Exosomal circular RNAs in tumor microenvironment: An emphasis on signaling pathways and clinical opportunities
Abstract
Exosomes can regulate the malignant progression of tumors by carrying a variety of genetic information and transmitting it to target cells. Recent studies indicate that exosomal circular RNAs (circRNAs) regulate multiple biological processes in carcinogenesis, such as tumor growth, metastasis, epithelial–mesenchymal transition, drug resistance, autophagy, metabolism, angiogenesis, and immune escape. In the tumor microenvironment (TME), exosomal circRNAs can be transferred among tumor cells, endothelial cells, cancer-associated fibroblasts, immune cells, and microbiota, affecting tumor initiation and progression. Due to the high stability and widespread presence of exosomal circRNAs, they hold promise as biomarkers for tumor diagnosis and prognosis prediction in blood and urine. In addition, designing nanoparticles targeting exosomal circRNAs and utilizing exosomal circRNAs derived from immune cells or stem cells provide new strategies for cancer therapy. In this review, we examined the crucial role of exosomal circRNAs in regulating tumor-related signaling pathways and summarized the transmission of exosomal circRNAs between various types of cells and their impact on the TME. Finally, our review highlights the potential of exosomal circRNAs as diagnostic and prognostic prediction biomarkers, as well as suggesting new strategies for clinical therapy.
1 INTRODUCTION
Cancer is the most important social issue in current society.1 According to the 2022 cancer statistics, the number of new cancer cases is close to 20 million, and there are approximately 10 million new cancer deaths.2 Researchers predict based on population data that the number of new cancer cases will be 35 million per year by 2050, an increase of 77% compared with the level in 2020.3 In addition, the comprehensive incidence rate of men in multiple cancers is significantly higher than that of women in 2022, while there is no significant difference in mortality between men and women in cancer.2, 4, 5 In 2022, lung cancer was the most prevalent cancer, representing 12.4% of all global cancer cases. Breast cancer and colorectal cancer represent 11.6 and 9.6% of global cancer cases, respectively, while prostate cancer (PCa) and gastric cancer (GC) account for 7.3 and 4.9% of the total cancer cases.2, 6 Thus, investigating the molecular mechanisms of cancer is crucial for developing new clinical diagnostic and therapeutic strategies.
Exosomes are nanoscale extracellular vesicles (EVs) formed by endocytosis and can regulate disease progression by carrying various substances including lipids, proteins, DNA, mRNA, ncRNA (noncoding RNA, including miRNA, lncRNA, and circRNA) and metabolites. Proteins such as CD9, CD63, CD81, ALIX, HSP70, and TSG101 have been considered as biomarkers for EV detection.7 Genetic material is an important component of exosomes, with extensive research on noncoding RNA in exosomes. Compared with other types of noncoding RNA, the structure of circRNA is very unique. CircRNAs do not have a 3′-terminal poly (A) tail and a 5′-terminal structure, but rather a circular covalent closure formed by reverse and selective splicing of mRNA precursors. And the special structure of circRNA enables it to resist the degradation of exonucleases both inside and outside the cell, thus possessing high stability and exerting long-lasting functions in the cell. In addition, circRNAs have a large number in exosomes and are not easily affected by external environments, which has attracted widespread attention.8 Currently, studies have found that multiple circRNAs play a crucial role in solid tumors (including lung cancer, esophageal cancer, GC, colorectal cancer, liver cancer, pancreatic cancer, renal cancer, bladder cancer, etc.) and have the potential to become therapeutic targets. For example, circSKA3 can maintain the stability of SLUG and activate the epithelial–mesenchymal transition (EMT) process in colorectal cancer by directly binding to SLUG in vivo and in vitro experiments. The use of ASOs (antisense oligonucleotides) to inhibit the level of circSKA3 can effectively weaken the interaction between circSKA3–SLUG and alleviate the metastasis of colorectal cancer.9, 10 In addition, circRNAs regulate key biological processes, including tumor proliferation, migration, apoptosis, autophagy, stemness, and drug resistance, which are crucial for tumor development and progression.11-13
Exosomal circRNAs mediate interactions between tumor cells and the tumor microenvironment (TME). The TME primarily consists of endothelial cells, cancer-associated fibroblasts (CAFs), T cells, natural killer cells (NK cells), macrophages, and neutrophils.14-16 The interactions between various cells and signaling molecules in the TME affect the progression of cancer.17 Exosomes are usually released into the microenvironment in a paracrine manner and act on receptor cells, thereby transmitting genetic materials such as circRNAs between cells and regulating tumor angiogenesis, immune escape, and chemotherapy resistance.18, 19 CAFs, abundant stromal cells, communicate with tumor cells via exosomes to influence tumor progression in the microenvironment.20, 21 And a few exosomal circRNAs are absorbed by immune cells, and then regulate the immune escape process and affect the efficacy of immunotherapy. Exosomal circIGF2BP3 is notably increased in non-small cell lung cancer (NSCLC) and impairs CD8+ T cell cytotoxicity by preventing programmed death ligand 1 (PD-L1) ubiquitination.22 Moreover, a small portion of gut microbiota can regulate the biosynthesis of exosomal circRNAs in cells, which affects the development of tumors.23
CircRNAs in EVs hold potential as tumor diagnostic and predictive biomarkers, as well as therapeutic targets.24 Researchers identified significant differences in the expression of circ_0056285, circ_0007761, and circ_0047921 in serum exosomes of NSCLC patients compared with healthy individuals, aiding in the clinical diagnosis of NSCLC.25 In addition, EVs exhibit better biocompatibility in delivering nucleic acid substances compared with artificial nanoparticles.26, 27 Recent studies have found that exosomes derived from LoVo cells overexpressing circFNDC3B can inhibit angiogenesis and exhibit better antitumor effects in animal models of colorectal cancer.28 Another study utilized small interfering RNA (siRNA) targeting exosomal hsa_circ_0005963 to reverse oxaliplatin resistance by regulating the miR-122/PKM2 axis and provide a new strategy for tumor therapy in colorectal cancer.29 Therefore, in-depth research on new specific therapeutic approaches targeting circRNAs in vivo has demonstrated strong superiority.
In this review, we will summarize the downstream target genes and signaling pathways of exosomal circRNAs involved in key biological processes, including tumor growth, metastasis, apoptosis, autophagy, drug resistance, and immune escape. In addition, we will elucidate the molecular mechanisms of exosomal circRNA interactions within various cells of the TME, including tumor cells, tumor-associated fibroblasts, immune cells, and gut microbiota. Finally, we will delve into the clinical application prospects of exosomal circRNAs in tumor diagnosis, patient prognosis prediction, drug efficacy prediction, and tumor therapy, providing promising new diagnostic and treatment strategies for cancer patients.
2 BIOGENESIS OF EXOSOMES AND EXOSOMAL circRNAs
EVs are membrane-derived vesicles secreted by cells and released into the extracellular environment. EVs are mainly divided into three types: exosomes, microvesicles, and apoptotic bodies.30 Generally speaking, cells can secrete EVs through budding or fuse with the cytoplasmic membrane through endosomes, thus forming exosomes.31 Given the significant impact of exosomal circRNAs on tumor progression, the EVs mentioned in this article specifically refer to exosomes. EVs are composed of phospholipid bilayers and can be released into blood and urine.32 Currently, EVs are usually extracted from blood and the culture supernatants from cells.33 The ultracentrifugation method is the most commonly used way for extracting EVs, which can remove cell debris through low-speed centrifugation and extract EVs through high-speed centrifugation.34 The conventional circRNA detection methods are also applicable to circRNA in EVs, including PCR, microarray, high-throughput sequencing, and northern blotting.35-37 Although researchers have studied various new methods for detecting exosomal miRNAs, further technological progress is still needed for the detection of circRNAs in EVs.38
Initially, EVs were seen as garbage bins for clearing redundant components within cells.39 With the increasing research on EVs, researchers have elucidated the crucial role of EVs in intercellular communication and their regulation of intracellular biological processes in various diseases.40-42 Exosomes start from endocytosis of the cell membrane and can produce early endosomes through the formation of buds within the cell. Subsequently, the early endosomes gradually mature and encapsulate proteins and nucleic acids, forming several intrinsic vessels and developing into multivesicular bodies.43 Finally, cell membrane fusion is mediated under the action of CD63, LAMP1, and LAMP2, followed by the release of EVs into the microenvironment.44
The contents of EVs mainly include DNA, mRNA, proteins, and ncRNA, which can act on receptor cells and regulate cancer progression.45-47 The content of these contents in exosomes may be closely related to the characteristics and progression of the disease.48 Previous studies on cancer have reported that EVs secreted by different tumor cells affect biological processes such as tumor growth, invasion, and drug resistance by fusing with receptor cell membranes.49-51 In addition, EVs have the ability to transmit antigens, regulate antigen presentation, and control tumor immune niches due to their surface facial membrane and special lumen structure. pMHCs (APCs present antigenic peptides) on EVs directly bind to antigen-specific T cells, which can induce T cell activation, including T cell proliferation, cytotoxicity, and cytokine production.52, 53 In vivo studies have shown that EVs can also transmit EVs to DC cells, leading to the production of CD4+ and CD8+ T cells. Moreover, EVs derived from DC cells can stimulate T cell activity in the absence of antigen-presenting cells (APCs) by carrying costimulatory molecules and pMHC.54, 55
Circular RNAs (circRNAs) are usually nonprotein coding RNAs that are spliced backward from their precursor mRNA into a loop.56 Due to its unique structure, circRNA can evade the action of exonucleases and has strong conservation, making it a potential biomarker for clinical applications.57-59 In the initial stage of tumors, the latest research has found that circRNAs can drive genetic mutations in hematological cancers, such as acute leukemia.60 CircRNAs can be enriched in MLL (the mixed linear leukemia) recombinants and bind to DNA, forming circRNA: DNA hybrids (circR loops). These circR loops promote chromosome recombination, induce mutations, and reduce genomic stability, which leads to the occurrence of acute leukemia.61 In addition, circRNAs are also involved in the stages of tumor metastasis. CircRNAs regulate the invasion, migration, angiogenesis, EMT process, and metastatic niche formation to achieve distant extravasation and colonization of cancer cells.62 The regulatory process of circRNAs can be achieved through various molecular mechanisms. Studies have demonstrated that circRNAs regulate tumor development through various molecular mechanisms.63 Most circRNAs can regulate downstream target proteins and pathways by competitively binding to miRNAs (also called competing endogenous RNA, ceRNA), thereby affecting the malignant progression of tumors.64 Certain circRNAs can directly interact with proteins to influence tumor growth, metastasis, and drug resistance.65 A small portion of circRNAs can form short peptides through translation and regulate cancer progression.66 Elucidating the molecular mechanisms of circRNA is crucial due to its involvement in biological processes like tumor growth and metastasis.
The biological process of circRNA encapsulation into EVs involves a large number of proteins, but its specific molecular mechanism remains unclear. It is speculated that the packaging of circRNAs into exosomes may be regulated by the following types of proteins: exosome-related proteins, RNA binding proteins (RBPs), and transmembrane transporters.67, 68 Among them, exosome-related proteins directly affect the synthesis of exosomal circRNAs by regulating membrane formation, circRNA loading and releasing.69 And RBPs can stabilize the generation of circRNA and regulate its entry into exosomes.70 Transmembrane transporters on EV membranes may regulate circRNA transport.71 Recent studies indicate that the protein hnRNPA2B1 can encapsulate various genetic materials (such as circRNAs) into exosomes.72, 73 For example, hnRNPA2B1 can directly bind to circNEIL3 and maintain the expression of circNEIL in exosomes, thereby delivering it to macrophages and promoting glioma development.74 In-depth research on the biosynthesis, composition and function of exosomal circRNAs can further understand cancer progression and provide new targets and strategies for the diagnosis and treatment of tumors.
3 DOWNSTREAM SIGNALING PATHWAYS IN TUMORIGENESIS REGULATED BY circRNAs
CircRNAs alter the activity of various downstream signaling pathways, including JAK/signal transduction and transcription activation factor 3 (STAT3), MEK–ERK, PI3K/AKT, Wnt/β-catenin, and Notch signaling pathways through these regulatory mechanisms in tumorigenesis.
3.1 JAK/STAT3 signaling pathway
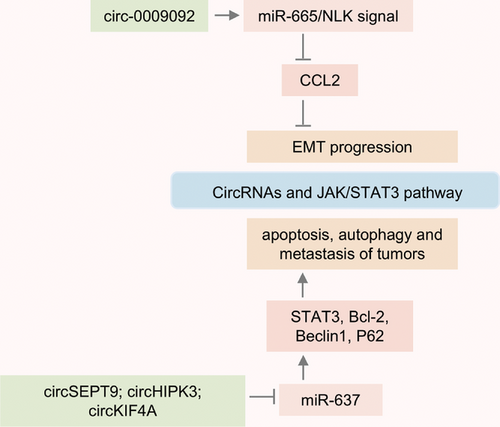
STAT3 plays a crucial role in cell proliferation, apoptosis, survival, and metastasis, acting as an oncogenic transcription factor.75 And STAT3 can act as a downstream target for various growth factors (TGF-α/β, EGF, and PDGF) and cytokines (IL-5, IL-6, IL-10, IL-11, IL-12, IL-20, TNF-α, and IFNγ).76 In recent years, research has found that circRNAs can regulate multiple target proteins of the STAT3 signaling pathway, and these STAT3-related proteins are upregulated in various types of tumors.77-79 Circ-0009092 regulates STAT3 modification and reduces CCL2 expression by targeting the miR-665/NLK signaling axis, which inhibits tumor-associated macrophage aggregation and slows down the EMT progression of tumor.80 In triple-negative breast cancer (TNBC), circSEPT9 is synthesized with the participation of E2F1 and EIF4A3 proteins and is significantly upregulated in tumor tissues. Research indicates that inhibiting circSEPT9 can induce tumor cell apoptosis and inhibit tumor growth by downregulating the LIF/STAT3 signaling pathway.81 CircRNAs also affect autophagy by regulating the STAT3 signaling pathway. The expression of circKIF4A in brain metastasis from TNBC was significantly increased, and it could promote the expression of STAT3 and p62 through competitive adsorption of miR-637, which induced tumor autophagy and metastasis.82 The expression of circHIPK3 is increased in tissues of colorectal cancer patients and is closely related to tumor volume, degree of metastasis, and survival.83 Also, researchers have identified that circHIPK3 targets the miR-637/STAT3 signaling axis to upregulate Bcl-2 and Beclin1 expression, thereby inhibiting autophagy and inducing oxaliplatin resistance in colorectal cancer.84 Therefore, STAT3 regulated by circRNA is involved in tumor cell proliferation, metastasis, apoptosis, and chemoresistance. And targeted inhibition of STAT3 activation has the potential to become one of the better strategies for reversing tumors (Figure 1).
3.2 MEK–ERK signaling pathway
The MEK–ERK signaling pathway is among the most extensively studied signaling cascades.85, 86 In humans, the effectors of signaling cascade reactions are mainly divided into three categories: (1) receptor tyrosine kinases of the HER family, such as RTKs, EGFR, and HER2-4; (2) A/B/CRAF (serine/threonine kinase of the RAF family); (3) MEK1/2 and ERK1/2 (dual specificity protein kinase family).87-89 The inactivation of the ERK signaling pathway can lead to the development of autoimmune and neurodegenerative diseases, while the activation of the MEK–ERK pathway promotes cancer metastasis and drug resistance.90 Numerous evidence suggested that over 40% of cancers were closely related to dysregulation of the MEK–ERK pathway.91
CircRNAs in tumors can regulate the MEK–ERK signaling pathway and affect cancer progression.92, 93 CircPDIA4 is induced by the protein Quaking (the RBP) and inhibits the dephosphorylation of ERK1/2 by interacting with it, maintaining the high activity of the MAPK signaling pathway in the cytoplasm. In the nucleus, circPDIA4 interacts with DHX9, enhancing circRNA biosynthesis and promoting GC growth and metastasis.94 And circUBE4B encodes a new procancer peptide (circUBE4B-173aa) and promotes the expression of phosphorylated MAPK1 by directly binding to MAPK1, which stimulates downstream MAPK/ERK signaling axis and promotes tumor malignant progression in the esophageal squamous cell carcinoma (ESCC).95 In addition, circRNAs affect chemoresistance by regulating the MEK–ERK signaling pathway in tumorigenesis. In TNBC, the overexpression of circZCCHC2 can lead to the decrease of miR-1200 expression and the increase of TPR expression, which induces the activation of RAS–RAF–MEK–ERK cascade signaling and inhibits the sensitivity of tumor cells to pirarubicin therapy.96 In pancreatic ductal adenocarcinoma (PDAC), circRPS29 can directly bind to miR-770-5p and increase TRIM29 levels in vivo and in vitro, subsequently activating the downstream MEK/ERK signaling axis and inducing the emergence of gemcitabine resistance.97 Moreover, circPCNXL2 is significantly upregulated in the tissues of patients with intrahepatic cholangiocarcinoma (ICC) and promotes the interaction of STEP and MEK1/2 by directly binding to the protein STEP, thereby upregulating the ERK/MAPK axis to accelerate tumor progression and reducing the antitumor effect of trametinib.98 Thus, in-depth research on the mechanism of ERK signaling dysregulation can provide new treatment options for alleviating tumor drug resistance.
3.3 PI3K/AKT signaling pathway
The PI3K/AKT signaling pathway initiates with PI3K activation, leading to elevated levels of PIP3 and AKT, thereby promoting tumor cell proliferation and survival.99, 100 The abnormal activation of the PI3K/AKT pathway enhances tumor cell migration, invasion and chemotherapy resistance.101, 102 CircRNAs can interact with the PI3K/AKT pathway. Specifically, circRNAs can directly bind to PI3K/AKT pathway-associated proteins and affect tumor growth by regulating phosphorylation processes.103-105 CircZNF215 expression is significantly elevated in postoperative metastatic ICC tumors and is closely associated with patient prognosis. Moreover, circZNF215 competitively binds to PRDX1, inhibiting the interaction between PRDX1 and PTEN, thereby reducing the activity of the PTEN/AKT signaling pathway and promoting tumor metastasis as well as ipatasertib resistance.106 Moreover, circRNAs can modulate the activity of the PI3K/AKT pathway by competitively binding to miRNA. CircRPPH1 synthesized under the action of ZNF460 protein can regulate the expression of ITGA5 by directly binding to miR-326, and then induce the activation of PI3K/AKT signal axis and accelerate the progress of TNBC.107 In addition, the latest research has found that a few circRNAs can also encode peptides and regulate tumor development by interacting with proteins in the PI3K/AKT pathway. CircTRIM1 can be translated into a new protein TRIM1-269aa due to its IRES element and an 810 nt open reading frame. Functionally, circTRIM1 encoded TRIM1-269aa enhances chemoresistance in TNBC by activating the PI3K/AKT/mTOR signaling pathway both in vivo and in vitro.108 CircPDE5A encodes a novel protein (PDE5A-500aa) that directly binds to PIK3IP1, thereby reducing PI3K/AKT signaling activity and inhibiting tumor proliferation and metastasis in ESCC. In addition, researchers used the Meo–PEG–S–S–PLGA platform to load circPDE5A and protein PDE5A-500aa for tumor therapy, and the results showed that it can effectively alleviate tumor progression.109 In general, further research on the circRNAs/PI3K/AKT signaling axis can provide new strategies for drug therapy and combination therapy in tumors.
3.4 Wnt/β-catenin signaling pathway
The Wnt signaling pathway regulates diverse biological processes, including cell proliferation, cell death, cell cycle, stemness maintenance, and embryonic development.110, 111 The Wnt signaling pathway mainly includes two signal transduction pathways: classical and nonclassical pathways. Mutations in genes related to the Wnt signaling pathway, such as APC, RNF43, AXIN, and CTTB1, typically induce the development of multiple tumors. Also, mutations in FZD7, WNT11, WNT5A, VANGL1, and VANGL2 facilitate tumor cell migration, exacerbating cancers such as colorectal cancer, breast cancer, PCa, and ovarian cancer.112-114 In bladder cancer, circNIPBL is significantly elevated and enhances Wnt5a expression and Wnt/β-catenin activity by directly binding to miR-16-2-3p. The key protein ZEB1 in the Wnt/β-catenin signaling pathway can bind to NIPBL pre-mRNA, thereby relying on a positive feedback mechanism to promote the biosynthesis of circNIPBL and tumor growth.115 Overexpression of circFBXO7 in ovarian cancer cells can increase the level of MTSS1 by downregulating the level of miR-96-5p, inducing the accumulation and nuclear transport of β-catenin, as well as phosphorylation of GSK3β, ultimately leading to the deterioration of ovarian cancer.116 Another circMMD enhances DVL1 levels by disrupting FIR and FUBP1 interaction and activates FZD6 expression by targeting miR-15b-5p. This results in continuous activation of the Wnt/β-catenin signaling pathway, thereby worsening glioma malignancy.117 In addition, circRNAs target the Wnt/β-catenin axis through binding with protein and then regulate the tumor progression. CircMTCL1 activates the expression of C1QBP and reduces the phosphorylation of β-catenin protein by directly recruiting protein C1QBP, which activates the Wnt/β-catenin signaling axis and exacerbates tumor growth and migration in laryngeal cancer.118 Reducing circ-CCT2 expression diminishes TAF15 recruitment, destabilizes PTBP1 mRNA, and downregulates Wnt/β-catenin axis activity, thereby influencing hepatoblastoma development.119 Thus, circRNAs lead to the abnormal activation of Wnt signaling, which promotes the malignant progression of various tumors.
3.5 Notch signaling pathway
The Notch signaling pathway exhibits high conservation during the evolutionary process, regulating tissue homeostasis, organ development, and determining cell fate.120 Recent scientific research indicates that the Notch signaling pathway can act as both a tumor suppressor and a protumor factor in cancer progression.121, 122 The dysregulation of the Notch signaling pathway enhances tumor invasiveness by accelerating angiogenesis and EMT progression.123, 124 And circRNAs participate in the regulation of the Notch signaling axis in tumor occurrence and development. Researchers conducted differential analysis between tumors and adjacent tissues and identified 11 circRNAs that were significantly differentially expressed in liver cancer. Among them, the expression of hsa_circ_001726 significantly increased in liver cancer tissues and cells, upregulating PRMT9 levels by binding to miR-671-5p. It subsequently enhanced liver cancer proliferation, metastasis, and EMT by activating the Notch signaling pathway.125 In addition, the upregulation of circ_0008532 in bladder cancer can inhibit the expression of miR-330-5p and miR-155-5p, induce the level of downstream target gene MTGR1 and activate Notch signal axis, which enhances the invasive ability of tumors.126 Moreover, the absence of circFBXW7 promotes leukemia cell proliferation by regulating MYC and NOTCH1 protein levels in both in vivo and in vitro experiments, indicating its crucial role in the progression of T-cell acute lymphoblastic leukemia.127 A few circRNAs inhibit tumor growth and metastasis by regulating the Notch signaling pathway. Recent studies indicate that circCRIM1 absorbs miR-146a-5p, leading to the upregulation of NUMB, which inhibits Notch signaling and reduces osteosarcoma (OS) cell proliferation, invasion, and migration.128 Therefore, circRNAs regulate the Notch signaling pathway in the TME, balancing protumor and tumor-suppressive effects, and offering new therapeutic strategies for malignant tumors (Table 1).
| Pathways | CircRNAs | Cancer type | Expression | Targets | References |
|---|---|---|---|---|---|
| JAK/STAT3 | circ-0009092 | Colorectal cancer | Down | miR-665/NLK | 80 |
| circSEPT9 | TNBC | Up | miR-637/LIF/STAT3 | 81 | |
| circKIF4A | TNBC | Up | miR-637/p62 | 82 | |
| circHIPK3 | Colorectal cancer | Up | miR-637/STAT3 | 84 | |
| MEK–ERK | circPDIA4 | Gastric cancer | Up | ERK1/2/STAT3 | 94 |
| circUBE4B | ESCC | Up | circUBE4B-173aa | 95 | |
| circZCCHC2 | TNBC | Up | miR-1200/TPR | 96 | |
| circRPS29 | PDAC | Up | miR-770-5p/TRIM29 | 97 | |
| circPCNXL2 | ICC | Up | STEP/MEK1/2 | 98 | |
| PI3K/AKT | circZNF215 | ICC | Up | PRDX1/PTEN | 106 |
| circRPPH1 | TNBC | Up | miR-326/ITGA5 | 107 | |
| circTRIM1 | TNBC | Up | TRIM1-269aa | 108 | |
| circPDE5A | ESCC | Down | PDE5A-500aa | 109 | |
| Wnt/β-catenin | circNIPBL | Bladder cancer | Up | miR-16-2-3p/Wnt5a | 115 |
| circFBXO7 | Ovarian cancer | Up | miR-96-5p/MTSS1 | 116 | |
| circMMD | Glioma | Up | miR-15b-5p/FZD6 | 117 | |
| circMTCL1 | Laryngeal cancer | Up | C1QBP/β-catenin | 118 | |
| circ-CCT2 | Hepatoblastoma | Up | TAF15/PTBP1 | 119 | |
| Notch | hsa_circ_001726 | Liver cancer | Up | miR-671-5p/PRMT9 | 125 |
| circ_0008532 | Bladder cancer | Up | miR-330-5p/miR-155-5p | 126 | |
| circFBXW7 | T-cell acute lymphoblastic leukemia | Down | MYC/NOTCH1 | 127 | |
| circCRIM1 | Osteosarcoma | Down | miR-146a-5p/NUMB | 128 |
4 CHARACTERISTICS AND FUNCTIONS OF EXOSOMAL circRNAs
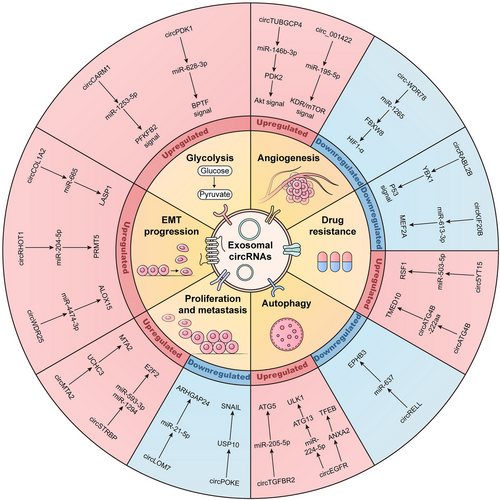
Exosomes carry various genetic molecules, including circRNAs. Numerous studies have demonstrated that the expression of circRNAs in exosomes is significantly dysregulated during tumor progression, which can affect tumor occurrence and development by regulating biological processes such as proliferation, metastasis, EMT progression, autophagy, glycolysis, and chemotherapy resistance (Figure 2).129, 130
4.1 Exosomal circRNAs regulate tumor proliferation and metastasis
Exosomal circRNA plays an oncogenic role in different types of tumors, which promotes tumor growth and metastasis. CircMTA2 is a circRNA transmitted by exosomes that can inhibit the ubiquitination process of MTA2 protein and stabilize the MTA2 structure by directly binding to UCHL3, thereby exacerbating the occurrence and invasion of GC.131 In addition, circ_0008717 in EVs can activate the levels of downstream target PAK2 in a miR-1287-5p-dependent manner, promoting the occurrence and metastasis of NSCLC.132 And the exploration in an in vivo xenograft tumor model indicates that the exosomal circSTRBP derived from GC cells was found to activate downstream E2F2 (E2F transcription factor 2) and enhance the progression of GC by absorbing miR-593-3p and miR-1294.133 Moreover, researchers screened circARID1A with significantly increased expression in glioblastoma through array analysis and found that circARID1A is abundant in exosomes. In-depth exploration revealed that exosomal circARID1A accelerates tumor invasion and migration by absorbing miR-370-3p and upregulating TGFBR2 levels.134
Certain exosomal circRNAs can suppress tumor cell proliferation and migration. In OS, the exosomal circLMO7 relies on miR-21-5p to increase the level of ARHGAP24, which acts as a tumor suppressor to slow down cancer progression.135 CircSTAU2, transmitted via exosomes, regulates CAPZA1 expression by absorbing miR-589, thereby inhibiting GC progression.136 Also, circPOKE exists in the exosomes of breast cancer cells and can reduce its binding with snail (the crucial regulator of the EMT process) by directly binding to USP10 protein, thus inhibiting the metastasis of breast cancer cells rather than proliferation.137
4.2 Exosomal circRNAs regulate angiogenesis and EMT progression
Exosomal circRNAs can regulate tumor migration and invasion through their regulatory role in angiogenesis. CircTUBGCP4 derived from exosomes of colorectal cancer cells can increase PDK2 expression and promote AKT signaling pathway activation by targeting miR-146b-3p, thereby promoting endothelial cell migration and angiogenesis.138 And the exosomal circ-WDR78 mediates the inactivation of the HIF1-α signaling pathway and inhibits the progression of NSCLC cells by absorbing miR-1265 and regulating the expression of FBXW8.139 Also, the expression of circ_001422 in serum exosomes of colorectal cancer patients is significantly increased and positively correlated with the degree of lymph node metastasis. And the exosomal circ_001422 increases the proliferation and migration process of endothelial cells and activates KDR/mTOR signaling and colorectal cancer progression by inhibiting miR-195-5p activity.140 Under hypoxic conditions, circ-ZNF609 in EVs of ESCC elevates VEGFA (vascular endothelial growth factor A) by decreasing miR-150-5p levels and directly binds to HuR protein to inhibit the translation of ZO-1, Occludin, and Claudin-1 mRNA, thereby exacerbating cancer progression.141
Also, the exosomal circRNAs transmitted between tumor cells can activate the metastasis of tumors by regulating the EMT progression. CircCOL1A2 is overexpressed in colorectal cancer cells and can be encapsulated in exosomes, facilitating tumor cell proliferation, metastasis, and EMT progression. Further mechanistic studies have found that the exosomal circCOL1A2 increases the expression of LASP1 by binding to miR-665, exacerbating the progression of colorectal cancer.142 Also, circRHOT1 in exosomes directly targets miR-204-5p, promoting PRMT5 expression and accelerating the growth, migration and EMT progression of breast cancer cells in both in vivo and in vitro experiments.143 Moreover, exosomal circWDR25 derived from hepatic stellate cells can upregulate the expression of ALOX15 in a miR-4474-3p-dependent manner, thereby promoting EMT and exacerbating the invasive characteristics of hepatocellular carcinoma (HCC).144
4.3 Exosomal circRNAs regulate autophagy and glycolysis
Autophagy can also serve as one of the most important biological processes regulated by exosomal circRNAs, thereby affecting the malignant progression of tumors. Exosomal circRELL activates autophagy by regulating the miR-637/EPHB3 signaling axis, thereby inhibiting the proliferation and metastasis of GC cells in both in vivo and in vitro experiments.145 Under the stress of starvation, the exosomal circTGFBR2 secreted by liver cells can competitively bind to miR-205-5p and increase the expression of ATG5, which induces protective autophagy in HCC cells and promotes tumor progression.146 During amino acid starvation-induced autophagy, exosomal circEGFR activates autophagy by regulating ANXA2 membrane translocation and forms a negative feedback loop to modulate autophagy in TNBC by targeting the miR-224-5p/ATG13/ULK1 signaling pathway.147
In addition, recent studies indicate that a subset of exosomal circRNAs can regulate metabolic processes like glycolysis, thereby influencing tumor development. Exosomal circCARM1 secreted by breast cancer stem cells targets the miR-1252-5p/PFKFB2 signaling axis, reprogramming tumor cell glycolysis.148 Also, the expression of circPDK1 is increased in pancreatic cancer tissues and serum exosomes, and it is related to the poor prognosis of patients. And exosomal circPDK1, activated by HIF1A, promotes migration and glycolysis of pancreatic cancer by inducing the activity of miR-628-3p/BPTF signal and the degradation of BIN1 protein.149
4.4 Exosomal circRNAs regulate chemoresistance
One of the most important challenges in current cancer therapy is chemoresistance.150 More and more studies have elucidated the close correlation between exosomal circRNAs, chemoresistance, and malignant progression of tumors (Figure 3).151, 152 Oxaliplatin resistance significantly affects the treatment efficacy in colorectal cancer patients.153 Researchers have found that the exosomal circATG4B is upregulated in oxaliplatin-resistant cells and regulates the expression of the TMED10 gene by encoding the circATG4B-222aa protein, leading to increased autophagy and induction of drug resistance.154 The elevated hsa_circ_0010467 levels in drug-resistant ovarian cancer tissues, cells, and serum exosomes can predict tumor grading and prognosis. Mechanistically, researchers have found that hsa_circ_0010467 maintains platinum resistance and promotes the growth of ovarian cancer by mediating downregulation of miR-637 and upregulation of LIF/STAT3 axis.155 And circSYT15 in EVs can competitively bind to miR-503-5p, regulate RSF1 expression, and promote cell proliferation and cisplatin resistance in cervical cancer.156 Moreover, exosomal circDCAF8 enhances HCC cell growth and metastasis via the miR-217/NAP1L1 signaling axis, promotes vascular endothelial cell angiogenesis, and induces regorafenib resistance.157 And circUPF2 in EVs can stabilize the expression of SLC7A11 by forming complexes with IGF2BP2 and SLC7A11, which reduces the sensitivity of HCC cells to ferroptosis and promotes resistance to sorafenib.158 Also, paclitaxel treatment significantly increased circBACH1 expression in exosomes of breast cancer cells. At the same time, exosomal circBACH1 enhances breast cancer cell stemness, angiogenesis, and metastasis by targeting the miR-217/G3BP2 axis, thereby promoting paclitaxel resistance and advancing breast cancer progression.159
The increase of several exosomal circRNAs can also enhance the sensitivity of chemotherapy in tumor cells. Researchers have found that overexpression of circRNA–CREIT can weaken resistance to doxorubicin treatment by degrading PKR proteins and promoting the activity of the apoptosis-related signaling pathway RACK1/MTK1. CircRNA–CREIT in exosomes can be transferred to tumor cells, potentially serving as an effective strategy to mitigate chemoresistance in TNBC.160 In patients resistant to gefitinib, the expression of circKIF20B in serum exosomes is significantly reduced and negatively correlated with tumor stage. Functionally, exosomal circKIF20B binds to miR-615-3p, modulating MEF2A expression, which inhibits the cell cycle, promotes apoptosis, reduces mitochondrial oxidative phosphorylation, and increases gefitinib sensitivity in NSCLC.161 Also, exosomal circRABL2B binds to YBX1, downregulates MUC5AC expression, reduces integrin β4/pSrc/p53 signaling pathway activity, inhibits tumor cell stemness, and alleviates erlotinib resistance in lung cancer.162
In short, exosomal circRNAs are transmitted between various cells and affect tumor malignant progression by regulating cancer-related biological processes and signaling pathways (Table 2), which provides potential molecular targets for tumor diagnosis and treatment.
| Exosomal circRNAs | Cancer type | Expression | Target | Function | References |
|---|---|---|---|---|---|
| circMTA2 | Gastric cancer | Up | MTA2 | Promote the proliferation and metastasis | 131 |
| circ_0008717 | NSCLC | Up | miR-1287-5p | Promote the occurrence and metastasis | 132 |
| circSTRBP | Gastric cancer | Up |
miR-593-3p/ miR-1294 |
Enhance GC cell growth and migration | 133 |
| circARID1A | Glioblastoma | Up | miR-370-3p | Accelerate tumor invasion and migration | 134 |
| circLMO7 | OS | Down | miR-21-5p | Suppress the growth and migration | 135 |
| circSTAU2 | Gastric cancer | Down | miR-589 | Inhibit proliferation and migration | 136 |
| circPOKE | Breast cancer | Down | snail | Inhibit the metastasis of breast cancer cells rather than proliferation | 137 |
| circTUBGCP4 | Colorectal cancer | Up | miR-146b-3p | Promote endothelial cell migration | 138 |
| circ-WDR78 | NSCLC | Down | miR-1265 | Induce ROS accumulation; inhibit NSCLC cell proliferation, invasion, and migration | 139 |
| circ_001422 | Colorectal cancer | Up | miR-195-5p | Promote endothelial cell migration | 140 |
| circ-ZNF609 | ESCC | Up | miR-150-5p | Facilitate angiogenesis and vascular permeability; enhance distant metastasis | 141 |
| circCOL1A2 | Colorectal cancer | Up | miR-665 | Promote proliferation and EMT progression | 142 |
| circRHOT1 | Breast cancer | Up | miR-204-5p | Accelerate growth and EMT progression | 143 |
| circWDR25 | HCC | Up | miR-4474-3p | Promote EMT and invasive characteristics | 144 |
| circRELL | Gastric cancer | Down | miR-637 | Activates autophagy; inhibit proliferation | 145 |
| circTGFBR2 | HCC | Up | miR-205-5p | Promote HCC progression | 146 |
| circEGFR | TNBC | Up |
miR-224-5p/ ANXA2 |
Promote autophagy, malignant progression | 147 |
| circCARM1 | Breast cancer | Up | miR-1252-5p | Reprogram the glycolysis process | 148 |
| circPDK1 | Pancreatic cancer | Up | miR-628-3p | Promote migration and glycolysis | 149 |
| circATG4B | Colorectal cancer | Up | circATG4B-222aa | Increase autophagy and induce oxaliplatin resistance | 154 |
| hsa_circ_0010467 | Ovarian cancer | Up | miR-637 | Maintain platinum resistance and promote ovarian cancer growth | 155 |
| circSYT15 | Cervical cancer | Up | miR-503-5p | Promote cell proliferation and cisplatin resistance | 156 |
| circDCAF8 | HCC | Up | miR-217 | Promote angiogenesis and regorafenib resistance | 157 |
| circUPF2 | HCC | Up | IGF2BP2 | Reduce ferroptosis sensitivity and promote sorafenib resistance | 158 |
| circBACH1 | Breast cancer | Up | miR-217 | Enhance the stemness, angiogenesis, and metastasis; promote paclitaxel resistance | 159 |
| circRNA–CREIT | TNBC | Down | PKR | Weaken resistance to doxorubicin; promote the activity of the apoptosis | 160 |
| circKIF20B | NSCLC | Down | miR-615-3p | Exacerbate gefitinib sensitivity | 161 |
| circRABL2B | Lung cancer | Down | YBX1 | Inhibit stemness and erlotinib resistance | 162 |
| circ-AHCY | Glioblastoma | Up | miR-1294 | Promote GBM cell growth | 169 |
| circ-0011536 | PDAC | Up | miR-451a | Promote tumor growth and increase peripheral nerve invasion and remodeling | 170 |
| circTFDP2 | Prostate cancer | Up | PARP1 | Promote proliferation and metastasis | 171 |
- Abbreviations: GBM, glioblastoma; ROS, reactive oxygen species.
5 EXOSOMAL circRNAs IN TME
The TME is a complex structure comprising various cells, exosomes, and extracellular matrix.163 The cells in TME mainly include tumor cells, various types of immune cells, cancer-related fibroblasts, pericytes, endothelial cells, and other tissue-resident cells.164 Due to their synergistic function in the TME, these cells form ecological niches that promote or inhibit tumor progression, thereby regulating the growth, migration, and invasion processes of tumors.165 Therefore, an in-depth discussion of the interactions between different types of cells in the microenvironment is of great significance for explaining tumor characteristics and finding therapeutic targets.
Exosomes in the TME are produced by paracrine cell types and can transmit various genetic molecules, including circRNAs. Usually, donor cells encapsulate circRNAs into exosomes and transport them to recipient cells, thus participating in intercellular communication.166 Tumor cells can promote tumor progression by transferring exosomal circRNAs to immune cells or activating nearby fibroblasts. Immune cells such as macrophages, T cells, and NK cells can communicate with tumor cells by transmitting exosomal circRNA to regulate immune cell function and promote the formation of an immunosuppressive microenvironment. For example, exosomal circRNA, as an important regulatory molecule for immune escape, can reduce T cell activation and enhance the immune tolerance of tumor cells.167 In the TME, angiogenesis is crucial for tumor proliferation and migration, and exosomal circRNA plays an important role in angiogenesis. Exosomal circRNAs can reshape the TME and activate cancer progression by competitively binding to miRNA, interacting with proteins and affecting endothelial cell function. In addition, exosomal circRNAs in the TME can also induce tumor cells to develop chemoresistance by regulating resistance-related molecular pathways.168 CircRNAs in exosomes facilitate information exchange between different types of cells in the TME, playing an important role in cancer metabolism and immune regulation (Figure 4).
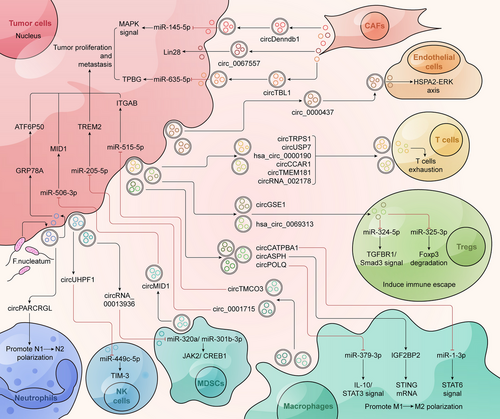
5.1 Tumor-derived exosomal circRNAs act on themselves
In the TME, tumor cells secrete exosomes that transmit genetic material (including circRNAs) to themselves or distant tumor cells, thus regulating tumor progression. Exosomal circRNAs regulate tumor-related signaling pathways and biological processes to influence the malignancy of tumors. Exosomal circ-AHCY can absorb miR-1294, leading to increased MYC and CTNNB1 expression and subsequently activating the Wnt/β-catenin signaling pathway. At the same time, it can also recruit EIF4A3 and stabilize TCF4 and β-catenin protein, which increases the expression of circ-AHCY through a positive feedback axis.169 In addition, Hedgehog-Gli1 mediates an increase in the expression of circ-0011536 in tumor exosomes, followed by targeting miR-451a/VGF signaling to promote tumor growth and increase peripheral nerve invasion and remodeling in PDAC.170 And circRNAs in exosomes can also affect DNA damage in tumor cells. Exosome-derived circTFDP2 is upregulated in PCa tissue and inhibits the cleavage of active caspase-3 by interacting with PARP1 protein, slowing down DNA damage in PCa and promoting cancer progression.171
Also, exosomes secreted by higher metastatic tumor cells can induce an advanced phenotype in lower metastatic tumor cells. Chen et al.172 demonstrated that exosomes from highly metastatic HCC cells can transfer to lower metastatic tumor cells, activating MAPK/ERK signals and intensifying EMT, thereby enhancing the metastatic potential of the lower metastatic cells. Among them, the exosomes derived from HCC cells with higher metastatic potential are rich in circRNA-100338. An in vitro experiment demonstrated that overexpression of exosomal circRNA-100338 can significantly enhance the migration and invasion abilities of HCC cells.173 In a word, these studies have found that exosomal circRNAs secreted by tumor cells have been shown to act on themselves and participate in regulating tumor growth and metastasis.
5.2 Exosomal circRNAs and CAFs
CAFs, derived from activated fibroblasts, are crucial components of the TME.174 CAFs can be categorized into myCAFs (characterized by myofibroblast traits and elevated α-SMA and FAP expression) and iCAFs (inflammatory CAFs, which secrete factors and regulate inflammation).175, 176 CAFs can interfere with tumor treatment and predict tumor prognosis by activating various cancer-related signals. Meanwhile, the transmission of exosomes between CAFs and tumor cells contributes to the formation of the TME and exacerbates tumor progression.177 By high-throughput sequencing of normal fibroblasts and CAFs in breast cancer, researchers screened the exosomal circTBPL1 that is significantly upregulated in CAFs. The exosomal circTBPL1 secreted by CAFs is absorbed by tumor cells and promotes TPBG expression by competitively binding to miR-653-5p, thereby accelerating breast cancer proliferation and invasion.178 Another study in pituitary adenomas found that circDennd1b in EVs derived from CAFs can inhibit miR-145-5p and upregulate ONECUT2 expression, subsequently activating the activity of the MAPK signaling pathway and promoting tumor metastasis.179 In colorectal cancer, exosomal-transported circ_0067557 derived from CAFs can regulate Lin28 (including Lin28A and Lin28B), which enhances proliferation, metastasis, and chemoresistance of tumor cells.180 Thus, exosomal circRNAs derived from CAFs are transported to tumor cells and regulate tumor progression by targeting downstream signaling pathways within tumor cells.
5.3 Exosomal circRNAs and endothelial cells
The endothelial-vesicular network is a novel mechanism regulating the tumor vascular system and microenvironment by altering the Notch signaling pathway and VEGFR2/3.181, 182 VEGFR3 is one of the key factors in lymphangiogenesis, which can increase the activity of lymphatic endothelial cells and promote their migration.183 Endothelial cells establish intercellular communication by releasing and absorbing EVs. Researchers have found that EVs secreted by endothelial cells can deliver protein DLL4 to nearby endothelial cells and improve their migration ability by regulating the Notch signaling pathway.184 Moreover, the EVs of endothelial cells can also load miRNAs and transfer them into tumor cells, affecting the sensitivity of chemotherapy.185, 186 In addition, the exosomal circRNAs derived from tumor cells can be taken up by endothelial cells, enhancing tumor metastasis by promoting endothelial cell migration. Exosomes secreted by GC cells transport hsa_circ_0000437 to human lymphatic endothelial cells. It promotes the formation of lymphatic vessels and lymph node metastasis by targeting the HSPA2–ERK cascade signal, which may become a new biomarker for predicting the prognosis and targeted treatment of GC.187 In a word, the vesicular transport of endothelial cells affects tumor angiogenesis and metastasis, which can become a promising new strategy for tumor therapy.
5.4 Exosomal circRNAs and T cells
The dysfunction of CD8+ T cells characterizes the tumor immune microenvironment.188, 189 This inhibitory tumor immune microenvironment is accompanied by depletion of CD8+ T cells, reduced cytokine release, and decreased cytotoxic function.190-192 Exosomal circRNAs regulate drug resistance in tumor immunotherapy across various cancer types. In lung cancer, circRNA-002178 is present in plasma exosomes of lung adenocarcinoma (LUAD) patients and upregulates PD-L1 levels by competitively binding to miR-34. Meanwhile, circRNA-002178, which relies on EVs for transmission, can be absorbed by CD8+ T cells and induce an increase in PD1, ultimately depleting T cells and promoting lung cancer development.193 And exosomal circUSP7 derived from NSCLC cells targets the miR-934/SHP2 axis, causing CD8+ T cell dysfunction and increasing resistance to anti-PD1 therapy in NSCLC patients.194 Another hsa_circ_0000190 is present in tissues, blood, urine, and exosomes of NSCLC patients. It can upregulate the level of sPD-L1 (soluble PD-L1), reduce the function of PD-L1 antibodies, and inhibit T cell activation, which promotes the emergence of immune therapy drug resistance and leads to poor prognosis in patients.195 In HCC cells, researchers have confirmed that circCCAR1 increases the expression of WTAP by absorbing miR-127-5p and is packaged into exosomes with the involvement of hnRNPA2B1 through the use of chromatin immunoprecipitation, RNA immunoprecipitation, and biotinylated RNA pull-down assays. Then, researchers explored the role of exosomal circCCAR1 in anti-PD1 resistance by constructing a mouse model containing recombinant human immune system components. It was found that circCCAR1 in EVs stabilizes PD-1 protein in CD8+ T cells, causing their dysfunction and enhancing resistance to anti-PD1.196 Also, researchers have found that circTMEM181 is significantly upregulated in anti-PD1-resistant HCC patients. Exosomal circTMEM181 enhances CD39 expression in macrophages by targeting miR-488-3p. The coexpression of CD39 in macrophages and CD73 in HCC cells enhances adenosine production, which inhibits CD8+ T cell activity and induces anti-PD1 resistance.197 In bladder cancer, the exosomal circTRPS1 directly combines with miR-141-3p to regulate GLS1-dependent glutamine metabolism, balance the level of ROS, and deplete CD8+ T cells in the TME, thus promoting the malignant progression of tumors.198
In addition, regulatory T cells (Tregs) produce immunosuppressive molecules, inhibiting CD8+ T cell activity and facilitating tumor immune escape.199-201 Exosomal circGSE1 regulates the TGFBR1/Smad3 signaling axis by targeting miR-324-5p, subsequently increasing the number of Tregs and promoting the progression of HCC.202 Has_circ_0069313 is upregulated in oral squamous cancer cells and induces immune escape by leading to the reduction of miR-325-3p expression and activating the degradation of Foxp3. Also, has_circ_0069313 in exosomes is transferred to Tregs and maintains the function of Treg cells.203 Altogether, exosomal circRNAs affect the efficacy of immunotherapy and tumor progression by regulating the function of T cells and Treg cells.
5.5 Exosomal circRNAs and macrophages
Macrophages are essential for antigen presentation and initiating inflammatory responses, positioning them as a key immune population in the body.204 Macrophages are usually divided into two subtypes: M1 and M2. Among them, tumor-associated macrophages have an M2 phenotype and account for about half of the tumor mass.205 Previous studies have identified that tumor-associated M2 macrophages and their secreted exosomes significantly contribute to tumor progression.206, 207 In ovarian cancer, the exosomal circTMCO3 is derived from M2 macrophages that competitively bind to miR-515-5p and increase the expression of ITGA8, thereby promoting malignant progression.208 Several exosomes derived from M1 macrophages contribute to antitumor therapy by modulating circRNA expression. Exosomes from M1 macrophages deliver miR-628-5p to liver cancer cells, inhibiting METTL14 expression and enhancing circFUT8 transport from the nucleus to the cytoplasm. Subsequently, circFUT8 inhibits the occurrence and development of liver cancer by targeting the miR-552-3p/CHMP4B signaling axis.209 In addition, overexpression of circ_0001715 in M0 macrophages can be transmitted to LUAD cells through exosomes and can also induce macrophage polarization toward M2 type and exacerbate lung cancer metastasis by targeting miR-205-5p and increasing TREM2 levels.210 Moreover, the expression of hsa_circ_0004658 in exosomes from RBPJ-activated macrophages was significantly elevated, and this exosomal hsa_circ_0004658 inhibited liver cancer progression by modulating miR-499b-5p and JAM3 expression.211 Also, the exosomal circBTG2 derived from activated macrophages can regulate the expression of PTEN protein by directly binding to miR-25-3p, and subsequently inhibit the growth and invasion of glioma.212
A large amount of exosomal circRNAs from tumor cells can modulate macrophage transition from M1 to M2 types and influence tumor malignancy. Recent studies indicate that the exosomal circATP8A1 is significantly overexpressed in the plasma of GC patients and is closely associated with TNM stage and prognosis. CircATP8A1 in EVs can activate the STAT6 signaling pathway rather than the STAT3 signaling pathway by targeting miR-1-3p, thereby activating the appearance of M2 macrophages and inducing cancer progression.213 And circNEIL3 relies on the action of hnRNPA2B1 protein to package into exosomes and stabilize the expression of IGF2BP3 protein through delivery to tumor-associated macrophages, producing immunosuppressive effects and subsequently promoting the growth of gliomas.74 Another exosomal circASPH enhances the stability of STING mRNA by directly binding to IGF2BP2, thereby increasing the transition of macrophages from M1 to M2 and promoting tumor growth.214 The exosomal cSERPINE2 from tumor cells acts on the tumor-associated macrophages and promotes the secretion of IL-6, thereby accelerating the proliferation and metastasis of breast cancer cells. At the same time, enhances EIF4A3 and CCL2 expression via positive feedback, induces cSERPINE2 production, and further recruits tumor-associated macrophages in breast cancer.215 And the exosomal circPOLQ derived from tumor cells increases IL-10 and STAT3 by absorbing miR-379-3p, thereby promoting polarization of M2 macrophages and exacerbating the occurrence of metastatic nodules in colorectal cancer.216 Also, circGLIS3 in tumor cells regulates the miR-1343-3p/PGK1 axis and directly binds to VIMENTIN, promoting GC proliferation and invasion both in vivo and in vitro. Additionally, circGLIS3 can be encapsulated into EVs and transmitted to macrophages, which induces polarization of macrophages toward M2 type.217
The exosomal circRNAs induced by stress also participate in the polarization of macrophages from M1 to M2. Recent research indicates that endoplasmic reticulum (ER) stress enhances tumor cell exosome secretion, facilitates the transfer of circ_0001142 into macrophages, and modulates the miR-361-3p/PIK3CB pathway, thereby affecting macrophage autophagy and polarization in breast cancer.218 And, exosomal circPLEKHM1 is produced by tumor cells under hypoxic conditions and can interact with macrophages in the TME. Specifically, circPLEKHM1 exacerbates tumor metastasis by enhancing the interaction between PAPC1 and eIF4G, increasing the translation of OSMR, and driving macrophage polarization toward the M2 type in NSCLC.219 Moreover, Kras mutation induces heterogeneity within tumors by increasing the expression of exosomal circHIPK3 and circPTK2, which induces the appearance of M2 macrophages, immune suppression, and exacerbates lymph node metastasis in lung cancer.220 Therefore, exosomal circRNAs derived from macrophages act on tumor cells and regulate tumor malignant progression, while the exosomal circRNAs secreted by tumor cells can be absorbed by macrophages and induce the appearance of M2 macrophages.
5.6 Exosomal circRNAs and NKs
NK cells similar to CD8+ T cells have the ability to kill target cells and are important effector cells in innate immunity.221 NK cells constitute approximately 5–10% of PBMCs (whole blood monocytes) and are primarily found in the blood, spleen, and bone marrow. The growth of NK cells typically requires transcription factors (including Tox, Nfil3, Id2, T-bet, and EOMES) and cytokines (including IL-2 and IL-15).222 NK cells have strong application prospects in clinical tumor treatment. On the one hand, researchers can enhance the practicality of NK cells by optimizing the source of therapeutic NK cells. On the other hand, enhancing NK cells' killing ability and persistence can improve the effectiveness of NK cell therapy.223-225 And EVs, as mediators of intercellular information exchange, can transmit circRNAs to NK cells, which affect tumor development by regulating the function of NK cells. For example, the exosomal circUHRF1 also originates from HCC cells, which mainly inhibits NK cell secretion of IFN-γ and TNF-α, and induces anti-PD1 resistance by decreasing miR-449c-5p and increasing TIM-3 expression.226 In summary, exosomal circRNAs can affect tumor progression by modulating the biological functions of NK cells, potentially offering a novel approach for tumor therapy.
5.7 Exosomal circRNAs and MDSCs
Bone marrow-derived suppressor cells (MDSCs) undergo extensive expansion in the TME and can interfere with the killing effects of NK and T cells.227 MDSCs can directly activate the immune escape of tumor cells and indirectly increase tumor invasiveness.228 While prior research has identified a role for MDSCs in castration resistance, the involvement of MDSC exosomes in castration-resistant prostate cancer remains uncertain. Researchers isolated MDSC exosomes and found that they can promote the proliferation and migration of PCa cells. Mechanistically, S100A9 in MDSC-secreted exosomes activates circMID1 expression, promoting PCa progression via the miR-506-3p/MID1 axis.229, 230
Polymorphonuclear MDSCs (PMN-MDSCs) have strong immunosuppressive effects and are activated in disease states.231 The presence of PMN-MDSCs in the TME is a critical factor in immunotherapy failure and tumor progression, and is inversely related to patient prognosis. Among them, the presence of PMN-MDSCs in the TME is one of the most important factors causing the failure of immunotherapy and intensified tumor progression, which is inversely related to patient prognosis. PMN-MDSCs are activated by factors such as VEGF, M-CSF, GM-CSF, and IL-6, and can upregulate the expression of ROS, iNOS, and Arg-1, leading to tolerance to immunotherapy.232, 233 On the one hand, the exosomal circRNA_0013936 derived from bladder cancer increases the expression of FATP2 by targeting the miR-320a/JAK2 axis. On the other hand, it also reduces RIPK3 levels by targeting the miR-301b-3p/CREB1 axis, thereby promoting the immunosuppressive function of PMN-MDSCs and becoming one of the important reasons for the failure of immunotherapy.234
5.8 Exosomal circRNAs and neutrophils
Neutrophils, as prevalent innate immune cells, are crucial for immune regulation and infection defense.235 Tumor-associated neutrophils (TANs) are the most important components of the TME, exhibiting a dual role in tumorigenesis.236 On the one hand, TANs activate tumor malignant progression by reshaping the extracellular matrix, promoting angiogenesis, driving immune suppression and tumor metastasis.237, 238 On the other hand, TANs can directly target tumor cells and perform antitumor functions. Similar to the naming for M1 and M2 macrophages, the N1 phenotype of neutrophils is an antitumor characteristic, while the N2 phenotype of neutrophils is a protumor characteristic.239, 240 Several exosomal circRNAs secreted by tumor cells can act on neutrophils and regulate their N1 to N2 transformation, which affects tumor growth and treatment. For example, recent studies indicate that exosomal circPACRGL originates from tumor cells and enhances the expression of downstream target TGF-β1 by competitively binding to miR-142-3p and miR-506-3p. It subsequently promotes neutrophil polarization from N1 to N2, intensifying colorectal cancer growth and metastasis.241
5.9 Exosomal circRNAs and microbiota
Microorganisms are involved in the malignant progression of tumors, and about 20% of human tumors are closely related to microbiota.242, 243 The microbiota mainly promotes the development of cancer in three ways, including regulating tumor cell proliferation and apoptosis, guiding factor production and body metabolism, as well as altering the function of immune cells.244 In general, the body barrier can establish a symbiotic relationship with microorganisms. When the body's barrier is disrupted, the microbiota can activate inflammatory responses and immunosuppressive functions, leading to the transformation of normal tissues into tumors.245 And the microbiota can influence tumor development by modifying the expression of circRNAs in EVs. Researchers have found that the infection with fusobacterium nucleatum can induce the synthesis of hnRNP L-dependent hsa_circ_0004085 and can also promote the encapsulation of hsa_circ_0004085 into EVs by regulating protein hnRNP A1. Subsequently, the exosomal hsa_circ_0004085 is delivered to the receptor cells, inhibiting ER stress and mediating resistance to 5-fluorouracil/oxaliplatin by targeting the GRP78/ATF6p50 axis.23 Although the microbiota contributes to the synthesis of exosomal circRNAs and holds promise as a novel target for cancer therapy, research in this area remains limited and requires further investigation (Table 3).
| Exosomal circRNAs | Cancer type | Expression | Parent cell | Receptor cell | Genes and pathways | References |
|---|---|---|---|---|---|---|
| hsa_circ_0004085 | Colorectal cancer | Up | Tumor cells | Tumor cells | GRP78/ATF6p50 | 23 |
| circNEIL3 | Glioma | Up | Tumor cells | Tumor-associated macrophages | IGF2BP3 | 74 |
| circTBPL1 | Breast cancer | Up | CAFs | Tumor cells | miR-653-5p/TPBG | 178 |
| circDennd1b | Pituitary adenomas | Up | CAFs | Tumor cells |
miR-145-5p/ ONECUT2 |
179 |
| circ_0067557 | Colorectal cancer | Up | CAFs | Tumor cells | Lin28 | 180 |
| hsa_circ_0000437 | Gastric cancer | Up | Tumor cells | Lymphatic endothelial cells | HSPA2–ERK signal | 187 |
| circRNA-002178 | Lung adenocarcinoma | Up | Tumor cells | T cells | miR-34/PD-L1 | 193 |
| circUSP7 | NSCLC | Up | Tumor cells | T cells | miR-934/SHP2 | 194 |
| hsa_circ_0000190 | NSCLC | Up | Tumor cells | T cells | sPD-L1 | 195 |
| circCCAR1 | HCC | Up | Tumor cells | T cells | miR-127-5p/WTAP | 196 |
| circTMEM181 | HCC | Up | Tumor cells | T cells | miR-488-3p/CD39 | 197 |
| circTRPS1 | Bladder cancer | Up | Tumor cells | T cells | miR-141-3p/GLS1 | 198 |
| circGSE1 | HCC | Up | Tumor cells | Treg cells |
miR-324-5p/ TGFBR1/Smad3 |
202 |
| has_circ_0069313 | OSCC | Up | Tumor cells | Treg cells | miR-325-3p/Foxp3 | 203 |
| circTMCO3 | Ovarian cancer | Up | M2 macrophages | Tumor cells | miR-515-5p/ITGA8 | 208 |
| circFUT8 | HCC | Down | M1 macrophages | Tumor cells | miR-552-3p/CHMP4B | 209 |
| circ_0001715 | Lung cancer | Up | M0 macrophages | Tumor cells | miR-205-5p/TREM2 | 210 |
| hsa_circ_0004658 | HCC | Up | Macrophages activated by RBPJ | Tumor cells | miR-499b-5p/JAM3 | 211 |
| circBTG2 | Glioma | Up | Macrophages activated by RBPJ | Tumor cells | miR-25-3p/PTEN | 212 |
| circATP8A1 | Gastric cancer | Up | Tumor cells | Macrophages | miR-1-3p/STAT6 | 213 |
| circASPH | Colorectal cancer | Up | Tumor cells | Macrophages | IGF2BP2/STING | 214 |
| cSERPINE2 | Breast cancer | Up | Tumor cells | Tumor-associated macrophages | IL-6 | 215 |
| circPOLQ | Colorectal cancer | Up | Tumor cells | Macrophages | miR-379-3p/IL-10 and STAT3 signal | 215 |
| circGLIS3 | Gastric cancer | Up | Tumor cells | Macrophages | miR-1343-3p/PGK1; VIMENTIN protein | 217 |
| circ_0001142 | Breast cancer | Up | Tumor cells | Macrophages | miR-361-3p/PIK3CB | 218 |
| circPLEKHM1 | NSCLC | Up | Tumor cells | Macrophages | PAPC1 and eIF4G | 219 |
| circHIPK3 | Lung cancer | Up | Tumor cells | Macrophages | / | 220 |
| circPTK2 | Lung cancer | Up | Tumor cells | Macrophages | / | 220 |
| circUHRF1 | HCC | Up | Tumor cells | NK cells | miR-449c-5p/TIM3 | 226 |
| circMID1 | Prostate cancer | Up | MDSCs | Tumor cells | miR-506-3p/MID1 | 229 |
| circRNA_0013936 | Bladder cancer | Up | Tumor cells | PMN-MDSCs | miR-320a/JAK2 axis; miR-301b-3p/CREB1 | 234 |
| circPACRGL | Colorectal cancer | Up | Tumor cells | Neutrophil | miR-142-3p/miR-506-3p/TGF-β1 | 241 |
6 POTENTIAL CLINICAL APPLICATION OF EXOSOMAL circRNAs
Exosomal circRNAs affect cancer progression through the modulation of various signaling pathways and their associated downstream target proteins. Meanwhile, exosomal circRNAs can transfer and share information between different cells, which makes them extremely useful in the diagnosis, prognostic prediction and therapy of cancer. And the three primary antitumor approaches to targeting exosomal circRNAs include chemical synthesis of siRNA/shRNA (short hairpin RNA), designing nanomedicines, and directly using exosomes originating from various cells (Figure 5).
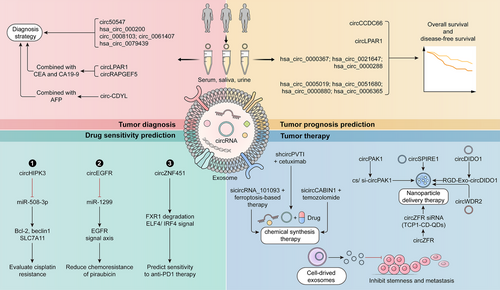
6.1 Diagnosis
Numerous scientific studies indicate that exosomal circRNAs hold significant potential as clinical diagnostic biomarkers for cancer patients in both tissue and blood samples.67 Circ50547, found in both tissue and serum exosomes of GC patients, enhances HNF1B expression and promotes GC progression by sequestering miR-217. And exosomal circ50547 has a higher diagnostic value compared with serum circ50547 in GC.246 Moreover, exosomal hsa_circ_000200 binds to miR-4659a/b-3p and upregulates HBEGF levels to promote the activity of the TGF-β/Smad signaling pathway, thereby exacerbating GC growth. Hsa_circ_000200 in EVs demonstrates superior diagnostic efficacy compared with tissues and serum, indicating its potential as a biomarker for GC diagnosis.247 Circ_0008103 and circ_0061407 in EVs are upregulated in NSCLC, enhancing tumor growth and invasion. And the downregulation of circ_0008103 and circ_0061407 in serum exosomes can reflect the stage and metastasis of tumors, providing new marker genes for the diagnosis of lung cancer.248 Researchers have found that the level of hsa_circ_0079439 in the exosomes of GC patients is significantly increased compared with normal individuals. In addition, the area under the curve (AUC) for hsa_circ_0079439 (with a value of 0.8595) significantly exceeded those of traditional cancer biomarkers, including CEA (0.5862), CA19-9 (0.5660), alpha-fetoprotein (0.5082), CA72-4 (0.5360), and CA125 (0.5018). Substantially, the exosomal hsa_circ_0079439 derived from plasma shows potential as a biomarker for both early and late diagnosis of GC.249
A few exosomal circRNAs can be combined with standard clinical diagnostic markers (including CEA, CA19-9, AFP, etc.), which further improves the reliability of diagnosis.250, 251 CircLPAR1 inhibits the direct effects of METTL3 and eIF3 h, reduces the expression of the oncogenic gene BRD4, and can be secreted into EVs, serving as a biomarker for colorectal cancer diagnosis. Research has shown that combining exosomal circLPAR1 with biomarkers CEA and CA19-9 significantly enhances the diagnostic value for colorectal cancer.252 CircRAPGEF5 was detected in the exosomes of LUAD and was found to promote the expression of ZEB1, enhancing the invasion process by directly binding to miR-1236-3p. In addition, researchers also found that the combined detection of serum CEA and serum exosomal circRAPGEF5 can significantly improve the effectiveness of diagnosis, providing a new strategy for minimally invasive diagnosis.253 Moreover, researchers detected and analyzed the tissues and plasma exosomes of 30 healthy individuals, 30 chronic gastritis patients, and 64 GC patients, and identified circLPAR1, which is negatively correlated with GC progression. The AUC values of plasma exosomal circLPAR1, CA19-9, and CEA in the diagnosis of GC were 0.836, 0.767, and 0.746, respectively, which were significantly lower than the AUC values of combined diagnosis (0.914). And the exosomal circLPAR1 is significantly associated with the survival of GC patients and holds substantial clinical value for diagnosis and prognostic assessment.254 Additionally, circ-CDYL levels are notably elevated in the plasma exosomes of early liver cancer patients, dependent on hnRNPA2/B1-mediated sorting for exosomal entry. The combination of exosomal circ-CDYL and plasma protein AFP can effectively diagnose liver cancer (the value of AUC is 0.896), providing a promising diagnostic strategy for liver cancer.255
6.2 Prognostic prediction
A considerable portion of exosomal circRNAs not only play an important role in tumor diagnosis, but also have practical value in predicting the prognosis of tumor patients.256 In patients with pituitary adenoma, the expression of circCCDC66 in serum exosome shows a significant increase compared with the healthy population. Importantly, patients with lower circCCDC66 levels in serum exosomes exhibit longer disease-free survival, indicating its potential as a biomarker for diagnosing and predicting the prognosis of pituitary adenomas.257 Deep sequencing and analysis of exosomes from high-grade astrocytoma cells revealed that circRNAs in serum exosomes, such as hsa_circ_0003828, hsa_circ_0075828, and hsa_circ_0002976, can act as clinical biomarkers for blood diagnosis. Furthermore, hsa_circ_0005019, hsa_circ_0051680, hsa_circ_0000880, and hsa_circ_0006365 show promise as biomarkers for prognostic monitoring.258 Recent studies have found a close correlation between circHNRNPU and poor prognosis in patients with multiple myeloma. The exosomal circHNRNPU can encode the circHNRNPU_603aa protein, stabilize the expression of c-Myc, and regulate the bone marrow microenvironment, which has the potential to become a new diagnostic and prognostic strategy for multiple myeloma.259 Moreover, microarray analysis of serum and bile samples identified hsa_circ_0000367, hsa_circ_0021647, and hsa_circ_0000288 are closely associated with the invasive phenotype of cholangiocarcinoma, suggesting their potential as diagnostic and prognostic markers superior to traditional markers.260
6.3 Drug sensitivity prediction
Some exosomal circRNAs can predict the effectiveness of chemotherapy and immunotherapy drugs.261, 262 The inhibition of exosomal circHIPK3 can regulate the expression of Bcl-2, beclin1, and SLC7A11 by absorbing miR-508-3p, and then regulate the process of autophagy-dependent ferroptosis and cisplatin resistance in GC. Further research indicates that exosomal circHIPK3 in serum is crucial for assessing cisplatin resistance.263 Also, the expression of circEGFR increased in tissues and plasma exosomes of TNBC patients and was negatively correlated with the poor prognosis of patients. CircEGFR promotes the growth, invasion, and EMT process by regulating the miR-1299/EGFR signal axis, while reducing the chemosensitivity of pirarubicin in TNBC. And circEGFR in plasma exosomes shows potential as a clinical marker for diagnosing and predicting the prognosis of TNBC.264 In addition, exosomal circZNF451 enhances macrophage polarization and reduces cytotoxic CD8+ T cells by promoting FXR1 degradation and activating the ELF4/IRF4 signaling pathway in macrophages. Also, the exosomal circZNF451 can modify the immune microenvironment in LUAD and may serve as a biomarker for predicting sensitivity to anti-PD1 therapy.265 In a word, exosomal circRNAs are pivotal in clinical diagnosis, prognosis and drug efficacy prediction, offering significant clinical value in carcinogenesis.
6.4 Tumor therapy
Numerous studies indicate that exosomal circRNAs regulate tumor progression through various molecular mechanisms, making them promising therapeutic targets.266, 267 CircPABPC1 is upregulated in colorectal cancer tissue and tumor cell exosomes, enhancing transcription in the nucleus by recruiting KDM4C to the HMGA2 promoter and decreasing H3K9me3 modification. Meanwhile, circPABPC1 in the cytoplasm regulates the expression of miR-874/miR-1292, increasing the expression of important proteins BMP4/ADAM19 in colorectal cancer metastasis and playing a role in antitumor metastasis therapy.268 Also, circRHOBTB3 is sorted into exosomes with the participation of protein SNF8 and slows down the progression of colorectal cancer by regulating metabolic signaling pathways and the production of ROS. Targeting circRHOBTB3 and elements for exosomal sorting will become a new strategy for responding to tumors.269
Targeting exosomal circRNAs in nanomedicine design can enhance tumor treatment efficacy and mitigate chemoresistance progression.270-272 Recently, researchers have used xenograft models to screen for circSPIRE1, which plays a potential role in tumor metastasis. The exosomal circSPIRE1 acts as a tumor suppressor by inhibiting angiogenesis and metastasis through upregulation of GALNT3 (enhancing glycosylation of E-cadherin) and QKI protein (promoting expression of circSPIRE1). And nanomedicines synthesized from the circSPIRE1 plasmid can alleviate the metastasis of renal cell carcinoma and provide new targets for the treatment of metastatic cancer.273 CircZFR levels rise in colorectal cancer tissues and blood exosomes, correlating with cancer progression and metastasis. Mechanistically, circZFR targets miR-3127-5p both in vivo and in vitro to elevate RTKN2 levels and promote tumor growth. Moreover, researchers utilized TCP1-CD-QDs nanoparticles to load si-circZFR (circZFR siRNA) and deliver it to the tumor area, which inhibited tumor growth in the patient-derived xenograft (PDX) model.274 Additionally, researchers have demonstrated that RGD-modified circDIDO1 packaged into exosomes (RGD–Exo–circDIDO1) inhibits the progression of GC in vivo and in vitro experiments. Mechanistically, RGD–Exo–circDIDO1 targets miR-1307-3p/SOSC2 signal in the treatment of GC, presenting a novel nanomedicine option for therapy.275 CircWDR62 is upregulated in temozolomide (TMZ)-resistant glioma cells and exosomes compared with the control, closely related to the progression of TMZ resistance. Functionally, circWDR62 regulates downstream target genes MGMT and tumor progression by competitively binding to miR-370-3p. At the same time, using EVs to deliver circWDR62 can promote TMZ resistance and tumor malignant progression.276 Moreover, circPAK1 binds to 14–3-3 ζ and reduces its recruitment of YAP, thereby increasing the nuclear localization of YAP, inducing Hippo activity, and promoting the progression of HCC. Researchers have also found that using CS/si-circPAK1 nanocomposites could better inhibit tumor growth and migration. In addition, the expression of circPAK1 in EVs induces the emergence of lenvatinib resistance, suggesting it as a potential target for HCC treatment.277
The combination of siRNA/shRNA targeting exosomal circRNAs and tumor therapeutic drugs can enhance antitumor effects.278 CircPVT1 is secreted by laryngeal cancer cells into exosomes and enters vascular epithelial cells. It then induces angiogenesis by absorbing miR-30c-5p, increasing Rap1b levels and upregulating VEGFR2 and PI3K/AKT signaling pathways. Meanwhile, researchers have found that the combination of circPVT1 shRNA and cetuximab in the PDX model can effectively inhibit vascularization and laryngeal cancer progression.279 And the exosomal circCABIN1 secreted by TMZ-resistant cells acts on receptor cells and activates the ErbB signaling pathway by targeting the miR-637/OLFML3 signaling axis, ultimately leading to the exacerbation of TMZ resistance. In a mouse xenograft model, the use of engineered exosomes targeting circCABIN1 and OLFML3 significantly enhances the therapeutic efficacy of TMZ, indicating their potential as a tool for clinical cancer therapy.280 In LUAD, the exosomal circRNA_101093 can maintain the level of circRNA within tumor cells and reduce cell sensitivity to ferroptosis by interacting with FABP3. And in preclinical research models, it was found that reducing exosomal circRNA_101093 can enhance the effectiveness of ferroptosis-based therapy.281
The exosomal circRNAs secreted by tumor cells can usually exert immunosuppressive functions and promote immune escape.282 And exosomal circRNAs in the TME can dysregulate immune cells, such as CD8+ T cells, NK cells and dendritic cells, influencing immune checkpoint therapy. In patients with ICC, the levels of exosomal circ-PTPN22 and circ-ADAMTS6 in plasma are significantly elevated, contributing to T cell depletion and inducing neutrophil extracellular trap formation. Exosomal circ-PTPN22 and circ-ADAMTS6 could serve as novel targets for liquid biopsy and contribute to immune checkpoint blockade therapy.283 In addition, exosomal circ_001264, derived from acute myeloid leukemia (AML) cells, activates the p38/STAT3 signaling axis by regulating RAF1 levels, followed by activating macrophage polarization toward the M2 type and elevating PD-L1 expression. At the same time, the combination of circ_001264 siRNA and aPD-L1 showed significant antitumor effects in AML.284
Some cancers, such as breast cancer, are closely related to obesity. Adipose-derived exosomal circCRIM1 exacerbates breast cancer progression by downregulating miR-503-5p and activating the OGA/FBP1 signaling pathway. Therefore, targeting the adipose-associated exosomal circCRIM1 is a promising strategy to alleviate breast cancer.285 Moreover, researchers have discovered that circ_0037104 in Hu-MSC-derived (human umbilical cord-derived mesenchymal stem cells) exosomes primarily binds to miR-620, elevates APAF1 levels, and suppresses the stemness and metastasis of cholangiocarcinoma, offering novel antitumor therapeutic strategies.286
Recently, researchers from Shanghai Jiao Tong University conducted clinical trials on the circRNA drug HM2002 injection. They administered HM2002 via epicardial myocardial injection in patients with ischemic heart failure undergoing Coronary Artery Bypass Grafting, in order to evaluate the safety and efficacy of HM2002. However, further clinical trials of circRNA drugs in tumor therapy are still needed. In the future, researchers can achieve effective delivery and expression of circRNAs in tumor tissues by developing new delivery strategies. In addition, circRNA may also be combined with other therapeutic strategies, such as targeted therapy and immunotherapy, to jointly combat the malignant progression of cancer and enhance treatment efficacy. In summary, we have summarized some exosomal circRNAs that play critical roles in tumorigenesis and may provide promising new strategies for cancer diagnosis and treatment (Table 4).
| Application | Exosomal circRNAs | Cancer type | Expression | Function | References |
|---|---|---|---|---|---|
| Diagnosis | circ50547 | Gastric cancer | Up | Diagnostic biomarkers | 246 |
| hsa_circ_000200 | Gastric cancer | Up | Diagnostic biomarkers | 247 | |
| circ_0008103; circ_0061407 | NSCLC | Up | Diagnostic biomarkers | 248 | |
| hsa_circ_0079439 | Gastric cancer | Up | Biomarkers for the early and late diagnosis | 249 | |
| circRAPGEF5 | Lung adenocarcinoma | Up | Minimally invasive diagnosis | 253 | |
| circ-CDYL | HCC | Up | Diagnostic biomarkers | 255 | |
| hsa_circ_0003828; hsa_circ_0075828; hsa_circ_0002976 | High-grade astrocytoma | Up | Diagnostic biomarkers | 258 | |
| Prognosis prediction | circLPAR1 | Colorectal cancer; gastric cancer | Down | Diagnostic biomarkers; prognostic biomarkers | 252 |
| circCCDC66 | Pituitary adenoma | Up | Diagnostic biomarkers; prognostic biomarkers | 257 | |
| hsa_circ_0005019; hsa_circ_0051680; hsa_circ_0000880; hsa_circ_0006365 | High-grade astrocytoma | Up | Prognostic biomarkers | 258 | |
| circHNRNPU | Multiple myeloma | Up | Diagnostic biomarkers | 259 | |
| hsa_circ_0000367; hsa_circ_0021647; hsa_circ_0000288 | Cholangiocarcinoma | Up | Diagnostic biomarkers; prognostic biomarkers | 260 | |
| Chemoresistance prediction | circHIPK3 | Gastric cancer | Up | Evaluate cisplatin resistance | 263 |
| circEGFR | TNBC | Up | Reduce pirarubicin sensitivity | 264 | |
| circZNF451 | Lung cancer | Up | Predict sensitivity to anti-PD1 | 265 | |
| Therapeutic target | circPABPC1 | Colorectal cancer | Up | Therapeutic targets for tumor metastasis | 268 |
| circRHOBTB3 | Colorectal cancer | Down | Inhibit tumor progression | 269 | |
| circSPIRE1 | Renal cell carcinoma | Down | Alleviate the metastasis | 273 | |
| circZFR | Colorectal cancer | Up | Promote metastasis | 274 | |
| circDIDO1 | Gastric cancer | Down | Inhibit tumor progression | 275 | |
| circWDR62 | Glioma | Up | Promote temozolomide resistance | 276 | |
| circPAK1 | HCC | Up | Induce lenvatinib resistance | 277 | |
| circPVT1 | Laryngeal cancer | Up | Induces angiogenesis | 279 | |
| circCABIN1 | Glioblastoma | Up | Promote temozolomide resistance | 280 | |
| circRNA_101093 | Lung adenocarcinoma | Up | Reduce sensitivity to ferroptosis | 281 | |
| circ-PTPN22; circ-ADAMTS6 | ICC | Up | Induce resistance to immune checkpoint therapy | 283 | |
| circ_001264 | Acute myeloid leukemia | Up | Activate M2 macrophages | 284 | |
| circCRIM1 | Breast cancer | Up | Promote tumor growth | 285 | |
| circ_0037104 | Cholangiocarcinoma | / | Inhibit the stemness and metastasis | 286 |
7 CONCLUSION AND PERSPECTIVES
Recent studies have shown that exosomal circRNAs play an important role in tumorigenesis and progression. Exosomal circRNAs can affect the malignant progression of tumors by regulating various biological processes including proliferation, metastasis, autophagy, angiogenesis, drug resistance, metabolism, and immune escape. And exosomal circRNAs regulate tumor development through various molecular mechanisms. Certain exosomal circRNAs translate into small peptides that regulate tumor-related gene expression, while others bind directly to target miRNAs and proteins, influencing tumor-related signaling pathways. For example, exosomal circRNAs (including hsa_circ_0010467, circSYT15, circDCAF8, and circBACH1) can promote the acquisition of chemoresistance in tumor cells by targeting miRNAs regulation, while others such as circRNA–CREIT and circRABL2B enhance the sensitivity of chemotherapy by downregulating proteins and resistance-related signaling pathways. Numerous studies indicate that exosomal circRNAs contribute to tumor malignancy by encoding novel peptides. Exosomal circATG4B and circHNRNPU regulate drug resistance and TME by encoding proteins circATG4B-222aa and circHNRNPU_603aa, respectively. Not all circRNAs are sorted into exosomes, but existing research has shown that a few circRNAs may have special sequence structures or bind to sorting-related proteins that allow them to be transported to other cells through exosomes, thereby regulating the growth and migration of tumor cells. By delving deeper into the function of circRNAs in cancer progression, researchers can design inhibitors or interfering RNAs targeting these circRNAs to slow down tumor proliferation and spread. Furthermore, due to the function of exosomes in transmitting biological regulatory molecules between cells, researchers can use exosomes to transport circRNA to tumor cells, thereby reversing cancer progression, improving targeted therapy efficacy and reducing side effects. Despite numerous studies on the role and mechanism of circRNAs in tumor progression, the molecular mechanism of their entry into exosomes remains unclear. We hope that researchers can elucidate the molecular mechanism by which circRNAs are sorted into exosomes and the sequence characteristics of circRNAs that can enter exosomes in the future.
With the development of single-cell sequencing technology, high-throughput sequencing technology, and various bioinformatics algorithms, research on circRNAs has made great progress. RNA-seq, a high-throughput sequencing technology, is widely used to comprehensively detect and identify circRNA expression profiles in cells and tissues.287 In recent years, the increasing sensitivity and accuracy of RNA-seq have provided technical support for the identification of circRNAs. Single-cell sequencing technology uncovers dynamic circRNA changes across developmental stages and disease states at the single-cell level, offering new insights into circRNA functions.288 In addition, advancements in bioinformatics have led to the development of efficient circRNA prediction algorithms, including find_circ, circRNA_finder, CIRI, CIRCexplorer, and MapSplice.289 Some emerging analytical methods like machine learning and deep learning can also explore the relationship between circRNAs and cancer progression, offering new strategies for their clinical application of circRNAs. In addition to the advancement of sequencing technology, numerous studies are investigating the molecular functions and mechanisms of circRNAs using PDX tumor mouse models and tumor organoid models. However, the current research is mainly based on cellular and animal experiments, and there is still a long way before achieving clinical application translation. Additional research is required to confirm these findings and establish practical clinical protocols.
Exosomal circRNAs in the tumor immune microenvironment modulate tumor growth by acting on various types of cells, including CAFs, endothelial cells, T cells, Treg cells, macrophages, neutrophils, NK cells, and microbiota. Exosomal circRNAs derived from tumor cells can be absorbed by immune cells and affect tumor immune escape by activating downstream tumor-related genes. Moreover, exosomal circRNAs secreted by immune cells and fibroblasts can be transmitted to tumor cells, regulating their growth, metastasis, and drug resistance. For example, exosomal hsa_circ_0004658 and circBTG2 derived from activated macrophages can inhibit tumor growth in liver cancer and glioma, respectively. Also, wang et al. collected plasma exosomes from healthy individuals and ICC patients for transcriptome sequencing, identifying circ-PTPN22 and circ-ADAMTS6 as potential biomarkers through differential gene analysis.283 And targeted inhibition of exosomal circ-PTPN22 and circ-ADAMTS6 can mitigate tumor progression by decreasing T cell exhaustion and neutrophil extracellular trap formation. A few exosomal circRNAs like circ_001264 can enhance antitumor effects when their siRNA is used in combination with aPD-L1. In addition, some harmful bacteria can activate the expression of exosomal circRNAs and stimulate tumor growth by transmitting genetic information between tumor cells. However, there is still limited research on the role of other types of cells, apart from T cells and macrophages, in regulating tumor progression through exosomal circRNAs. Also, the relationship between probiotics and exosomal circRNAs in carcinogenesis is still unknown.
Exosomal circRNAs have great clinical application prospects. Exosomal circRNA, known for its high abundance and stability, is found in various body fluids like blood and urine, making it an ideal diagnostic and prognostic biomarker. Researchers have identified some exosomal circRNAs as potential biomarkers for cancer diagnosis, and their combination with traditional biomarkers (such as CA19-9 and CEA) can enhance diagnostic accuracy. For instance, exosomal circ50547, hsa_circ_0079439, circ_0008103, and circ_0061407 are linked to cancer progression and survival, indicating their potential to serve as biomarkers for cancer diagnosis. Exosomal circLPAR1, circRAPGEF5, and circ-CDYL can be combined with traditional markers to improve diagnostic accuracy. The level of exosomal circRNAs may be closely associated with the prognosis of patients. Researchers can assess disease progression and therapy efficacy by monitoring changes in exosomal circRNAs, and develop personalized therapy plans for patients. Through in-depth research on exosomal circRNAs, early signals of tumor occurrence can be revealed, providing new methods for tumor prevention and a scientific basis for the formulation of public health policies.
Functional circRNAs may serve as future therapeutic targets for cancer. Researchers can target circRNAs for tumor therapy using ASOs, siRNA, and CRISPR-Cas13a. Researchers can also reverse the drug resistance of tumor cells by regulating the synthesis and secretion of exosomes, as well as affecting the uptake of target cells. To achieve precise treatment of tumors, the combination of various exosomal circRNAs with other noncoding RNAs, proteins, and DNAs has become a new possibility. In addition, the development of nanomedicines targeting exosomal circRNAs has the potential to become a new strategy for tumor therapy. However, this method faces extensive limitations, including the loading efficiency of circRNAs, the tumor-targeting ability of nanoparticles, and immunogenicity. In order to improve these shortcomings, researchers have also attempted to develop exosomal circRNAs derived from immune cells for tumor therapy, which has become a promising therapeutic strategy.
Currently, there is still much debate about the application of exosomal circRNAs in the clinical therapy of tumors. Despite the stable structures and high abundance of exosomal circRNAs in various body fluids, some researchers argue that false positives may occur in their identification, posing a challenge to their standardization as diagnostic markers. And exosomes, as one of the natural nanocarriers, have the advantages of low toxicity and high targeting. However, the delivery efficiency of exosomal circRNA remains one of the current controversies. Although exosomal circRNAs have made some progress in clinical diagnosis and therapy, more research is still needed for their clinical application.
AUTHOR CONTRIBUTIONS
Junshu Li, Wencheng Zhou, and Huiling Wang drafted the manuscript. Hongxin Deng and Meijuan Huang designed and revised the manuscript. All authors have reviewed and endorsed the final manuscript.
ACKNOWLEDGMENTS
This work was supported by the Sichuan Science and Technology Program, China (grant number 2024NSFSC1951), the Science and Technology Foundation of Sichuan, China (grant number 2022NSFSC0762), and the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (grant number ZYGD23023).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




