Oncogenic viral antigens for engineered T cell immunotherapy: Challenges and opportunities
Abstract
Viruses that cause malignancies such as hepatocellular carcinoma and cervical cancer are the cause of approximately 20% of all human cancers. In recent years, engineered T cell immunotherapy targeting tumor-associated antigens (TAAs) has had some success against virus-associated cancer, although these treatments are associated with side effects. TAA-specific-modified T cells may kill cancer cells but they also react with and damage healthy tissue. During an oncogenic virus infection, viral DNA integrates into the host genome, leading to the expression of viral-specific antigens in the tumor in a restricted and durable manner. The cross-reactive side-effects of conventional TAA-specific engineered T cell treatment can be avoided by creating engineered T cells that target oncogenic viral antigens. To chart a course for the discovery of additional viral-specific antigens and their combination with immune checkpoint inhibition therapies, this review summarizes the development, preclinical research, and clinical application of oncogenic viral antigen–specific T cell immunotherapy. This review also addresses challenges such as virus mutation and diverse integration, which can result in the loss of the target.
Abbreviations
-
- ACT
-
- adoptive T-cell therapy
-
- ATL
-
- adult T-cell leukemia
-
- CAR-T
-
- chimeric antigen receptor T Cell
-
- CMV
-
- cytomegalovirus
-
- CR
-
- complete response
-
- CRS
-
- cytokine release syndrome
-
- CTL
-
- cytotoxic T cells
-
- DAMPs
-
- damage-associated molecular patterns
-
- DLBCL
-
- diffuse large cell lymphoma
-
- EBV
-
- Epstein-Barr virus
-
- EBViNT
-
- EBV-induced natural T cell
-
- FLT3L
-
- Fms-like tyrosine kinase 3 ligand
-
- GC
-
- gastric carcinoma
-
- GLPD
-
- germinotropic lymphoproliferative disorder
-
- GVHD
-
- graft-versus-host disease
-
- HBV
-
- hepatitis B virus
-
- HBZ
-
- the HTLV-1 basic leucine zipper factor
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- HL
-
- Hodgkin lymphoma
-
- HLA
-
- human leukocyte antigen
-
- HNSCC
-
- head and neck squamous cell carcinomas
-
- HPV
-
- high risk human papillomavirus
-
- HTLV-1
-
- human T-cell lymphotropic virus-1
-
- iPSCs
-
- induced pluripotent stem cells
-
- KS
-
- Kaposi sarcoma
-
- KSHV
-
- Kaposi sarcoma herpesvirus
-
- LC
-
- liver cirrhosis
-
- LMP
-
- latent membrane protein
-
- LP
-
- leader protein
-
- MAGE-A
-
- Melanoma-associated antigen A3
-
- MCC
-
- Merkel cell carcinoma
-
- MCD
-
- multicentric Castleman disease
-
- MCV
-
- Merkel cell polyomavirus
-
- MHC
-
- major histocompatibility molecules
-
- NA
-
- nucleoside analogs
-
- NK
-
- natural killer
-
- NPC
-
- nasopharyngeal carcinoma
-
- PCR
-
- partial complete response
-
- PD
-
- progressive disease
-
- PEL
-
- primary effusion lymphoma
-
- PLC
-
- peptide loading complex
-
- PR
-
- partial response
-
- PTLD
-
- post-transplant lymphoproliferative disorder
-
- SCC
-
- squamous cell carcinoma
-
- TAA
-
- tumor associated antigens
-
- TCR-T
-
- T-cell receptor–modified T cell
-
- TME
-
- tumor microenvironment
-
- WGS
-
- whole-genome sequencing
-
- WHO
-
- World Health Organization
1 INTRODUCTION
Numerous investigations on the characteristics of neoplastic development have shown that oncogenic viruses are one of the causes of cancer. Research has indicated that viruses are responsible for 10%–20% of all human malignancies [1]. Examples of viruses that have been linked to the development of malignancies include the Epstein-Barr virus (EBV), hepatitis B and C viruses (HBV and HCV), human papillomavirus (HPV), human T-lymphotropic virus type 1 (HTLV-1), Kaposi sarcoma herpesvirus (KSHV), and Merkel cell polyomavirus (MCV) [2]. The effectiveness of traditional treatments such as radiotherapy and chemotherapy against virus-associated cancers is insufficient [1]. Engineered T cell therapy is a cutting-edge and effective cancer treatment. However, the application of this treatment remains challenging because of adverse events, particularly the cross-reactive (on-target/off-tumor) toxicity. For example, a deadly off-target cross-reactivity with a peptide from the Titin protein was observed with an affinity-matured T cell receptor (TCR) that recognized the promising tumor antigen Melanoma-associated antigen A3 [3]. To simultaneously increase anticancer effectiveness and decrease cross-reactive toxicity, it is essential to select optimal antigens that are tumor-specific, carcinogenic, and immunogenic.
For effective engineered T cell treatment, targeting an oncogenic viral antigen that is specifically expressed in virus-associated tumors and plays a significant role in the formation of tumors is ideal. For example, when a cell enters the S phase, the phase of DNA synthesis, oncogenic proteins encoded by small DNA oncogenic viruses such as HPV and large oncogenic herpesviruses such as EBV and KSHV can gain access to the cellular replication system and the nucleotides needed for viral DNA synthesis. Furthermore, through a variety of mechanisms, both the HTLV-1 oncoprotein transactivator from the X-gene region (Tax) and the basic leucine zipper factor (HBZ) may predispose cells to oncogenesis. Additionally, the HBV-X protein from HBV forms a complex with p53 that prevents it from acting as a transcription factor and DNA binder [3]. These oncogenic viral proteins are essential for the development of tumors linked to viruses. Engineered TCR-modified T cells (TCR-Ts), which can target viral peptides, can enable action against tumor cells of designed adoptive T-cell therapy (ACT) transgenic CD8+ T cells [4]. Seven oncogenic viruses have been identified in studies from 1970s to 2010s (Figure 1). For the treatment of cancer caused by viruses, modified T cell immunotherapy targeting oncogenic viral antigens may be a long-lasting and unique therapeutic strategy with few adverse effects. Many clinical trials for engineered T cell treatment have focused on oncogenic viral antigens (Figure 1), which may advance the development of engineered ACT immunotherapy.
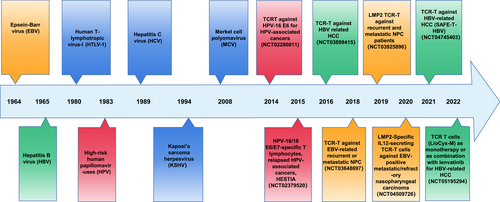
The history TCR engineered T cells therapy for oncogenic viral tumor.
2 ENGINEERED T CELLS
TCR-Ts and chimeric antigen receptor T cells (CAR-Ts) are engineered T cells whose structure has been genetically altered to target certain tumor antigens or to release cytokines that improve the immunosuppressive tumor microenvironment (TME). TCR-Ts and CAR-Ts have benefits and drawbacks.
2.1 T cell receptor-modified T cells
TCR-Ts are generated by a gene-engineered method to change an autologous or allogenic T cell such that it expresses a particular tumor antigen and causes the lysis of cancer cells. The main goal of TCR-T treatments is to guide CD8+ T cells, the main agents of antitumor immune responses, to human leukocyte antigen (HLA) class I restricted sites [5]. Endogenously processed linear peptide epitopes, presented by major histocompatibility (MHC) molecules, are recognized by the TCR, so that both surface-bound proteins and intracellular targets are covered [6]. TCR-Ts elicit lower peak cytokine levels at high antigen densities. However, certain CAR-T cells can cause major immunological toxicities that may result in the cytokine release syndrome (CRS), which can be fatal [3]. Additionally, the use of a particular TCR in immunotherapy is always dependent on the presence of the patient's matching HLA molecule and the level of MHC class I molecule expression in the target cells. However, MHC class I can be downregulated in viral infections, and its expression is low in a number of tumors that induce immune evasion [7].
2.2 Chimeric antigen receptor T cells
T cells have the capacity to identify tumor antigens without the aid of HLA because of CAR-Ts. The critical component of CAR-T therapies is the CAR, which is composed of four essential parts: an extracellular hinge domain, a trans-membrane domain, an intracellular signal domain, and a binding domain specific to the tumor-associated antigen (TAA). This binding domain is typically derived from the scFv fragment of the antigen-binding region in monoclonal antibodies [8]. Most aberrant cancer proteins are present within cells and only presented by MHC complexes after proteasomal degradation. CAR-Ts only recognize surface TAAs, making it difficult to discover appropriate antigens in many cancers [3].
Four generations of CAR-T therapy have been produced, and these mainly differ in the co-stimulatory domain. In the first generation of CAR, the tyrosine activation motif on the CD3ζ chain without a co-stimulatory domain activates T cells. In the second generation of CAR, a second co-stimulatory domain, often CD28 or 4-1BB (CD137) moieties, was included to enhance the ability of T cells to survive and proliferate. In the third generation of CAR-T, an additional co-stimulatory domain (CD28 and 4-1BB or TLR2) was introduced to significantly increase the effectiveness of infused CAR-T cells. The addition of the intracellular region of the cytokine receptor to the CAR was included in the fourth generation of CAR and significantly aids in the promotion of T cell growth [5]. The antigen-binding site retained its distinctive property, allowing CARs to recognize their target in a manner similar to that of an antibody by binding molecules in a conformation-dependent manner, without the need for intracellular antigen processing and MHC pathway presentation. This increases the application of CAR-T cells but also has a drawback. The use of CARs is restricted to molecules that are present on the cell surface, encompassing only approximately 30% of all known proteins. Additionally, soluble versions of the target in the peripheral circulation can interfere with CAR-T cells. A concerning possibility is that these soluble antigens might “capture” CAR-T cells and prevent them from traveling to their target location. Furthermore, they might cause CAR-T cells to spontaneously activate off-target and cause unfavorable side effects [6].
3 THE MOLECULAR MECHANISMS INVOLVED IN THE ONCOGENESIS OF VIRUSES
The seven viruses known to cause cancer in humans have diverse genomes, cellular tropisms, cancer pathologies, and disease prevalence. However, they share many features that lead to cancer in humans [7]. Viral infection usually includes an acute infection period and a chronic infection period. During the acute infection period, patients experience a relatively rapid virus clearance associated with the expansion of virus-specific T cells and production of specific antibodies. Patients who are unable to completely eliminate the virus, including those with a compromised immune system, may experience periods of chronic infection [8]. For example, approximately 90% of HPV-infected patients present with innate and humoral immune-mediated viral clearance within several months after acute viral infection. During the regression process, infiltration of CD4+ and CD8+ lymphocytes and macrophages and an increase in pro-inflammatory cytokines and neutralizing antibodies occurs. However, this does not prevent reinfection by the same HPV type or other viral types. Furthermore, in 10% of patients, HPV can persist, increasing the risk of cancer, which is observed in approximately 1% of all infected patients [9]. During the chronic infection, some physiological changes in cellular properties occur and lead to cancer initiation and progression. For example, viral-derived oncoproteins such as E6 and E7 of papillomavirus in a manner by inactivating a particular cellular protein known as retinoblastoma that is essential for regulating cell replication.
This change can cause the accumulation of DNA mutations, which in turn, leads to rapid cell division and the development of tumors [10].
Viral integration is an important event for carcinogenesis and can affect DNA methylation patterns and gene expression. In a study using whole-genome sequencing, the authors identified 3666 HPV integration breakpoints, showing a large possibility of virus integration into host genome. Another study using a dataset of 279 head and neck squamous cell carcinomas reported HPV integration in 103 samples and 56 (54%) showed integration in a known gene [9]. Transcriptionally active HBV integration has been suspected as a driver of hepatocellular carcinoma (HCC) and can extend to the entire liver in some HBe- patients. This can lead to ubiquitous hepatitis B surface antigen (HBsAg) expression independent of HBV replication [11].
Tumor antigens are processed by the proteasome and converted into peptides. These peptides are then loaded onto HLA-I in the endoplasmic reticulum with the help of various auxiliary proteins and chaperones in the peptide loading complex. Once the peptide and HLA-I form a strong complex, it is then transported to the cell surface. To attract conventional dendritic cells (cDC1) to the tumor, chemokines are used. Fms-like tyrosine kinase 3 ligand promotes cDC1 differentiation and survival. When tumor cells start to die, they release damage-associated molecular patterns that attract cDC1s to the draining lymph nodes. The cDC1s then process and load cancer antigens onto class I HLA-I and HLA-II. This is done to present the antigens to CD8+ and CD4+ T cells, respectively (Figure 2) [12]. However, the virus can hide from the immune system by turning off unnecessary viral proteins that might be sensed by cell-mediated immune recognition, which serves as an immune evasion strategy [13]. There is an emerging idea that viral infections could in principle contribute to cancer initiation and/or progression without being consistently detectable in every tumor cell (“hit-and-run”) [14]. A study using three independent detection approaches (immunohistochemical visualization, flow cytometric analysis, and quantitative polymerase chain reaction) showed that HBV-HCC cells can express short HBV-specific mRNA without antibody detection of HBV antigens [15]. Selecting a viral antigen that is persistent in the tumor for engineered T cell immunotherapy is critical and remains a challenge.
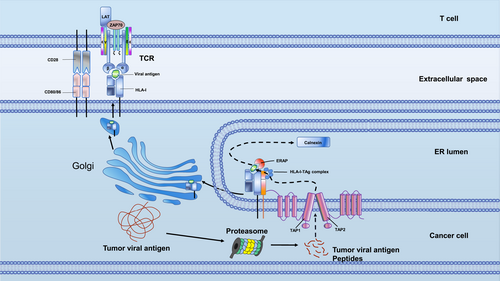
The TCR-T recognize the tumor antigen presented by HLA-I pattern. ERAP, endoplasmic reticulum aminopeptidase; HLA-I, human leukocyte antigen; TAP, transporters associated with antigen processing; TCR, T cell receptor.
4 STUDIES OF ONCOGENIC VIRAL ANTIGEN–SPECIFIC ENGINEERED T CELLS IN COMMON HUMAN CANCERS
One of the biggest challenges in ACT treatment is selecting the appropriate engineered T-cell targets. Target tumor antigens are chosen using two standard criteria: antigens are selected depending on whether the antigen is solely expressed in cancers and whether there are sufficient numbers of patients who might benefit from the therapy. Therefore, engineered T cell therapy using oncogenic viral antigens may be a suitable strategy for cancer treatment [16]. A series of clinical trials on potential treatments are ongoing (Table 1).
| Cancer | Antigen | Type | Stage and result | Host | NCT |
|---|---|---|---|---|---|
| HBV | |||||
| Hepatocellular carcinoma | HBV-envelop | TCR-T | Phase 1 (recruiting) | Beijing 302 Hospital, China | NCT03899415 |
| HBV-envelop | TCR-T | Phase 1, phase 2 (withdrawn) | The Third Affiliated Hospital of Sun Yat-sen University, China | NCT03634683 | |
| HBV-envelop | TCR-T | Phase 1 (recruiting) | Singapore General Hospital, Singapore | NCT04745403 | |
| HBV-envelop | TCR-T | Phase 1b/2 (not yet recruiting) | Multi-center sponsor by Lion TCR Pte. Ltd. | NCT05195294 | |
| HPV | |||||
| Cervical cancer | E6 | TCR-T | Phase 1 (recruiting) | Department of Oncology, Xinqiao Hospital, China | NCT03578406 |
| E7,E6 | TCR-T | Phase 1 (active, not recruiting) | Houston Methodist Hospital, United State | NCT02379520 | |
| E6 | TCR-T | Phase 1, phase 2 (completed, has results) | National Institutes of Health Clinical Center, United States | NCT02280811 | |
| 2 pts had PR for 1.5 years | |||||
| E7 | TCR-T | Phase 1, phase 2 (recruiting) | National Institutes of Health Clinical Center, United States | NCT02858310 | |
| E7 | TCR-T | Early phase 1 (suspend) | National Institutes of Health Clinical Center, United States | NCT04476251 | |
| E7 | TCR-T | Phase 1 (withdrawn) | National Institutes of Health Clinical Center, United States | NCT04411134 | |
| Vulvar squamous cell carcinoma | E7 | TCR-T | Phase 1 (terminated) | National Institutes of Health Clinical Center, United States | NCT03197025 |
| E7 | TCR-T | Phase 1, phase 2 (recruiting) | National Institutes of Health Clinical Center, United States | NCT02858310 | |
| E7 | TCR-T | Phase 1, phase 2 (terminated) | National Institutes of Health Clinical Center, United States | NCT03937791 | |
| EBV | |||||
| Nasopharyngeal Neoplasms | LMP1 | CAR-T | Phase 1, phase 2 (recruiting) | Second Nanjing MU, China | NCT02980315 |
| LMP1, LMP2 and EBNA1 | TCR-T | Phase 2 (recruiting) | Fujian Cancer Hospital, China | NCT03648697 | |
| LMP2 | TCR-T | Phase 1 (recruiting) | Sun Yat-sen University Cancer Center, China | NCT03925896 | |
| LMP2 | TCR-T | Phase 1, phase 2 (not yet recruiting) | Department of Oncology, Xinqiao Hospital, China | NCT04509726 | |
| MCV | |||||
| Merkel cell carcinoma | MCPyV TAg | TCR-T | Phase 1, phase 2 (terminated, has results) | Fred Hutch/University of Washington Cancer Consortium Seattle, United States | NCT02584829 |
| 2 pts had CR, 1 had PR, 3 had PD, 1 had PCR | |||||
| HTLV-1 | |||||
| Not found | |||||
| HCV | |||||
| Not found | |||||
| KSHV | |||||
| Not found | |||||
- Abbreviations: ACT, adoptive T-cell therapy; CR, complete response; PCR, partial complete response; PD, progressive disease; PR, partial response.
4.1 Hepatitis B virus
HBV mainly causes human HCC. Despite the fact that the global immunization campaign has reduced the infection incidence of HBV in children under the age of five to 1%, a World Health Organization (WHO) research study indicated that there are still an estimated 257 million chronic HBV carriers worldwide. Furthermore, according to WHO's report in 2020, as of 2015, the mortality from this virus was over 8.8 million, mostly from liver cirrhosis and HCC [17]. Several HBV-specific TCRs were produced after the creation of a CAR that was specific for the HBV envelope protein; this strategy served as the foundation for the use of T cell immunotherapy for HBV infection [6]. The nucleoside analogs (NA) used in current HBV antiviral treatments prevent the synthesis of HBV-DNA but have no effect on the generation of antigen from HBV-RNA templates. Because they display HBV antigens similar to untreated infected cells, HBV-infected hepatocytes treated with NA can be specifically targeted by CAR/TCR-T cells [8]. HCC cells produce a peptide chain that can attach to major MHC molecules, making it possible for T lymphocytes to identify the cells. HCC cells often have integrated HBV-DNA fragments. For CAR/TCR-T cell treatment, HBsAg may be a possible target [16]. In vitro recognition of HBV-positive cell lines and HBsAg particles by HBsAg-specific CAR-T cells was assessed in a preclinical investigation. However, in cytotoxicity experiments, HBsAg-specific CAR-T cells failed to kill HBV-positive cell lines. When HBsAg-CART cells were adoptively transferred into HBV-infected humanized mice, the levels of HBsAg and HBV-DNA in the liver significantly dropped in comparison with those in control animals. Notably, after treatment with HBsAg-specific CAR-T cells, the proportion of HBV core–positive hepatocytes among all human hepatocytes significantly decreased, suggesting noncytopathic viral elimination [18].
Eight patients with advanced HBV-HCC were included in a phase I clinical trial (NCT03899415) that used adoptively transplanted short-lived autologous T cells that had HBV-specific TCR. One of the patients experienced a partial recovery that lasted for 27.7 months. Importantly, following HBV-TCR-T cell infusion, the majority of the patients showed a decrease in circulating HBsAg and HBV DNA levels or their stability, demonstrating on-target effects [18]. In another phase I clinical trial (NCT04677088), autologous T cells that expressed specific TCRs encoded by the incorporation of HBV-DNA into the host DNA were introduced. A small HBV peptide, rather than the whole HBV antigen, is encoded by the HBV-DNA integration. By transfecting mRNA into cells, autologous T cells were made to express the selected TCRs, and these TCR T cells were adoptively transplanted into two patients in increasing quantities (1 × 104–10 × 106 TCR-T cells/kg) weekly for 112 days or 1 year, with no negative side effects. Five of six lung metastases in one patient showed volume reduction during the first year of the T-cell treatment [15]. The few permanently infected hepatocytes that are present in these individuals may be the only target for CAR/TCR T cells [8]. Research has indicated that immunotherapeutic IL-2 is capable of rescuing CD8+ T lymphocytes that have been rendered dysfunctional by hepatocellular priming. Therefore, when anti-progressive disease (PD)-L1 therapy is not immediately effective, IL-2-based techniques should be taken into account for the combination treatment of chronic HBV infection with CAR/TCR T cells [19].
4.2 Hepatitis C virus
The HCV, which affects 180 million individuals globally, is one of the main causes of chronic liver disease [20]. With over 90% cure rates, direct antiviral medicines have changed patient care [21]. However, some patients are still unable to completely eliminate the HCV, and therefore, a novel therapy is urgently needed for these patients. In preclinical ACT research, hepatocytes infected with HCV derived from cell culture (HCVcc) were destroyed by HCV-specific TCR-Ts targeting the HCV E2 protein. Target cells expressing HCV/E2 of various genotypes and subtypes may be identified by anti-HCV CAR-T cells, and these cells can then be killed. HCV/E2-transfected and HCVcc-infected target cells exhibited cytotoxic activity when exposed to anti-HCV CAR-T cells. Additionally, T cell degranulation and the release of pro-inflammatory and antiviral cytokines occurred concurrently with antigen detection [21]. This preclinical study indicated that engineered E2-specific T cells may be a powerful treatment for HCV-associated HCC.
4.3 High risk human papillomavirus
HPV, the most common oncogenic virus, is the cause of 30% of all malignancies caused by infectious agents [9]. HPV-18 and HPV-16 make up most of the carcinogenic HPV subpopulation. Recent data from in vitro and in vivo experiments have raised the possibility that HPV-positive epithelial malignancies might benefit from TCR T cells made to destroy HPV-infected cells by focusing on HLA-A*02:01 linked to a portion of the HPV-16 protein E7. The HPV E7 antigen, an alluring therapeutic target, is constitutively expressed by HPV+ malignancies but not by healthy tissues [22]. In a preclinical study, T cells that were genetically modified to target HPV-16 E7 caused regression of HPV-16+ human malignancies in an animal model. A preclinical foundation for an ongoing clinical study of E7 TCR T cells treating patients with metastatic HPV+ malignancies was supported by these findings [23]. Nagarsheth and colleagues conducted a first-in-human, phase 1 clinical trial of T cells engineered with a TCR targeting HPV-16 E7 for the treatment of metastatic human papilloma virus–associated epithelial cancers (NCT02858310) [23]. Six of the 12 patients who received the therapy had objective tumor responses, providing preliminary proof of the effectiveness of this treatment. One or more tumors completely regressed in three individuals. In some patients, cancer regression was extensive with durable regression of some tumors. One patient developed retroperitoneal, pelvic, thigh, and more than 80 lung metastases as a result of metastatic vulvar squamous cell carcinoma (SCC). She had previously received seven systemic anticancer medications. Following therapy, she had a partial response (PR) lasting 8 months, with 25 lung tumors completely regressing and being undetectable on imaging 8 months later. Another patient with metastatic SCC had more than 90 metastatic tumors affecting the thorax, retroperitoneum, bones, and kidney. Chemoradiation and PD-1-based treatment had been previously used to treat this patient. He had a PR lasting 9 months, with 80 tumors completely regressing and being undetectable on imaging 14 months following therapy. These findings highlight immune editing as a constraint on the curative potential of cellular treatment and perhaps other immunotherapies in advanced epithelial cancer. Furthermore, the results show that viral antigen–specific engineered T cells can induce the regression of common carcinomas.
4.4 Merkel cell polyomavirus
Merkel cell carcinoma (MCC), which has neuroendocrine characteristics, is an uncommon yet extremely aggressive skin cancer [24]. The disease-associated mortality of MCC has been calculated to be as high as 46% [24]. In 80% of MCC cancers, the MCV, which was identified in 2008, is clonally integrated. Several UV-related mutations are present at high frequencies in the remaining 20% of MCC cancers [25]. At least 60% of all MCC is caused by clonal integration of MCV DNA into the tumor genome with sustained production of viral T antigens [26]. The maintenance of MCC cell lines in vitro as well as virus-mediated carcinogenesis depend on MCV-encoded T-antigens (TAgs) [27]. Thus, TAgs may be a viable target for MCC treatment using modified T cells. Preclinical research identified naturally occurring MCV TAg epitopes and isolated TAg-specific TCRs. HLA-A2-positive cells that were loaded with the cognate peptide or cells that consistently expressed MCV TAgs were able to activate T cells that expressed these TCRs. TCR therapy resulted in the regression of existing tumors in a mouse model and proved the cytotoxic capacity of T cells modified to express these TCRs in vitro [27]. Patients with MCC that spread to other areas of the body were examined in a phase I/II clinical trial (NCT02584829) to determine the side effects and effectiveness of targeted radiation therapy, recombinant interferon beta, and avelumab with or without MCV TAg-specific polyclonal autologous CD8+ T cells. However, the lack of financing forced the end of the investigation. The limited results demonstrated that in the MCV TAg-specific polyclonal autologous CD8+ T cell treatment group, two patients had complete response, one had PR, three had PD, and one had partial complete response. In the 1-year follow-up period, four of the seven patients treated with MCV TAg-specific polyclonal autologous CD8+ T cells developed new metastases. Three of the seven patients who received MCV TAg-specific polyclonal autologous CD8+ T cell therapy died of all-cause mortality. The cellular treatment group did not show significant adverse events, but six of the seven patients had experienced other moderate adverse events, such as fever, skin infection, decreased lymphocyte count, skin ulceration, and hypertension. The outcome of the clinical trial is promising. Only one clinical trial is in progress, and thus more research is required to determine the effectiveness and safety of the MCV-TAg-specific engineered T cell immunotherapy.
4.5 Epstein-Barr virus
EBV is associated with the development of a broad range of malignancies, including Burkitt's lymphoma, Hodgkin lymphoma (HL), B-, T-, and natural killer (NK)-cell lymphomas, post-transplant lymphoproliferative disorder, nasopharyngeal carcinoma (NPC), and gastric carcinoma [28]. Over 90% of people worldwide are infected with EBV, but there are still no effective treatments or vaccinations [29]. These EBV-associated cancers exhibit viral antigens such as leader protein, latent membrane protein (LMP)-1, LMP-2, EBNA-1, EBNA-2, and EBNA-3A/B/C; the various tumor types have varied expression patterns [30]. The first proof that LMP-1166-TCR engineered T-cells allow efficient recognition to display potent cytotoxicity toward engineered LMP-1 overexpressing tumor cells in vitro and in vivo was presented in a preclinical study that demonstrated a novel HLA-A2-restricted TCR that specifically recognizes the LMP-1166 epitope [31]. The EBV-induced Natural T cell (EBViNT) clinical investigation examined autologous EBV/LMP2A-specific CD8+ T cells in patients with relapsed/refractory EBV-positive malignancies. Of the 11 recruited patients, 8 patients (4 with NPCs, 1 with HL, 2 with extranodal NK/T lymphomas, and 1 with diffuse large B-cell lymphoma) received a single infusion of EBViNT. All patients responded favorably to a single infusion of EBViNT, and three had demonstrable antitumor effects. This study demonstrated the safety of EBViNT therapy and suggests that the therapy may represent a new alternative for treating EBV-positive cancer patients with recurrent disease who have not responded to standard treatment [32]. A clinical trial (NCT04509726) for the LMP2-specific TCR-T in combination with IL-12 for the treatment of EBV-positive metastatic/refractory nasopharyngeal cancer has been filed; however, no participants have been recruited. The LMP2 antigen–specific high-affinity TCR-transduced autologous T-cell treatment for recurrent and metastatic nasopharyngeal cancer is being tested in another open-label, single-center, phase I clinical study (NCT03925896) and is now accepting participants. The high affinity TCR targeting the aforementioned EBV antigen was screened from healthy donors using the sorting and single-cell cloning approach in a phase II clinical study (NCT03648697) that focused on the NPC highly expressed EBV antigens such as LMP1, LMP2, and EBNA1. The TCR gene is then transferred to autologous T cells using lentivirus. In this trial, NPC patients who have previously received treatment but have progressed or relapsed will be examined for the safety and tolerability of EBV-TCR-T cell therapy.
4.6 Human T-cell lymphotropic virus-1
Adult T-cell leukemia (ATL), an aggressive form of T-cell cancer, may be induced by HTLV-1 infection. Between 5 and 10 million people were estimated to be carrying the HTLV-1 virus worldwide in 2012, with high endemicity clusters [33]. While the majority of HTLV-1-infected people remain asymptomatic carriers for the entirety of their lives, only approximately 5% eventually acquire ATL after a lengthy latency period of 50–60 years [34]. Despite an active immune response that includes cytotoxic T cells and NK cells, HTLV-1 survives in the host, indicating that the virus has created efficient defenses against host immune surveillance [35]. Tax and HBZ are two essential HTLV-1 oncoproteins; the expression of Tax varies among ATL cases, and the expression of HBZ is constant [36]. There is no preclinical or clinical trial of engineered T cell therapy targeting HTLV-1 specific oncogenic viral antigens. However, a study of the long-term survivors with ATL demonstrated that Tax-specific memory cytotoxic T-lymphocytes, together with anticancer agents, eradicated ATL cells and exhibited long-term preventive effects from relapsed ATL [37]. The study indicated that it is possible to use engineered CAR or TCR T cells specific for the HTLV-1 Tax antigen to treat ATL patients infected with HTLV-1.
4.7 Kaposi sarcoma herpesvirus
KSHV (also known as human herpesvirus 8) was identified as the pathogen of Kaposi sarcoma (KS). Immunosuppressed individuals have been reported to develop KS (iatrogenic KS). Epidemic KS (HIV1KS) refers to KS in the presence of HIV infection and AIDS. KS rates in persons living with HIV are 500 times higher than those in the general population [38]. The KSHV is also pathogenetically linked to a number of lymphoproliferative diseases, including diffuse large cell lymphoma (DLBCL), germinotropic lymphoproliferative disorder, multicentric Castleman disease, and primary effusion lymphoma (PEL)/extra cavitary-PEL [39]. In contrast to the strong T-cell responses to other human herpesviruses, T cell responses to KSHV are at a modest level [40]. When CD8+ T lymphocytes bind viral antigens expressed on MHC-I by infected cells, cytokines are secreted, cytotoxic granules are released, or the target cell is induced to undergo apoptosis. This process cannot take place if the target cell's MHC-I expression is impaired. T cell-mediated killing is thwarted by many KSHV proteins. Both KSHV K3 and K5 are viral ubiquitin ligases that promote MHC-I degradation and prevent CD8+ T lymphocytes from activating and recognizing infected cells. Pomalidomide is an immunotherapy used to treat KSHV-associated disorders that focuses on increasing MHC-I expression to improve CD8+ T cell identification of cancer cells [41]. The MHC-I molecule restricts TCR-T cell therapy functionality. TCR-T treatment for KSHV-associated disorders may be possible when combined with pomalidomide, although there is no clinical trial to support this possibility. Selecting a particular tumor-related antigen is crucial for TCR-T and/or CAR-T cell treatment for KSHV-associated malignancy. KSHV induces a variety of IFN-T cell responses; however, they are rarely as potent as those against EBV, Cytomegalovirus, and influenza. Reactions to some antigens (like K8.1) may offer more protection against viral reactivation than others (like ORF73/LANA). These findings shed light on the possible efficacy of KSHV-specific T cell immunotherapy using modified T cells to target K8.1 [40].
5 THE CHALLENGE WITH ENGINEERED T CELLS IN ONCOGENIC VIRAL ANTIGENS
While the use of oncogenic viral antigens in engineered T cell immunotherapy is generally safe and well tolerated, there are still some challenges regarding this novel therapy. There is an inherent danger with TCR-T-cell treatment that an engineered TCR will identify healthy tissues that express the same antigen, leading to an immune response, and that TCR-T cells will cross-react with comparable antigens. Prior to human administration, comprehensive cell line and animal research is always carried out; however, it can often be challenging to foresee potential effects [4]. Furthermore, patients often show poor physical conditions, and HBV-specific engineered T cell treatment may cause significant liver inflammation due to the death of HBV-infected normal hepatocytes (on target off-tumor consequences) [18].
The most frequent adverse event after CAR-T infusion is CRS, which is brought on by T-cell activation upon engagement of CAR-T with their targets and subsequent induction of a systemic inflammatory response [42]. Fever, tachycardia, hypoxia, nausea, headache, rash, shortness of breath, mild or severe hypotension requiring vasopressors, respiratory failure, coagulopathy, and/or multi-organ system failure are possible symptoms of CAR T-cell-induced CRS [43]. Significant toxicities that are directly linked to the activation of potent immune effector responses occur in up to one-third of patients [44]. Grade 3 to Grade 5 CRS may threaten the patients' lives.
Traditional definitions of tumor heterogeneity have focused on the genetic components; however, tumor heterogeneity is now understood to encompass a wide range of factors, including geographic heterogeneity, intratumoral heterogeneity, and epigenetic plasticity [45]. Antigen escape is caused by tumor heterogeneity, which is manifested in several locations within the same tumor or its recurring lesion. One of the main mechanisms for the tumor's return following the ACT is the lack of the target antigen [46].
The ecosystem or community in which malignant cells live and grow is defined as the TME [47]. Through a sophisticated network made up of both cellular and noncellular elements, the TME creates a physical barrier around the tumor cells. Studies have revealed that rather than being spectators, the TME components actively participate in the onset and maintenance of carcinogenesis. Through persistent proliferation and immune escape, the TME plays a crucial role in a number of tumor biological processes, including pathogenesis, progression, metastasis, and treatment resistance [48]. For example, when a patient with HCC has HBsAg in their blood circulation, the TME causes T-cell fatigue, which impairs the immune system's ability to remove the virus and virus-infected cells [18].
6 THE FUTURE OF VIRAL ANTIGEN–SPECIFIC ENGINEERED T CELLS IN ONCOGENIC VIRUS–ASSOCIATED HUMAN CANCER
A more standardized and uniform manufacturing method is fundamentally required for the further deployment of engineered ACT technology. However, although reinvigorating or expanding autologous virus-specific T cells is attempted, it is often not effective in completely curing cancer. This is most likely because of immune-evasion mechanisms, such as the deletion of T cells with the highest affinity. However, because neither negative thymic selection nor clonal deletion by the tumor has taken place, the isolation of such antiviral TCRs with high affinity is possible from healthy donors. Adoptive immunotherapy for viral infection has been successfully proven by the transfer of viral-specific T cells from a stem-cell donor or from third-party cell banks in the setting of allogeneic hematopoietic stem cell transplantation [5]. The production of ACT using allogenic T cells that have been modified to be slightly immunoreactive to the host is a straightforward and universal approach. Increased scalability of CAR-based cell treatments will help reduce the burden of cancer incidence as a result of the development of off-the-shelf techniques. However, the host immunological rejection of allogeneic implants must be circumvented through the use of standardized donor-derived cells. One study showed that it is possible to lower the risk of graft-versus-host disease by disrupting MHC expression with K3 and K5 proteins from HHV8 [49]. T-iPSCs, which maintain the assembled “endogenous” TCR gene after nuclear reprogramming, are iPSCs that have been induced by T cells. Notably, the TCR gene arrangement in T cells that were generated from T-iPSCs was identical to that in native T cells. As a result, T-iPSCs represent an effective starting material for the growth of many T cells, each of which expresses TCRs specific to an antigen [16].
7 SUMMARY
Oncogenic viral antigen–specific engineered T cell therapy for virus-associated cancer may not be likely to induce “off-target” effects to related peptides or when it recognizes the cognate as antigen “off-organ” in healthy tissue. Viral antigen targets are unique and differ from “self-antigens”, as they are expressed only in virus-associated tumors. However, effectively addressing the practical challenges of selecting viral antigens that maintain expression in the tumor during viral infection is of utmost importance. Other challenges such as the CRS, tumor heterogeneity, immunosuppressive TME, and high financial cost are of concern and should be addressed. A series of phase I/II clinical trials on the basis of numerous preclinical studies is ongoing. Extensive research is currently being conducted to determine the optimal dosage and administration route for viral antigen–specific ACT products. To decrease the recurrence and metastasis rates, reduce adverse drug reactions, and improve the quality of life of patients, many treatments including viral specific engineered ACT combined with surgery, radiotherapy, chemotherapy, or other immunotherapies should be studied to facilitate the treatment of virus-associated cancer.
AUTHOR CONTRIBUTIONS
Haipeng Zhang: Conceptualization and draft preparation; writing/original draft preparation. Sha Wu and Xiaohong Li: Supervision. Haipeng Zhang, Xiaohong Li, Sha Wu and Jing Chen: Review and editing. Sha Wu and Xiaohong Li: Project administration. Xiaohong Li: Funding acquisition. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable.
CONSENT TO PARTICIPATE
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.




