Molecular residual disease: A new clue for individualized approach in non-small cell lung cancer
Abstract
The prognostic value of molecular residual disease (MRD) in non-small cell lung cancer (NSCLC) cases using high-depth circulating tumor DNA (ctDNA) sequencing has been well documented. The utility of MRD to direct individualized therapy has increasingly emerged in the clinical trial design of solid tumors, such as escalation or de-escalation of adjuvant therapy based on MRD. And the efficiency of MRD assay is a key determinant to the success of clinical trials, especially the limitation of detection and predictive value. Here, we review the progress made in evaluating the clinical validity of ctDNA-MRD test and provide insight into exploiting these developments to future clinical scenarios for improving the individualized therapy of NSCLC.
Abbreviations
-
- cfDNA
-
- cell-free DNA
-
- CRT
-
- chemoradiotherapy
-
- ctDNA
-
- circulating tumor DNA
-
- LOD
-
- limit of detection
-
- MRD
-
- molecular residual disease
-
- NPV
-
- negative predictive value
-
- NSCLC
-
- non-small cell lung cancer
-
- PPV
-
- positive predictive value
1 INTRODUCTION
Molecular residual disease (MRD) refers to the hidden state under the traditional medical imaging modalities (including positron emission tomography and computed tomography) after treatment. Tumor-derived molecular can be detected by liquid biopsy, and they represent persistent cancer and potential disease progression [1]. The significance of MRD lies in its strong correlation with disease recurrence or progression in patients who were presumed to have achieved a tumor-free status. Monitoring of MRD is essential to assess treatment effectiveness and establish the individualized therapy for patients who achieve complete response after curative treatment in hematologic tumors, such as acute lymphoblastic leukemia, multiple myeloma, and acute [2-8].
In recent years, there is growing evidence supporting the value of MRD in non-small cell lung cancer (NSCLC) through the ultra-deep sequencing of circulating tumor DNA (ctDNA) [9-15]. And it is increasingly clear that patients with detectable ctDNA-MRD indicate the presence of residual tumor and insidious progression. Currently, two well-known ctDNA-based detection strategies are available for MRD: (1) tumor-informed strategy, which uses the primary tumor's mutational compendium to design the individualized panel of genetic locus and analytic filtering process (e.g., Signatera [9] and ArcherDx assays [16]); (2) tumor-agnostic strategy, which designs a fixed but high-coverage gene panel by a bioinformatics approach for a specific cancer type of interest (e.g., cancer-personalized profiling by deep sequencing (CAPP-Seq) [10] and Guardant Reveal assay [17]). Although these platforms are not directly comparable, several essential evaluation indexes of ctDNA-based MRD detection should be noted, such as limit of detection and sensitivity, which set them apart from the ctDNA-based genotyping test that is frequently used in the advanced stage [18, 19].
Here, we propose the particularities of MRD test in NSCLC and various evaluation parameters of ctDNA-MRD assay, including technically and clinically relevant parameters. And we further discuss the future clinical scenarios of MRD in NSCLC and highlight the concept of drug holiday based on the negative predictive value of MRD.
2 DETECTING MRD IN PATIENTS WITH LUNG CANCER
MRD assessment in cases of lung cancer should need to be treated differently from other tumor types. First, oncogenic driver mutations are responsible for a distinct subgroup of NSCLC, and some of these can be targeted using small-molecule inhibitors. Up to 70% of advanced NSCLC cases have at least one potentially actionable mutation (e.g., EGFR, ALK, and ROS1) [20-22]. Second, the detectability of ctDNA varies according to cancer types with the greatest detectability observed for small cell lung cancer (91.1%) and prostate cancer (87.9%) but only moderate detectability for NSCLC (allelic fractions of 0.1–0.01) [23]. Furthermore, the NSCLC genotype can significantly affect the ctDNA-based variant allele frequency, and TP53 and EGFR mutations markedly increase ctDNA shedding [24].
Therefore, MRD detection for lung cancer has its unique uncertainties (Box 1), which originates from the understanding of tumor heterogeneity and individualized treatment of NSCLC. Moreover, due to different detection principles for MRD assay in lung cancer, no unified standards and thresholds are available. And it is a great challenge to evaluate the effectiveness of a commercial MRD test. Thus, we summarized the basic technically and clinically relevant parameters of currently available MRD assays in Table 1.
Box 1 Uncertainties about ctDNA-MRD in NSCLC.
-
#1. Differences between targetable and untargetable ctDNA variants.
-
The majority of NSCLC cases have at least targetable mutation (e.g., involving EGFR, ALK, and ROS1). the narrow definition of MRD for lung cancer is still controversial. Thus, whether untargetable ctDNA variants derived from the tumors with targetable mutation has any clinical significance is unknown. For example, ctDNA detected variants containing wild-type or mutant d EGFR might affect the selection of adjuvant treatment, TKIs, or chemotherapy.
-
-
#2. The meaning of tier II/III ctDNA variants in NSCLC.
-
According to the four-tiered system for cancer somatic variants, tier I represents variants with strong clinical significance; tier II, variants with potential clinical significance; tier III, variants of unknown clinical significance; and tier IV, variants deemed benign or likely benign. Thus, from a clinical perspective, the patient-specific tier II/III somatic variants detected by MRD assay are not well understood.
-
-
#3. MRD detection through the cerebrospinal fluid.
-
Current approaches for MRD detection in lung cancer rely on ctDNA circulating in the peripheral blood. Previous studies have suggested its limited efficiency for those with central nervous system metastases due to the blood–brain barrier. Thus, it is unclear that whether cerebrospinal fluid could be used as source for MRD detection.
-
-
#4. MRD detection in the context of radiotherapy.
-
Radical radiotherapy, another important curative treatment for many cancer types, is known to trigger apoptosis, necrosis, senescence, and mitotic catastrophe in cancer cells by inducing DNA damage, which differs completely from the all-or-nothing effect of surgery. There is a lack of evidences elucidating the optimal time and prognostic value for ctDNA detection in the process of radiotherapy.
-
-
#5. ctDNA methylation as MRD detection source in NSCLC.
-
In colorectal cancer, previous study suggested that integration of epigenomic signatures could increase the sensitivity versus genomic alterations alone. Whether ctDNA methylation could be a reliable MRD detection source in NSCLC remain unknown.
-
ctDNA, circulating tumor DNA; MRD, molecular residual disease; NSCLC, non-small cell lung cancer; TKI, tyrosine kinase inhibitor.
| Individualized panel | Fixed panel | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Index/Technical approaches | Signatera-Natera [9] | Archer Dx-Invitae [16] | RaDaR-Inivata [12] | brPROPHET-Burning Rock [28] | Roche Avenio [10] | OncoMRD-Geneplus [14] | SHIELDING-Geneseeq [15] | MinerVa-Genecast [13] | |
| Technical parameter | Blood volume/cfDNA input | 20 mL | 20 mL | 20 mL | 20–60 ng | 10 mL | 20 mL | 20 mL | 20 mL |
| Variants tracked/panel coverage | 16 | 200 | 48 | 50 | 197 genes | 338 genes | 139 genes | 769 genes | |
| Depth (×) | 100,000 | 100,000 | 100,000 | 100,000 | 10,000 | 30,000 | 30,000 | 30,000 | |
| Limit of detection (sample level) | 0.01% | 0.0011% | — | 0.004% | 0.01% | 0.02% | 0.02% | 0.007% | |
| Clinical parameter | PPV | 93% | — | 90% | 88% | 100% | 89.1% | 77.1% | 80.7%a |
| NPV | 90% | (Sensitivity: 85.3%) | 82.5% | 68% | 100% | 96.8% | 87% | 83.8%a | |
| Lead time (median) | 70 days | 136 days | 212.5 days | 318 days | 5.2 mos | 3.4 mos | 88 days | — | |
- Abbreviations: mos, months; NPV, negative predictive value; PPV, positive predictive value.
- a Landmark detection.
2.1 Blood draw
A previous study has indicated that the median cell-free DNA (cfDNA) concentration in plasma was 7.69 ng/mL among patients with early stage NSCLC who received a definitive therapy [25], which suggests that approximately 20,000 haploid genome equivalents (3.3 pg) are contained in 10 mL of plasma. Assuming a library preparation efficiency of 50% and that at least two molecules are needed to confidently identify a variant, the physical detection limit of ctDNA analysis would be approximately 0.02% in 10 mL of plasma. In addition, a previous study has predicted that patients who have a 1 cm [3] tumor would have two tumor DNA molecules in 10 mL of plasma [9]. Thus, these data serve as a reminder that high-depth ctDNA sequencing should guarantee a sufficient amount of blood draw. And most of the MRD assays require 20 ml blood (Table 1).
2.2 Limit of detection (LOD)
LOD is defined as the ctDNA concentration at which 95% of clinical samples will be called positive [26]. For mutation-based next-generation sequencing (NGS) ctDNA-MRD assays, the number of mutations tracked, the sequencing depth at mutant positions and the cfDNA input can all affect the LOD performance. In fact, most ctDNA analysis approaches have not published reliable LOD. Meaningful LODs should be determined in settings that match clinical samples as closely as possible, including in the amount of cfDNA input.
2.3 Predictive value
Most of the previous studies focused on the positive predictive value (PPV) of MRD. However, in our previous study [14], we found that the negative predictive value (NPV) of longitudinal undetectable MRD was 96.8% for those localized NSCLC patients after definitive resection, and we further conclude that these might represent the potentially cured population. However, as shown in Table 1, both PPV and NPV showed a large heterogeneity among different assays.
2.4 ctDNA lead time
It is well known that ctDNA-MRD detection generally precedes radiographical relapse by several months, which refers to ctDNA lead time. In a recent meta-analysis of nine eligible ctDNA studies [27], the average ctDNA lead time was 179 ± 74 days of 148 patients suffering disease progression. To date, no specific studies have explored the clinical significance of this valuable time frame. And it remains unknown whether the more sensitive MRD detection could bring longer lead time.
As mentioned earlier, these parameters cannot directly used to compare these assays. But it is essential to propose these basic parameters to distinguish MRD detection from other liquid biopsies. Uniform external quality assessment is required in the future, such as National Center for Clinical Laboratories (NCCL), Asia Pacific Laboratory Accreditation Cooperation (APLAC), and College of American Pathologists (CAP).
3 FUTURE CLINICAL SCENARIOS OF MRD IN NSCLC
3.1 Escalation therapy based on positive predictive values
Hitherto, several analyses suggest the predictive value of MRD on the adjuvant/consolidative therapy for lung cancer. Among patients from the TRACERx study with detectable ctDNA after surgery, three patients had an increased number of detectable ctDNA variants after adjuvant chemotherapy and two patients experienced recurrence within 1 year after surgery, although one patient who achieved ctDNA clearance after adjuvant chemotherapy remained disease-free for 688 days after surgery [9]. Another CAPP-Seq-based study provided additional data to support the predictive value of ctDNA-based MRD detection based on 65 patients who received definitive chemoradiotherapy (CRT) for locally advanced NSCLC, including 28 patients who received consolidation ICI [11]. In the study, there was no significant difference in freedom from progression according to the consolidation ICI treatment status among patients with undetectable ctDNA after CRT (p = 0.23) although ICI treatment provided significantly better freedom from progression among patients with detectable ctDNA after CRT (p = 0.04). In our previous study [14], adjuvant therapy was given to 55 patients of whom 10 with detectable MRD after surgery. These 10 patients had significantly longer disease-free survival compared with all other patients who were MRD-positive immediately after surgery but did not receive adjuvant therapy. Conversely, patients with undetectable MRD after surgery cannot benefit from adjuvant therapy. And this finding was further confirmed by a meta-analysis of 5 studies [27]. Taken together, these findings strongly imply that the MRD status can guide the individualized therapy for NSCLC although randomized trials are needed to confirm its predictive value. And the MRD positivity could act as a sign to escalate or enhance the therapy. This strategy might especially benefit patients who exhibit an increasing ctDNA mutation burden and help increase their clinical cure rate. Currently, multiple studies are evaluating this strategy, including the MERMAID-1 (NCT04385368), MERMAID-02 (NCT04642469), and LUN0114 (NCT04585490) trials.
3.2 Drug holiday based on negative predictive values
Long-term maintenance treatment inevitably creates long-lasting side effects and an economic burden. Therefore, a strategy of active, rational, and temporary drug discontinuation, which is described as a “drug holiday,” might be a promising application for MRD detection in patients with lung cancer (Figure 1). It is generally unusual to contemplate a drug holiday when treating most malignant diseases as the therapeutic effects often rapidly diminish when the drug is discontinued. However, high-precision MRD detection could make this strategy feasible as it can more accurately diagnose the tumor burden. Discontinuation of TKIs in chronic myeloid leukemia (CML) based on MRD negativity is a classic example of this approach. Since 2007, multiple studies have confirmed that a subset of CML patients can safely stop TKIs and achieve considerable treatment-free remission [29-35]. The key criteria for TKI discontinuation included TKI therapy for at least 3 years and a stable molecular response for at least 2 years (BCR-ABL1 transcripts ≤0.01% on the International Scale) [36-38].
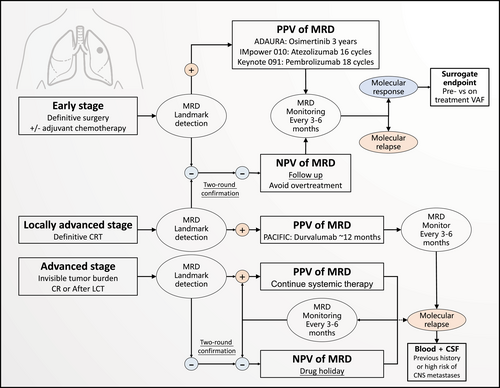
Future scenario: molecular residual disease-guided drug holiday. A proposed future scenario where molecular residual disease (MRD) can be used to guide drug holidays for different stages of non-small cell lung cancer (NSCLC). The scenario for early-stage NSCLC is based on the design of the ADAURA trial, and the scenario for locally advanced NSCLC is based on the design of the PACIFIC trial.
Delaying the expansion of intratumoral drug-resistant clones may also provide a potential theoretical basis for this approach [39]. A single-center exploratory trial enrolled 11 patients with metastatic castration-resistant prostate cancer to receive adaptive abiraterone and prednisone treatment [40]. And the drug was withdrawn when the prostate-specific antigen (PSA) concentration falls below 50% of the original value according to an evolutionary game theory model using Lotka–Volterra equations, and it was withheld until the PSA returns to its initial value [40]. The interim results revealed that only one patient experienced progression at 11 months and the median time to progression was ≥27 months, which was a significant improvement over the results from previous studies. Moreover, these patients received a cumulative drug dose that was 47% lesser than the standard dose in this setting [40]. Based on the above evidence, our team previously reported a pilot study at the 2021 World Conference on Lung Cancer (WCLC) preliminarily exploring the feasibility of drug holidays for TKI-treated oligopersistent NSCLC patients after local consolidative therapy (LCT) [41]. It is well known that LCT can improve the progression-free survival (PFS) and OS of oligometastatic NSCLC [42-45]. Hence, we sought to investigate whether MRD-guided drug holidays are feasible for TKI-treated oligopersistent NSCLC patients who achieve a complete remission status after LCT [41]. We used the tumor-informed, fixed NGS panel to sensitively detect ctDNA, with an LOD of 0.02%. Indicators of drug retreatment included radiographical relapse, detectable MRD, and elevated carcinoembryonic antigen (CEA). After screening by the invisible tumor burden and undetectable MRD, 34 patients were enrolled and undertook a drug holiday. The median duration of frontline TKI was 9.8 months. During the median follow-up of 6.9 months, 19 patients (56%) were still on the drug holiday with no evidence of disease progression; 15 patients (44%) received retreatment due to positive indicators. The median length of drug holiday was 6.3 months in the overall cohort, and the response rate of all retreated patients was 100% (all confirmed partial remission) [41]. These results suggest that an MRD-guided drug holiday is a viable option for a select population. Several trials following this strategy are ongoing, such as BESPOKE (NCT04264702) and CTONG 2101 (NCT04841811).
3.3 MRD as a surrogate endpoint
Given the strong correlation between achieving an MRD-negative status and survival outcomes in hematological malignancies, MRD has gradually become a meaningful end point in clinical trials [7, 46-48]. A surrogate endpoint that can support accelerated approval of a promising therapy is clearly needed. In breast cancer, several studies have demonstrated dynamic changes in ctDNA in the neoadjuvant setting. Despite a strong prognostic value, a decrease in or clearance of ctDNA is significantly related to tumor response after neoadjuvant therapy, especially pathologic complete response [49-52]. In a pan-cancer analysis, Zhang et al. [53] used the Guardant360 ctDNA assay to detect ctDNA VAF changes in 171 pan-cancer patients before (pretreatment) and during (on-treatment) ICI therapy, and on-treatment reductions in VAF and lower on-treatment VAF were independently associated with longer PFS and OS and an increased objective response rate. Moreover, the investigators proposed the concept of “molecular response” by integrating pretreatment and on-treatment VAF, which successfully predicted long-term survival, similar to the initial radiologic response. In the Checkmate 816 trial [54], the complete pathological response rate of patients who achieved ctDNA clearance was significantly higher than those did not. The pathological complete response rate was significantly higher among those with ctDNA clearance than among those without ctDNA clearance in both treatment groups, 46% versus 0% in the nivolumab plus chemotherapy group, and 13% versus 4% in the chemotherapy-alone group. Hence, MRD as a surrogate endpoint in lung cancer is a promising direction.
3.4 Molecular relapse
As mentioned above, ctDNA-MRD detection generally precedes radiographical relapse by several months. The rational therapeutic strategy at this molecular relapse stage remains unknown. A close observation seems to constitute expedient care for patients in this context. In general, this is a novel, conceptually challenging issue. In a multicenter, randomized controlled trial on nasopharyngeal cancer, Chan et al. [55] randomized 104 NPC patients with detectable plasma EBV DNA after CRT to adjuvant chemotherapy or observation. However, no significant difference in the 5-year relapse-free survival rate was found between the two arms. This finding suggests that not all existing treatment modalities show sufficient efficacy for residual tumors at the molecular level. From the clinical perspective on lung cancer, a clear distinction between detectable MRD of driver gene variants or nondriver gene variants is necessary. Because a low-toxicity targeted therapy can be used for druggable driver gene variants, this issue may be particularly problematic for patients with a wild-type gene.
3.4.1 MRD in the advanced stage of NSCLC
The treatment of metastatic NSCLC has evolved based on findings from landmark studies, and some patients can now expect long-term survival. For example, the KEYNOTE-024 study revealed that pembrolizumab for advanced NSCLC with a programmed-death ligand-1 (PD-L1) tumor proportion score of ≥50% provided a 5-year overall survival (OS) rate of 31.9% [56]. In the ALEX study, first-line alectinib for ALK-positive NSCLC showed a 5-year OS rate of 62.5% [57]. In the FLAURA study, osimertinib for untreated EGFR-positive NSCLC provided a 5-year OS rate of 53.7%, and 28% of the patients are still under treatment [58]. However, along with treatments becoming more effective, long-lasting treatment side effects and economic burdens must also be considered.
In an international cohort study by Lee et al., ctDNA profile of 1127 advanced NSCLC patients demonstrated that detectable ctDNA was associated with shorter OS (HR = 2.05, 95% CI: 1.74–2.42, p < 0.001) independently of clinicopathologic features and metabolic tumor volume. For those 722 (64%) patients with detectable ctDNA, 255 (23%) matched to the targeted therapy by ctDNA sequencing had longer OS than those not treated with the targeted therapy (HR = 0.63, 95% CI: 0.52–0.76, p < 0.001). These data confirmed the prognostic value of MRD in the advanced stage of NSCLC.
At present, no ctDNA-related studies are available targeting the ctDNA-MRD detection for those patients with metastatic NSCLC disease. But it is worth considering for oligometastatic disease, which is known as a distinct cancer state between locally confined and systemically metastatic diseases in terms of the clinical cure rate [59-61]. Several early clinical studies confirmed that survival improved when a radical local therapy was added to a standard systemic therapy for oligometastatic disease [43-45, 62]. Furthermore, continued improvements in the effectiveness of the targeted therapy and long-term survival have increasingly allowed patients to undergo the radical local therapy for oligometastatic NSCLC [42]. Therefore, when the definitive therapy is used for all residual diseases, it is rational to use MRD to guide subsequent maintenance treatment. A ctDNA profile analysis from a phase II trial revealed that relative to maintenance therapy or observation, the local consolidative therapy was associated with a decreased ctDNA burden, including a lower number of detected mutations and a decreased average VAF value [63]. Moreover, among six patients with serial ctDNA data, an increase in the number of ctDNA-detected mutations preceded clinical progression by 6.7 months [63]. In addition, a recent study reported the value of ctDNA in 31 NSCLC patients with long-term benefit from ICI, which was identified as progression-free survival over 12 months [64]. In this study, Hellmann et al. used CAPP-Seq to detect the ctDNA in these patients and found that 25 out of 27 patients with undetectable ctDNA (93%) remained progression-free; by contrast, all four patients with detectable ctDNA eventually progressed [64]. Hence, advanced NSCLC patients with a long-term survival benefit from the targeted therapy or immunotherapy are worthy of further exploration.
4 CONCLUSIONS
There is increasing interest in the unique role of MRD detection, which is related to the improved responses to treatment for NSCLC. However, MRD detection in patients with lung cancer still faces many challenges that need to be overcome. Prospective clinical trials are needed to confirm that MRD detection assays produce valid results in different treatment contexts.
AUTHOR CONTRIBUTIONS
Jia-Tao Zhang wrote the manuscript. Wen-Zhao Zhong and Yi-Long Wu revised the manuscript. Yi-Long Wu supervised the study.
ACKNOWLEDGMENTS
This work was supported by the Guangdong Provincial People's Hospital Young Talent Project (Grant No. GDPPHYTP201902 to Wen-Zhao Zhong). The funding sources had no role in the preparation of this manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interests.
ETHICS STATEMENT
Not applicable. No patients or animal was involved in the present review.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as all datasets were generated from published research.




