. Because NT-proBNP was the main predictor with two cut-off values, separately combined with other predictors as secondary predictors were reformed to compare the prediction value (Figure S5), and finally, five compound models were formed. On the maximal chi-square statistics approach result, predictors significantly associated with poorer OS were NT-proBNP ≥1850 or ≥9020 pg/mL, SBP <98 mmHg, cTNI ≥0.07 ng/mL, eGFR ≤60 mL/min/1.73 m2, total bilirubin ≥17.3 μmol/L, dFLC ≥99.2 mg/L and bone marrow plasma cells ≥8.5%. Multivariate Cox analysis of the risk coefficient for patients with AL amyloidosis is given in Table 4. The integrating models of NT-proBNP combined with cTNI or eGFR were superior to other combinations in predicting prognosis (with an AUC of 0.756 for the combination of NT-proBNP and cTNI, and an AUC of 0.753 for NT-proBNP and eGFR). No difference was seen in ROC analysis between the other two combined models (Figure 2a).
| Prognostic factor | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Sex (female) | 0.811 (0.646–1.018) | 0.102 | Not included | |
| Age (per 1 yr older) | 1.017 (1.005–1.030) | 0.005 | 1.025 (1.002–1.046) | 0.034 |
| SBP (per 1 mmHg greater) | 0.976 (0.968–0.981) | <0.001 | 0.972 (0.955–0.989) | 0.001 |
| DBP (per 1 mmHg greater) | 0.972 (0.962–0.982) | <0.001 | 1.026 (0.997–1.056) | 0.055 |
| cTnI (per 1 ng/mL greater) | 1.408 (1.219–1.626) | 0.001 | 1.005 (1.001–1.008) | 0.006 |
| cTnT (per 1 ng/mL greater) | 0.959 (0.735–1.250) | 0.624 | Not included | |
| NT-proBNP (per 100 pg/mL greater) | 1.121 (1.089–1.241) | <0.001 | 1.103 (1.101–1.104) | <0.001 |
| LV ejection fraction (per 1% greater) | 0.960 (0.952–0.969) | <0.001 | 0.991 (0.975–1.008) | 0.837 |
| Urine protein (per 1 g/24 h greater) | 0.998 (0.997–1.001) | 0.028 | 0.967(0.917–1.018) | 0.103 |
| Creatinine (per 1 μmoL/L greater) | 1.001 (1001–1.002) | 0.003 | 0.998 (0.994–1.001) | 0.352 |
| eGFR (per 1 mL/min/1.73 m2 greater) | 0.990 (0.986–0.994) | <0.001 | 0.989 (0.979–0.999) | 0.037 |
| Serum albumin (per 1 g/L greater) | 1.014 (1.001–1.026) | 0.032 | 0.980 (0.956–1.004) | 0.103 |
| Total bilirubin (per 1 μmoL/L greater) | 1.010 (1.008–1.013) | <0.001 | 1.006 (1.002–1.012) | 0.016 |
| Alkaline phosphatase (per 1 U/L greater) | 1.005 (1.003–1.007) | <0.001 | 1.032 (1.021–1.042) | 0.003 |
| dFLC (per 1 mL/L greater) | 1.011 (1.008–1.015) | 0.692 | 1.009 (1.002–1.026) | 0.013 |
| κ/λ (per 1% greater) | 1.000 (0.999–1.001) | 0.761 | Not included | |
| Bone marrow plasma cells (per 1% greater) | 1.025 (1.025–1.051) | <0.001 | 1.07 (1.041–1.099) | <0.001 |
| Bortezomib-based regimen | 1.004 (0.802–1.257) | 0.969 | ||
- Abbreviations: CI, confidence interval; cTnI, cardiac troponin I; cTnT, cardiac troponin T; DBP, diastolic blood pressure; dFLC, difference of free light chain; eGFR, estimated glomerular filtration rate; κ/λ, kappa/lambda ratio; LV, left ventricular; No., number; NT-proBNP, N-terminal pro-brain natriuretic peptide; SBP, systolic blood pressure.
| Prognostic factor | Comparison | HR (95% CI) | P |
|---|---|---|---|
| NT-proBNP (ng/L) | ≥1850 vs. <1850 | 3.902 (2.939–5.180) | <0.001 |
| ≥9020 vs. <9020 | 4.918 (3.791–6.380) | <0.001 | |
| SBP (mmHg) | ≤98 vs. >98 | 1.860 (1.508–2.512) | <0.001 |
| eGFR (mL/min/1.73 m2) | ≤60 vs. >60 | 2.147 (1.677–2.748) | <0.001 |
| cTnI (ng/L) | ≥0.07 vs. <0.07 | 3.048 (2.310–4.023) | <0.001 |
| Total bilirubin (μmol/L) | ≥17.3 vs. <17.3 | 2.808 (2.166–3.640) | <0.001 |
| dFLC (mg/L) | ≥99.2 vs. <99.2 | 3.120 (2.237–4.353) | <0.001 |
| Bone marrow plasma cells (%) | ≥8.5 vs. <8.5 | 2.420 (1.786–3.281) | <0.001 |
- Note: p value were compared using Chi Square test.
- Abbreviations: AL, light chain; CI, confidence interval; cTnI, cardiac troponin I; dFLC, difference of free light chain; eGFR, estimated glomerular filtration rate; No., number; NT-proBNP, N-terminal pro-brain natriuretic peptide; OS, overall survival; SBP, systolic blood pressure.
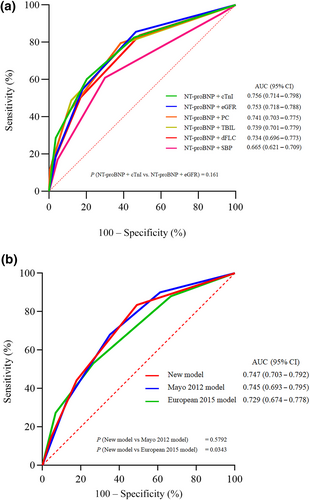
Comparison of the performance of different prediction models. (a) Comparison of NT-proBNP combined with different biological indicators. (b) Comparison between new model and two classical Mayo models. p values were compared using the deLong test. AL, light chain; AUC, area under curve; CI, confidence interval; cTnI, cardiac troponin I; dFLC, difference of free light chain; eGFR, estimated glomerular filtration rate; NT-proBNP, N-terminal pro-brain natriuretic peptide; PC, bone marrow plasma cells; SBP, systolic blood pressure; TBIL, total bilirubin.
3.4 Stage model using NT-proBNP and eGFR
Because the predictive effectiveness of NT-proBNP combined with cTNI or eGFR was equivalent in ROC analysis, we focused on which staging system could distinguish more high-risk patients with different organ involvement because both cardiac and renal involvements were of utmost importance in determining the prognosis. As depicted in Table S2, neither baseline NT-proBNP nor cTNI could identify 70% of high-risk patients with only renal involvement. The 10/25/50/75/90th percentile of cTNI was <0.01/<0.01/0.02/0.04/0.52 ng/mL and NT-proBNP was 133/213/460/878/2133 pg/mL. Patients with NT-proBNP less than 1850 pg/mL exhibited the longest OS, followed by those with NT-proBNP between 1850 and 9020 pg/mL; the patients with NT-proBNP more than 9020 pg/mL demonstrated the worst prognosis (Figure 1b). Besides, our data also demonstrated that patients with eGFR more than 60 mL/min/1.73 m2 (Figure 1c) exhibited a better prognosis compared to those with eGFR less than 60 mL/min/1.73 m2, which is one of the criteria for diagnosis of chronic kidney disease (CKD) and the cut-off value to distinguish the stage 3 from stage 2 of CKD. Therefore, we defined NT-proBNP value >1800 pg/mL or >9000 pg/mL taking 1 or 2 points, respectively, and the eGFR value was lower than 60 mL/min/1.73 m2 taking 1 point. As shown in Figure 3, the patients were accordingly divided into stages I to IV based on the scores of 0 to 3, and with the median OS of not reached, 57, 15, and 6 months, respectively.
OS of the patients in CRENAL cohort stratified by the model with NT-proBNP and eGFR at diagnosis. The number of patients at risk (survival proportions) at each time point is shown below the x-axis. Survival curves were compared using the log-rank test. AL, light chain; CRENAL, China Registration Network for light-chain Amyloidosis; eGFR, estimated glomerular filtration rate; NT-proBNP, N-terminal pro-brain natriuretic peptide; OS, overall survival.
3.5 Comparison of the prognostic staging system
The performance of the new model was compared with the Mayo 2012 model and the European 2015 model. Because most data were with cTNI rather than cTNT, the cut-off value of cTNI in 0.07 ng/mL, which was defined by the maximal chi-square statistics approach, replaced the criteria of cTNT = 0.025 ng/mL in the Mayo 2012 model. Finally, there were 980, 851, and 968 patients stratified by a new model, Mayo 2012 and European 2015 model, respectively. 812 patients were staged by three models simultaneously. The ROC curve (Figure 2b) showed that the AUC of the new model was superior to the European 2015 model (0.747 vs. 0.729, p = 0.0343), and was the same as that of the Mayo 2012 model (0.747 vs. 0.745, p = 0.5792).
3.6 Concordance analysis of new model and Mayo models
Using the net reclassification index (NRI), the Mayo 2012 model and the European 2015 model were compared to the new models in a pair-wise fashion by examining the change in net improvement in survival prediction for each pair of models (Table S3, Figure 4). In the entire study population analysis, the net improvement for the new models was higher than the two models, with a net improvement of 10.6% over the Mayo 2012 model (p < 0.001) and 10.0% (p < 0.001) over the European 2015 model. The new model had the best predictive capacity in 1-year landmark, with a net improvement of 7.4% over the Mayo 2012 model (p = 0.004) and 4.4% (p < 0.001) over the European 2015 model. In the 2-year landmark, the new model had no difference compared to the Mayo 2012 model, with a net improvement of 1% (p = 0.851), and the European 2015 model, with a net improvement of 0.6% (p = 0.981). In the 3-year landmark, the new model outperformed Mayo 2012 with a net improvement of 11.2% (p = 0.001) and the European 2015 with a net improvement of 8.6% (p = 0.009). The proportional hazard for overall survival by stage for each staging model is shown in Table S4.
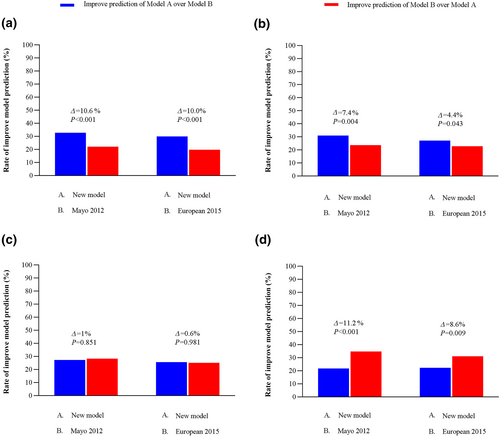
Relative likelihood of correct vital status prediction with net reclassification index between the staging models. (a) Entire study population (n = 812). (b) 1-year survival (n = 652). (c) 2-year survival (n = 524). (d) 3-year survival (n = 426). Results are represented as improved and worse predictions. The difference in these results is summarized as the net improvement (Δ).
The survival of patients treated with or without a bortezomib-based regimen was analyzed by the three models (Figure S6). In the Mayo 2012 model stage I, the median OS was not different between patients with a bortezomib-based regimen in NR and those without in NR. The median OS of patients with bortezomib-based regimen was better than that of those without in Mayo 2012 model stage II (NR vs. 79), III (NR vs. 19) and IV (26 vs. 5). In the new model, the median OS was not different between patients with bortezomib-based regimen or without in stage IV (6 vs. 7). The median OS of patients with bortezomib-based regimen was better than that of those without in the new model stage I (NR vs. NR), stage II (NR vs. 29) and III (30 vs. 5). In the European 2015 model, the median OS was not different between patients with bortezomib-based regimen or without in stage IV (7 vs. 3). The median OS of patients with bortezomib-based regimen was better than that of those without in the European 2015 model stage I (NR vs. NR), stage II (NR vs. 79) and III (NR vs. 19).
4 DISCUSSION
This study reports the treatment outcomes of a large cohort of patients with AL amyloidosis from CRENAL. Our study shows that the median OS of AL amyloidosis patients who received comprehensive treatment schemes has markedly improved in the past decade. Although traditional stage systems based on cTnI, NT-proBNP, and dFLC can be used for restaging the patients after comprehensive treatment [11], the newly developed model using two thresholds of NT-proBNP combined with eGFR less than 60 mL/min/1.73 m2 is superior to identify patients with the best and worst prognosis even in those who received bortezomib-based regimen in the CRENAL cohort.
Over the past few decades, the overall survival of patients has improved significantly due to improvements in supportive care and the availability of new treatments. In our cohort, a multidisciplinary approach was widely used to avoid red flag ignorance of non-timely diagnosis and unfavorable outcomes from the beginning. Nutritional counseling was applied to preserve body weight and improve quality of life [4]. Integral therapy includes diuretics, antiarrhythmic drugs, agents that control bowel habits, and medications used to control neuropathic pain. Serious side effects such as hypotension, electrolyte disturbances, and creatinine elevation were effectively avoided. For patients with serious kidney or heart involvement, renal replacement or implantable cardioverter-defibrillators were applied, respectively. In our cohort, 48.3% of patients received a bortezomib-based regimen, and 15.9% of patients received treatment including ASCT, melphalan, lenalidomide, thalidomide with dexamethasone, and others 9.7% of patients did not receive treatment.
A better stratification is important to develop treatment strategies and predict the outcomes, though the clinical course of patients with AL amyloidosis is heterogeneous and depends on which organs are involved and to what extent [12]. In the current study, 875/980 patients were diagnosed after 2012%, and 54.4% of patients were stratified to stage I or II according to Mayo 2012, but the median OS of each stage was markedly prolonged compared to the previous study [7]. In the patients with stage IV cancer, the median OS of patients who were treated with a bortezomib-based regimen was extended to more than 2 years. Those results far deviated from the definition of high-risk patients. To identify the high-risk patients accurately, we screened all the independent risk factors, with two thresholds of NT-proBNP more than 1800 pg/mL and 9000 pg/mL, and eGFR less than 60 mL/min/1.73 m2, a four-stage score system was developed. By the new stage model, 41.8% of patients were reclassified to stage I, 32.5% to stage II, 16.5% to stage III, and 6.8% to stage IV disease with a median OS not reached, 57-, 15-, and 6 months respectively. The new stratification model exhibited improved consistency with the clinic practice, by which more patients were stratified to an earlier stage, and can also identify the true high-risk patients at stage IV classified by Mayo 2012 even for the patients who received a comprehensive treatment, including bortezomib-based regimen.
As a multisystemic disorder, AL amyloidosis can affect the heart, kidneys, liver, nerves, lungs, and bowel [13]. However, the heart and kidney are the two most commonly involved organs [14]. The morbidity and mortality of patients with AL amyloidosis are closely related to the severity of cardiac involvement. Previous studies [15, 16] showed that NT-proBNP is a sensitive marker of heart dysfunction and the most powerful prognostic factor in AL. Increased NT-proBNP levels can detect inapparent heart involvement and are useful in monitoring heart dysfunction caused by the amyloidogenic light chains and response to therapy. Consistent with previous studies that NT-proBNP>8500 pg/mL identify a very poor risk subgroup of AL amyloidosis [17] though a mild increase in NT-proBNP had been regarded as a risk [7, 18, 19], our study shows that extremely increased NT-proBNP indicates a worse prognosis.
Although the prognosis value of lower eGFR is controversial [20-36], renal response has been shown to be associated with overall survival [22]. Superior OS was observed in patients with proteinuria reduction >75% [23]. Gertz et al. [24] found that serum creatinine level was an independent predictor of overall survival when corrected for cardiac involvement. Rezk et al. [25] indicated that the outcome of patients with renal and cardiac involvement death depends on the NT-proBNP and eGFR values at the time of diagnosis. Importantly, the renal response rate after treatment is lower than that of the heart [26], indicating that patients with renal involvement may need more active treatment. In the group of patients with eGFR <60 mL/min/1.73 m2 in our cohort, 75% of them exhibited the values of NT-proBNP and cTNI below the cut-off. Therefore, eGFR less than 60 mL/min/1.73 m2 is helpful in discriminating high-risk patients in such a population with renal involvement and especially without cardiac patients.
In addition, NT-proBNP, cTNI, bilirubin, and dFLC were mainly excreted by the kidney; late-stage renal failure can result in an increase of those biomarkers and a reduction of sensitivity and specificity in the diagnosis of myocardial involvement for persons with eGFR <60 mL/min/1.73 m2 [27-29]. The levels of BNP are also known to be significantly increased for patients on hemodialysis, and associated with an increase in volume overload, kidney disease and heart failure [30, 31]. Despite that the factors including cTnI, total bilirubin, dFLC, and the bone marrow plasma cells were demonstrated to be significantly associated with OS, and nearly all had a higher HR than eGFR in current cohort, eGFR less than 60 mL/min/1.73 m2, which is a sign of late stage of kidney involvement and contribute mostly to the increase of NT-proBNP, was therefore chose to be in our new stage model as opposed to one of the others.
Compared with the Mayo 2012 model and the European 2015 model, the new model allowed the evaluation of cardiac and renal function, the most commonly affected organs, and has a superior ability to classify the highest-risk patients more accurately. Besides vigorous internal validation, NRI analysis demonstrated that the entire group prediction of the new model is more valuable than the Mayo 2012 model and the European 2015 model. Further analysis of the diagnostic power of the three models in 1-, 2- and 3-year survival. The new model had the highest diagnostic ability for 1-year survival. This is because the new model distinguishes stage I, stage II, and stage III for more patients who survived longer than 1 year. Significantly, a few patients were discriminated into stage IV, but most of those patients were predicted to reach the end point of death within 1 year accurately. In 2-year survival, there was no difference in diagnostic power among the three models. In 3-year survival, the diagnostic power of the new model is surpassed by the Mayo 2012 and European 2015 models. Interestingly, it remains a question whether the patients who reached the endpoint of death after 2 years were properly classified as stage IV. With the treatment of bortezomib, the survival time of amyloidosis patients has been prolonged significantly. The loose cut-off value of parameters of the current staging system leads to patients who are defined as stage IV having long-term survival. Our analysis revealed that increasing the cut-off value of NT-pro BNP was the most sensitive parameter for distinguishing high-risk patients. eGFR is of great significance as a secondary indicator. The increase in the afterload of CKD patients is related to the increase in NT-pro BNP [28]. Furthermore, CKD patients reduce the sensitivity of treatment [26], and it is difficult to obtain the expected treatment effect and indicates poor prognosis. The role of eGFR expands the predictable population, providing a new model suitable for screening patients with heart and kidney involvement. The advantages of the new model are also exhibited by fewer parameters, lower prices, and more convenience for clinical practice. Importantly, the new model categorized the poorest patients, which would require more advanced treatment for those who had acquired rapid and complete hematologic responses but still difficult to reverse the early high mortality of refractory patients [32-34].
The strength of the CRENAL cohort is that it reflects the real world of 980 patients with AL amyloidosis from 29 centers, with minimal loss to follow-up, and it was specifically designed to evaluate overall outcomes and its independent risk factors such as treatment and the number of the organs involved. Our population appears to be a representative AL population since renal involvement was present in 74.6% of the patients and heart involvement in 66.2% compared with the prevalence rate in other AL populations. In addition, we were able to assess the influence of main treatment regimens including ASCT, proteinase inhibitors and other traditional drugs, objectively, which is more reliable than a single center data from a specific population. Another strength is that we were able to adjust for baseline kidney function on the basis of cystatin C-based eGFR, which is independent of muscle mass, although follow-up data on cystatin C-based renal function were not available.
This study is limited by its retrospective nature, heterogeneous treatment regimens, long spans of following time, and the lack of results for 1 or more parameters of staging systems in a subset of patients, which may create selection bias. With the emergence of new drugs and the advancement in treatment technology [3, 35, 36], it was evident that the patients diagnosed in the early years had a shorter survival period, and their median survival period was underestimated in the survival analysis [6]. Secondly, this study did not carry out data decentralization, which led to too many groups with few cases and made this study difficult. However, our data reflect real-world research that has irreplaceable reference significance. Thirdly, internal validation of the new model has been confirmed but not the external validation.
5 CONCLUSION
In summary, the median OS of AL amyloidosis patients who received comprehensive treatment schemes has been markedly improved in the CRENAL cohort. The new model based on two thresholds of NT-proBNP more than 1800 pg/mL and 9000 pg/mL, and eGFR less than 60 mL/min/1.73 m2 is superior to identify the patients with the best and worst prognosis even in those who received comprehensive treatment.
AUTHOR CONTRIBUTIONS
Wenjian Wang and Liye Zhong conceived the study. Jie Li, Liwen Li, Hui Liu, and Hongwen Fei participated in its design and coordination and developed the protocol. Hokhim Yau, Pengjun Liao, Jianteng Xie, and Sheng Li collected the data. Hokhim Yau, Sheng Li, Weiting He, and Yaxi Zhu performed statistical analysis. Hokhim Yau, Sheng Li, Jianteng Xie, and Wenjian Wang edited all tables, prepared all figures and drafted the manuscript. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors acknowledge all the clinical staff and the hospital for participating in the Plasma Cell Disease Collaborative Group Plan. We wish to thank those involved in the clinical care of our patients.
CONFLICT OF INTEREST STATEMENT
None of the authors have any conflicts of interest that are relevant to the content in this manuscript.
ETHICS STATEMENT
This research adheres to the principles outlined in the Declaration of Helsinki. All procedures involving human subjects were approved by the Medical Ethics Committee of Guangdong General Hospital, Guangdong Academy of Medical Sciences (No. GDREC2019844H) and were conducted in accordance with their ethical standards. We confirm that this manuscript has not been submitted to or is under review at another journal or other publishing venue.
INFORMED CONSENT
All participants provided written informed consent.
Open Research
DATA AVAILABILITY STATEMENT
We regret to inform that the data underlying this research cannot be made available. Due to privacy and confidentiality concerns, the dataset used in this study contains sensitive information that cannot be shared publicly. Furthermore, the data were obtained under strict ethical guidelines that prohibit their dissemination beyond the scope of the current research project.




