Therapeutic potential of Parabacteroides distasonis in gastrointestinal and hepatic disease
Abstract
Increasing evidences indicate that the gut microbiota is involved in the development and therapy of gastrointestinal and hepatic disease. Imbalance of gut microbiota occurs in the early stages of diseases, and maintaining the balance of the gut microbiota provides a new strategy for the treatment of diseases. It has been reported that Parabacteroides distasonis is associated with multiple diseases. As the next-generation probiotics, several studies have demonstrated its positive regulation on the gastrointestinal and hepatic disease, including inflammatory bowel disease, colorectal cancer, hepatic fibrosis, and fatty liver. The function of P. distasonis and its metabolites mainly affect host immune system, intestinal barrier function, and metabolic networks. Manipulation of P. distasonis with natural components lead to the protective effect on enterohepatic disease. In this review, the metabolic pathways regulated by P. distasonis are summarized to illustrate its active metabolites and their impact on host metabolism, the role and action mechanism in gastrointestinal and hepatic disease are discussed. More importantly, the natural components can be used to manipulate P. distasonis as treatment strategies, and the challenges and perspectives of P. distasonis in clinical applications are discussed.
1 INTRODUCTION
Gastrointestinal and hepatic diseases such as luminal, liver, and pancreatic diseases pose serious threat to the quality of people's life and account for a significant portion of health care expenditures.1 Numerous factors could affect digestive system diseases, mainly genetic and environmental factors.2, 3 Environmental factors such as diet, obesity, lifestyle, and microbiota could lead to the onset and progression of disease.4-7 Microbial genome sequencing of stool samples revealed significant changes of gut microbiota in fatty liver disease8 and inflammatory bowel disease (IBD).9
Intestine commensal microbiota plays a crucial role in host nutrient uptake, metabolism, establishment of mucosal immune system,10, 11 and help to construct intestinal epithelial barrier, defend against pathogens, support energy metabolism, and shape the immune system.12, 13 The secreted proteins, small molecular metabolites, or the bacteria themselves are directly or indirectly involved in the regulation of human health. For example, short-chain fatty acids (SCFAs) and bile acids (BAs) produced by gut microbiota are able to affect host metabolism and immune function.14, 15 Imbalance of gut microbiota is closely related to intestinal tract and liver disease, and manipulating microbiota holds promise as an approach for treating them.16
Parabacteroides distasonis was first isolated from human stools and classified as a member of the genus Bacteroides.17 However, analysis of its 16S rRNA sequence indicated that 90% gene sequence similarity to Tannerella forsythensis.18 Additionally, the major menaquinones found in P. distasonis are MK-9 and MK-10, which are different from those typically found in Bacteroides species.18 Based on these characteristics, P. distasonis has been reclassified as the genus Parabacteroides and the family Tannerellaceae. It is recognized as the type species of genus Parabacteroides.18 Noteworthily, P. distasonis shows the protective effects against diseases such as obesity19 and colitis20 and is expected to be one of the next-generation probiotics.21 Recent studies reported that P. distasonis regulated host metabolism,19 gut barrier, and immunity.22, 23 Its metabolites are mainly classified into SCFAs, amino acid, BAs, and other active compounds, which contribute to various physiological processes.24
Multiple studies have demonstrated that P. distasonis plays an important role in diseases. Understanding its ability to generate metabolites and action mechanism is able to better facilitate utilization of this bacteria. However, the relationship between P. distasonis, gastrointestinal and hepatic disease, and the treatment strategies has not been comprehensively discussed in previous study. In this review, we focused on the reported metabolic pathways of P. distasonis and its metabolites both in vivo and in vitro. We mainly discussed the role of P. distasonis in gastrointestinal and hepatic disease and its mechanisms of immune and metabolic regulation. We also summarized the natural components that are able to regulate P. distasonis, which could be potentially used as effective agents for diseases treatment. Additionally, clinical trials related to P. distasonis mainly through dietary interventions and prebiotics to regulate its abundance have been summarized. Finally, we discussed the prospect and limitations of its clinical application.
2 FEATURES OF P. DISTASONIS
P. distasonis exhibits a protective role in various diseases, but has been also observed negatively involved in some infectious diseases. The dichotomous role of P. distasonis may be attributed to strain variability. Here, we discuss the features of P. distasonis including the basic structural composition, utilization of polysaccharides, antibiotic resistance and biofilm formation. These features are associated with the colonization, adhesion, and invasion of P. distasonis in the host.
2.1 Characteristics of structure
P. distasonis belongs to gram-negative anaerobic bacteria. Its structure is consistent with the structural characteristics of gram-negative bacteria. Lipopolysaccharides (LPS) is the main component of outer membrane of gram-negative bacteria. In the lipid portion of LPS from strain ATCC 8503, large amounts of anteisobranched pentadecanoic acid were found, while 3-hydroxyheptadecanoic acid was not detected.25 O-antigen is a component of LPS, which is related to bacterial virulence. The rfbA gene encoding the enzyme that catalyzes the formation of O-antigen in the initial step, and the variation in this gene may be used to classify different strains of P. distasonis.26 The production and structure of O-antigen may help us distinguish pathogenic strains in P. distasonis, but more experimental evidence is needed.
The structure of outer-membrane protein (OMP) contributes to bacterial resistance. An OMP was detected from a clinical strain of P. distasonis. Test of pore-forming activity in liposomes indicates that this protein may form pore.27 It has the characteristic of OmpA protein, and the ompA gene was identified in P. distasonis.28 Further studies are needed to elucidate the impact of OMP on P. distasonis resistance and its variation among resistant strains.
Nine glycoproteins have been identified as surface layer (S-layer) glycoproteins on the P. distasonis.29 The composition of glycoproteins is the result of its adaptation to the host environment, which may contribute to its own survival. The function of S-layer glycoproteins is still unclear. Previous studies have demonstrated that S-layer proteins is able to contribute to adhesion,30 immune response,31 and the form of biofilm.32, 33 Those may render some P. distasonis strains to adhere and invade cells.
Capsules are the outermost structures of Bacteroides fragilis, which are composed of acidic polyanionic exopolymer.34 Encapsulated bacteria have enhanced ability to adhere to rat peritoneal mesothelium35 and resist host phagocytosis,36 which is related to the enhancement of virulence. Capsules were also detected in other Bacteroides species, and P. distasonis has been found not to be encapsulated through India ink wet mounts, electron microscopy, and ruthenium red staining.37, 38 However, the clinically isolated strains of P. distasonis have been observed with capsules structures by transmission electron microscopy.39 This indicated that some strains of P. distasonis have capsules, which may be related to their pathogenicity.
2.2 Utilization of polysaccharides
P. distasonis ATCC 8503 is the typical strain. The complete genome sequences of P. distasonis ATCC 8503 describes its metabolic profile. Gene Ontology analysis shows that polysaccharide metabolism, protein degradation, and cofactor biosynthesis are the main metabolic pathways.40 Polysaccharides are one of the nutritional sources for P. distasonis, and the utilization of polysaccharides helps the colonization of P. distasonis in the gut. Here is a summary of the ability of P. distasonis to utilize polysaccharides and the enzymes involved.
Compared with other Bacteroidetes in the gut, P. distasonis has the minimal number of genes involved in carbon source degradation. A total of 97 glycoside hydrolases belonging to 31 glycoside hydrolases families (as described in Carbohydrate-Active Enzymes database) were identified.40 P. distasonis ferments carbohydrates such as galactose, inulin, raffinose, and trehalose, but lacks the ability to break down l-arabinose, glycerin, and cellulose.17, 18, 40 Mucopolysaccharidase was achieved from a clinical separated strain of P. distasonis, hyaluronidase and chondroitinase like activity was detected in the purified enzyme.41 Neuraminidase belongs to glycoside hydrolase, metabolizes glycoconjugates, and removes sialic acids.42 Three strains of P. distasonis are positive for neuraminidase assay, when cultured in digest broth and 5% proteose peptone water broth.43 The result of genome sequencing also showed that P. distasonis has polysaccharide deacetylases and enzymes to degrade proteins, but lack polysaccharide lyases.40
Colonic mucus layer is mainly composed of mucin O-glycan, and the relative abundance of P. distasonis is correlated with mucin O-glycans decorated with terminal fucose.44 P. distasonis possess α-fucosidases to utilize l-fucose and may degrade mucin O-glycans as carbon source.40, 45 Additionally, P. distasonis forms fucosylated glycan decorated glycoproteins, which is able to enhance the colonization ability of microorganisms.46, 47
The similar metabolic pathway of N-glycan was found in P. distasonis. Glycoside hydrolase in P. distasonis degrades N-glycan to β-1,4-d-mannosyl-N-acetyl-d-glucosamine, which may be phosphorylated by a specific enzyme and directly enter to glycolysis.48 Apart from 2-β-d-glucooligosaccharide sophorohydrolase, laminarinase and mucopolysacharidase were obtained from P. distasonis.41, 49, 50 The final products of fermentation include succinic acid, acetic acid, propionic acid, and isovaleric acid.51 The metagenomic data have revealed that P. distasonis codes phosphotransacetylase, an enzyme that is capable of producing acetic acid.52
Overall, P. distasonis has various glycoside hydrolases which degrade substances that are difficult to degrade by the host. In this process, P. distasonis produces small molecules that are capable of influencing the intestinal environment. In addition, polysaccharides are also used to stimulate the growth of P. distasonis and exert therapeutic effects on diseases.
2.3 Antibiotic resistance
P. distasonis was the most resistant species in the isolated B. fragilis group when exposed to penicillin, cefoxitin, and cefotaxime.53 Among 2673 isolated B. fragilis group, P. distasonis exhibited the highest resistance to beta-lactam antibiotics.54 The resistant strains express beta-lactamase.53 The genes encoding this enzyme including blaTEM-1, blaBIL-1,55 cepA,56 cfiA,56 and a unique gene similarity with cfxA and cfxA257 were detected in P. distasonis.
However, clavulanic acid, the inhibitor of beta-lactamase, cannot change the susceptibility of P. distasonis to cefamandole, cefoxitin, and cephalothin. On the other hand, ethylenediaminetetraacetate enhances the barrier permeability, resulting in increased minimal inhibitory concentrations of beta-lactam antibiotics. The combination of clavulanic acid and ethylenediaminetetraacetate shows a synergistic effect, indicating that lower permeability is the major factor in producing resistantance.58 The components of penicillin-binding proteins (PBPs) were reduced, absent, or altered in molecular weight in resistant strains. This alteration in PBPs is correlated to cefoxitin resistance.59
The percentage of clindamycin, amoxicillin/clavulanic acid, and imipenem-resistant strains were increased in recent years.60 The resistance to clindamycin, erythromycin, streptogramins, and tetracycline in a strain of clinically isolated P. distasonis is able to be transferred to B. fragilis, indicating that the antibiotic resistance is specifically mediated by plasmid.61 The resistance to clindamycin was correlated with the erythromycin ribosomal methylase F (ermF) gene, which encodes methyltransferase.62 The tetQ gene, which encodes protein to protect bacteria from tetracycline, has been detected in P. distasonis 8503 through PCR assay, and the tetQ gene may be transferred to Enterococcus faecalis.63 The analysis of antibiotic resistance and resistance genes in different strains of P. distasonis is one aspect of safety assessment. It is also necessary to evaluate the impact of transferred antibiotic resistance genes on the host.
2.4 Formation of biofilm
In the process of biofilm formation, the planktonic bacteria initially adhere to surface by physicochemical processes. Then, the bacteria secrete extracellular polymeric substances, which mainly contains polysaccharides, lipid, and enzymes. This secretion leads to the adhesion from reversible to irreversible.64 In a study, microscopic observation was used to evaluate the biofilm formation capacities. All 14 strains of P. distasonis have the ability to form biofilm, although the biofilm structure is different due to its variation.33 Chronic stress reduces the abundance of P. distasonis in the cecum65; the stress-induced molecules are one of the factors that affect the biofilm formation, and most molecules reduce the biofilm formation capacity.33 Interestingly, the natural component celastrol was reported to promote the biofilms formation of P. distasonis and increase its abundance in cecum.66 Biofilms formation may also affect the abundance of P. distasonis in the intestine, but there is still limited research on the substances and mechanisms that stimulate biofilms formation.
In general, antibiotic resistance, the presence of capsules, and the biofilm structure are related to the strain of P. distasonis. These characteristics not only affected the colonization of P. distasonis in the intestine, but also were relate to its potential for invasion and infection within the body. Further investigation into the relationship between strains and these characteristics will provide valuable direction for the secure research on P. distasonis.
3 METABOLIC PATHWAYS REGULATED BY P. DISTASONIS
The active metabolites produced by gut microbiota are able to directly affect intestinal function or regulate physiopathological processes by circulating to other organs.67 Multiple studies have found that P. distasonis generates various metabolites to participate in the regulation of the body. Metabolomic data have provided a more comprehensive understanding of its metabolic capacity. Limitations by the functional annotation of metabolic enzymes and the actual metabolites of P. distasonis still require experimental verification. Based on these premises, we have compiled literatures on the metabolites affected by P. distasonis and analyzed its metabolic capacity (Figure 1). This section focuses on the metabolites that may be produced in vivo or in vitro.
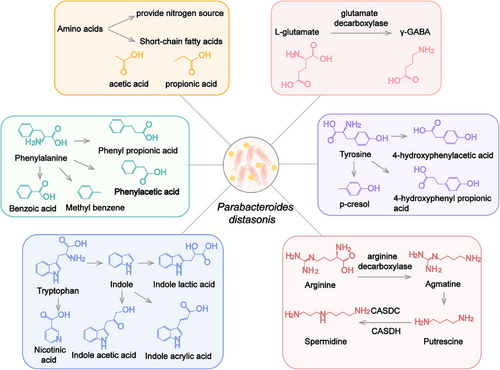
3.1 Amino acid metabolism
Gut microbiota play an important role in amino acid metabolism. Proteins from the diet are hydrolyzed by microbiota into amino acids and peptides, which further absorbed by the host or bacteria.68 Amino acids and peptides are able to enter bacteria cells through specific transport proteins, participate in protein synthesis, or be metabolized to small molecules. P. distasonis is capable of utilizing most amino acids and dipeptides for self-reproduction or producing active molecules. For example, asparagine may be used as the sole nitrogen sources for P. distasonis, and the related genes (ansA and aspA) are found in its genomes.69 Amino acids are degraded to small bioactive molecules through multiple steps such as decarboxylation and deamination.68 Gamma-aminobutyric acid (γ-GABA) is an inhibitory neurotransmitter. P. distasonis decarboxylates l-glutamate to γ-GABA by glutamate decarboxylase,70 which may affect neuroendocrine in female.71
SCFAs are not only derived from the decomposition of carbohydrates, but also come from the metabolism of amino acids. Amino acids are transformed to corresponding keto acids or saturated fatty acids through transamination or deamination reaction by bacteria. SCFAs are generated as final products through a series of steps,68, 72 P. distasonis mainly produces acetic acid and propionic acid in this process.73, 74
P. distasonis has the ability to metabolize aromatic amino acids in the presence or absence of glucose.75 P. distasonis transforms phenylacetic acid, benzoic acid, phenyl propionic acid, and methyl benzene from phenylalanine.75 The supplement of glucose promotes the production of phenyl pyruvic acid and phenyl lactic acid, which are the oxidative products of phenylalanine.75 Tyrosine may be decomposed into 4-hydroxyphenylacetic acid and 4-hydroxyphenyl propionic acid by P. distasonis. Furthermore, p-cresol, a bioactive microbial metabolite from tyrosine, has been detected when P. distasonis was cultured in yeast extract, casitone, and fatty acid (YCFA) medium.75 However, in another report, no p-cresol was detected in medium when P. distasonis was cultured in modified Gifu anaerobic medium.76 This process may be influenced by other substances in culture medium. Tryptophan only generates indole and indole derivatives under the action of bacteria.77, 78 Tryptophan is metabolized by P. distasonis to indole,73 indole-3-acetic acid,75 indolelactic acid,66, 69, 75, 79 and indoleacrylic acid,80 while indole was not detected in P. distasonis cultured in brain heart infusion (BHI) medium in another study.51 Nicotinic acid (NA) is also the product of tryptophan metabolism by gut microbiota. Quinolinate, which is derived from tryptophan metabolism, serves as a precursor substance in the synthesis of NA.81 The data of genome sequencing indicate that P. distasonis possesses the genes for NA synthesis. P. distasonis carries genes encoding nicotinate-nucleotide pyrophosphorylase, 2′,3′-cyclic nucleotide 2′-phosphodiesterase, and purine nucleoside phosphorylase, which are involved in the pathway from quinolinate to NA. After administering P. distasonis to mice, the levels of NA in feces were increased, and the increased NA has been detected in multiple P. distasonis strains cultured in vitro. These findings indicated that P. distasonis indeed has the ability to generate NA. However, P. distasonis cannot increase the level of nicotinamide.82
The different results of those in vitro experiments may be attributed to differences in strain diversity, culture medium, and detection sensitivity. In summary, P. distasonis utilizes multiple amino acids and metabolize amino acids into small bioactive molecules. Among them, p-cresol,83 indole,84 indole-3-acetic acid,84 indole lactic acid,85 and indole acrylic acid80 are natural ligands of aryl hydrocarbon receptor (AhR). The AhR pathway affects the integrity of the intestinal barrier.86 In addition, SCFAs and NA have been found to exhibit a protective effect on the intestinal barrier, which may be achieved through G protein coupled receptors. Those metabolites may relate to the function of P. distasonis and contribute to the function of P. distasonis in maintaining intestinal barrier integrity.
3.2 Polyamine metabolism
Polyamines, mainly including putrescine and spermidine, belong to small polycationic molecules. Many bacteria have the ability to produce, utilize or degrade polyamines.87 This process is common reaction in bacteria and may be related to their classification.87, 88 The distribution of polyamines may serve as a chemotaxonomic marker in bacterial classification.88 Strains in phylum Bacteroidetes were cultured in anaerobic environment to evaluate the level of polyamines by high-performance liquid chromatography. It was found that spermidine is the predominant polyamine in Parabacteroides.89
It is reported that arginine decarboxylation is the dominant pathway for the biosynthesis of polyamines.90 Arginine may be converted into putrescine through either the arginine deiminase or arginine decarboxylase catalyzed pathway, with ornithine or agmatine serving as intermediates, respectively.91 In a metabolomics analysis of gut microbiota, the arginine levels decreased and agmatine levels increased in the culture medium of P. distasonis,69 which may be related to the biosynthesis of polyamines.92 Compared with reported polyamine biosynthetic and transport proteins, P. distasonis contains genes that encode homologous proteins of agmatine deiminase and N-carbamoylputrescine amidohydrolase in putrescine synthesis.93 The homologs of carboxyspermidine decarboxylase and carboxyspermidine dehydrogenase metabolize putrescine into spermidine.93 Putrescine, spermine, and spermidine were found in the cell of P. distasonis.89, 93 The levels of polyamines in the body are influenced by the gut microbiota.94 Transplantation of P. distasonis increased the spermine and putrescine and reduced N-acetylspermidine in the cecum of mice.95 This further demonstrates the ability of P. distasonis to regulate polyamine metabolism.
3.3 Nucleotide and fatty acid metabolism
Nucleotide metabolism is the other major metabolic pathway influenced by P. distasonis. It has been observed that P. distasonis increases the level of uracil in polyamine-free media,69 mega media,69 and BHI medium.66 Uracil from microbiota stimulates chronic inflammation in the intestine and activate innate immunity response.96 This may explain the regulatory effects of P. distasonis on intestinal inflammation and immunity.
Bacteria are the main source of exogenous purines in the gut, which promotes mucous barrier integrity.97 P. distasonis increases the levels of xanthosine, xanthine, hypoxanthine, and guanine and decreases the levels of adenine and adenosine.66, 69 In nucleotide metabolism pathway, adenine and adenosine serve as upstream components that may provide raw materials for the synthesis of hypoxanthine, xanthosine, xanthine, and guanine. Supplementing intestinal hypoxanthine restores energy metabolism disorders caused by inflammation and improves barrier function.98 This suggested that the nucleotide metabolism regulated by P. distasonis may contribute to its beneficial effects on the intestine.
Gut microbiota also participates in fatty acid metabolism, and the changes in microbiota affect the composition of lipid in the host. P. distasonis participates in the metabolism of polyunsaturated fatty acids. γ-Linolenic acid was increased in the cecum and liver after the transplantation of live P. distasonis.99 P. distasonis produces γ-linolenic acid in high-fat diet (HFD) extract, indicating its ability to metabolize fatty acids.99 The regulation of fatty acid metabolism by P. distasonis may be one of the mechanisms for treating metabolic diseases, but there is currently limited research on the metabolites of fatty acid.
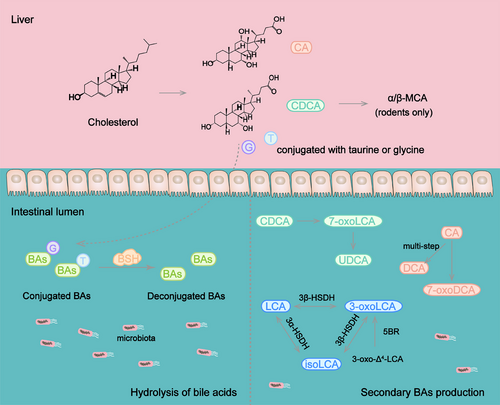
3.4 BA metabolism
BAs are synthesized in the liver from cholesterol and modified by microbiota in the distal gastrointestinal tract. Conjugated and deconjugated BAs are absorbed to blood through BA transporter or passive diffusion in the enterohepatic circulation. BAs function as signaling molecules regulating many important physiological processes including metabolism100 and immune responses.101 BAs conjugated with taurine or glycine are secreted into small intestine and converted to secondary BAs in distal ileum, cecum, and colon through deconjugation, dehydrogenation, dihydroxylation and epimerization.102, 103 Gut microbiota is necessary for the hydrolysis of conjugated BAs and biotransformation of free BAs.
Recent studies have identified several enzymes involved in shaping BA pool in P. distasonis. Target BAs metabolomics and RNA-Seq analysis revealed that P. distasonis possesses bile salt hydrolase (BSH),104 an enzyme deconjugating the conjugated BAs. BSH controls a crucial step in the conversion of BAs transformation in intestine and is essential for maintaining BAs homeostasis in the host. P. distasonis isolated from human has the ability to deconjugate taurocholic acid in vitro and vivo through BSH.105 The administration of P. distasonis alleviates hepatic fibrosis by increasing the activity of BSH and reducing the level of taurochenodeoxycholic acid (TCDCA), which induces pyroptosis in the liver.66
The deconjugated BAs further transform to new BAs in intestine. In a study, no secondary BAs were detected in the cecum of germ-free mice-inoculated P. distasonis strain K-5 the mainly BAs were β-muricholic acid (β-MCA). Additionally, the results in vitro showed that P. distasonis deconjugated tauro-β-muricholic acid (T-β-MCA) but lacked 7α-dehydroxylating activity.106 Interestingly, strain K-5 and its extract were able to stimulate 7α-dehydroxylation and 7β-dehydroxylation, which expressed by Eubacterium sp. strain 36S.107
P. distasonis produced deoxycholic acid (DCA) and 7-oxodeoxycholic acid (7-oxoDCA) from cholic acid (CA) when sodium taurocholate served as a substrate in YCFA medium.19 With the supplement of sodium TCDCA, TCDCA undergoes hydrolysis to produce chenodeoxycholic acid (CDCA), then CDCA is converted to ursodeoxycholic acid (UDCA) through 7-oxolithocholic acid (7-oxoLCA).19 7α-Hydroxysteroid dehydrogenase (7α-HSDH) and 7β-hydroxysteroid dehydrogenase (7β-HSDH) are known to catalyze these processes108 and may participate in the production of UDCA. Live P. distasonis increased the levels of UDCA, glycoursodeoxycholic acid, lithocholic acid (LCA), and taurolithocholic acid (TLCA) in the cecum of mice with calorie restriction diet or HFD.109
3-Oxolithocholic acid (3-oxoLCA) and isolithocholic acid (isoLCA), the isoforms of LCA, have been identified as inhibitors of T helper 17 cells (Th17) differentiation.110, 111 The synthesis of 3-oxoLCA and isoLCA is associated with gut microbiota.111 Recent research has clarified that P. distasonis generated LCA, 3-oxoLCA, and isoLCA through 5β-reductase, 3α-hydroxysteroid dehydrogenase (3α-HSDH), and 3β-hydroxysteroid dehydrogenase (3β-HSDH) in vitro.112 P. distasonis produced 3-oxoLCA from 3-oxo-Δ4-LCA and converted 3-oxoLCA, isoLCA, and LCA into each other through the action of 3α-HSDH and 3β-HSDH.112 The levels of 3-oxoLCA, isoLCA, LCA, and DCA were increased in mice treated with live P. distasonis.113 The transplantation of P. distasonis ameliorated rheumatoid arthritis and inhibited the generation of 3-oxoLCA, isoLCA, LCA, and DCA,113 indicating that the change of BA pool is the key for the function of P. distasonis. The latest research has found that P. distasonis converted CA into a newly detected BA called 3-acetylcholic acid in vitro.114
The BAs produced by P. distasonis are related to the raw materials provided in vitro, which may lead to different production of BAs in vivo and in vitro. P. distasonis is able to utilize BAs from other bacteria to produce new BAs in the gut. Thereby, its regulation of BA pool also involves the interaction with other gut microbiota, which is also worth exploring.
Overall, researches on P. distasonis have focused on its impact on BA metabolism (Figure 2), as its production of active metabolites that affect various physiological processes. It is worth noting that its metabolic capability is not limited to those reported. Limited by culture conditions or detection techniques, there may be additional metabolites yet to be explored. In addition, its effect on the metabolites is not only limited to the metabolism of substances by its own metabolic enzymes, but also involves the cometabolic effects with other intestinal microbiota. It may be the reason why part results of in vivo and in vitro experiments are not consistent.
4 ROLE OF P. DISTASONIS IN GASTROINTESTINAL AND HEPATIC DISEASE
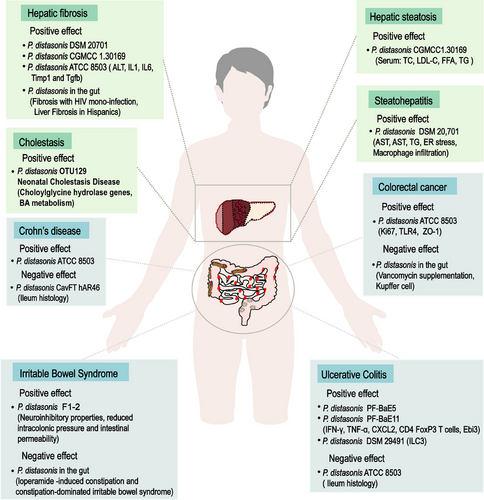
Dysbiosis of the gut microbiome is associated with many systemic diseases. More attention has been paid to the connection between P. distasonis and diseases, especially for gastrointestinal and hepatic disease. Here, we conducted a literature search for recent reports on the treatment of P. distasonis (Table 1), with a focus on the changes of P. distasonis in six types of liver and intestinal diseases, in order to provide clinical references and theoretical support for the application of P. distasonis.
| Strain | Disease | Pathway | Mechanism of action | References |
|---|---|---|---|---|
| P. distasonis CGMCC1.30169 | Obesity and metabolic dysfunctions | Bile acid metabolism | P. distasonis and its production (UDCA, LCA, and succinate) improved the Claudin-1 and Zo-1, increased succinate to activate FBPase, and promoted IGN in jejunum and increased FGF15 in ileum and serum. | 19 |
| P. distasonis PF-BaE5 and PF-BaE11 | TNBS-induced colitis | Inflammatory reaction | P. distasonis primed dendritic cells to induce regulatory T lymphocytes from naïve CD4 T cells, reduced the expression of proinflammatory factors, increased levels of IL-10 producing CD4+ FoxP3+ T cells and enhanced expression of the gene encoding Ebi3. | 22 |
| P. distasonis ATCC-8503 | Hepatic fibrosis | Bile acid metabolism | P. distasonis increased the gene level of Oatp4 and Bsep to reverse liver BAs in mice with hepatic fibrosis. P. distasonis promoted the BSH activity in cecum and reduced the level of TCDCA, which increased in hepatic fibrosis and induced pyroptosis to damage hepatocytes and HSCs. | 66 |
| P. distasonis ATCC 8503 | Type 2 diabetes | Inflammatory reaction | P. distasonis increased indoleacrylic acid and promoted the secretion of Il22 to maintain the intestinal barrier. | 80 |
| P. distasonis NSP007 | Insulin resistance | Nucleotide metabolism | P. distasonis and its product nicotinic acid enhanced the integrity of the intestinal barrier, reduced systemic inflammation. | 82 |
| P. distasonis ATCC-8503 | Testicular dysfunction | Polyamine metabolism | P. distasonis ameliorated triptolide-induced testicular dysfunction and increased spermine that upregulate Hsp70s and improved oxidative stress. | 95 |
| P. distasonis ATCC-8503 | Nonalcoholic fatty liver disease | Fatty acid and bile acid metabolism | P. distasonis-derived γ-linolenic acid enhanced lipid catabolism and changed the BA synthesis pathway in NAFLD. | 99 |
| P. distasonis ATCC 8503 | Obesity after calorie restriction | Bile acid metabolism | P. distasonis enhanced glucose and energy metabolism, decreased fasting blood glucose levels, improved glucose tolerance and insulin sensitivity, as well as increased thermogenesis, promoted the synthesis of UDCA and LCA to induce the secretion of GLP-1 and increased the level of UCP1. | 109 |
|
P. distasonis BAA-1295 |
Arthritis | Bile acid metabolism | P. distasonis derived BAs promoted M2 polarization of macrophages, inhibited the differentiation of Th17 cells and reversed the elevation of inflammatory factors, inhibited the differentiation of Th17 cells in vitro through suppressing the expression of RORγt. | 113 |
| P. distasonis DSM 29491 | Ulcerative colitis | Immune system | P. distasonis promoted intestinal ILC3, enhanced the expression of mucins and claudins proteins, and increased the number of goblet cells. | 116 |
| P. distasonis ATCC 8503 |
KRAS p.G12D-mutant colorectal cancer |
Immune system | P. distasonis inhibited the invasion of F. nucleatum, prevented it from binding to DHX15, reduced the expression of Ki67 in colonic organoids, inhibited intestinal epithelial over proliferation. | 125 |
| P. distasonis ATCC 8503 | Colorectal cancer | Inflammatory reaction | P. distasonis decreased the expression of Il-4, Il-6, and Tnf-α, and increased the expression of Il-10 and Tgf-β. | 129 |
| P. distasonis DM-PD18 | Alcohol-induced liver injury | Amino acid metabolism | P. distasonis-mediated regulation of the gut–liver axis ameliorated alcohol-induced dysbiosis of microbiota metabolites profile, primarily affecting amino acid metabolism and bile acids metabolism. | 140 |
| P. distasonis DSM 108218 | Nonalcoholic steatohepatitis | Fatty acid metabolism | P. distasonis reduced serum LPS, promoted the expression of tight junction proteins E-cadherin, restored intestinal barrier function. Additionally, P. distasonis metabolized inulin to produce pentadecanoic acid, which has a therapeutic effect on intestinal barrier. | 148 |
| P. distasonis DSM 20,701 | Chronic alcohol-related liver disease | Fatty acid metabolism | P. distasonis and P. distasonis- derived propionic acid activated MUP1 expression in the liver, and significantly reduced macrophage infiltration in mice fed with alcohol. | 156 |
| P. distasonis | Bladder cancer | Immune system | P. distasonis augmented the infiltration of CD4T and CD8T cells into the tumor. | 167 |
| P. distasonis F1-2 | Chronic abdominal pain | Inflammatory reaction | P. distasonis F1-2 relieved visceral pain associated with leaky gut by reducing nociceptor response to capsaicin, inflammatory soup, and bradykinin stimulation. | 135 |
| P. distasonis ATCC 8503 | Rheumatoid arthritis | Inflammatory reaction | P. distasonis used β-glucosidase to release glycitein from the diet, modulated inflammatory rhythms in rheumatoid arthritis. | 179 |
4.1 P. distasonis and IBD
IBD is an autoimmune inflammatory disease that requires a lifelong management, including ulcerative colitis (UC) and Crohn's disease (CD). Numerous literature reports have documented the presence of gut microbiome imbalances in IBD populations, featured by an enrichment of intestinal germs and a loss in probiotics. Transformation in the constitution and function of intestinal flora directly affect the intestinal environment, which may be a critical factor in the progression of IBD.
Several strains of P. distasonis were reported to show immunomodulatory and intestinal barrier strengthening efficacy in vitro and induce the conversion of dendritic cells (DCs) from naive CD4 T cells to regulate T cells and exert anti-IBD effects in vivo.22 In a prospective observational study involving 64 volunteers, it was found that the acute phase protein C reaction protein, LPS-binding protein, serum amyloid A1, and orosomucoid 1 in overweight/obese IBD patients were significantly lower than ordinary IBD patients, and overweight/obesity could further promote the microbial diversity of UC, as indicated by the increased abundance of some probiotics, including P. distasonis, Alistipes indistinctus, and Ruminococcus bromii.115 Therefore, it was speculated that the reduced inflammatory response in IBD patients was related to the increased abundance of probiotics. Mice with obvious resistance to dextran sulfate sodium (DSS)-induced colitis had significantly increased the abundance of Akkermansia muciniphila and P. distasonis among the gut microbiota, which were subsequently colonized to demonstrate synergistic improvement of intestinal epithelial integrity and protect against acute colitis.116 In addition to live P. distasonis alleviating IBD, bacterial components and bacterial by-products are also able to reduce inflammation. The insoluble membranous fraction isolated from P. distasonis (mPd) after centrifugation was fed to mice, which inhibited the expression of proinflammatory factor, prevented the decrease of serum antibodies and gut microecological imbalance induced by DSS.20 Colonization of the intestine of SAMP1 mice with P. distasonis did not increase intestinal inflammation and other behavioral indicators, but induced depression-like behaviors.117 Some drugs or natural products exert anticolitis effects by regulating intestinal flora. Enteromorpha clathrata polysaccharide enhanced the level of Parabacteroides spp. in vivo. Enteromorpha clathrata polysaccharide could promote the development of P. distasonis F1-28 in vitro and increase its generation of SCFAs.118
Although many studies suggested the positive character of P. distasonis in IBD, different opinions still exist. Pglyrp regulates intestinal flora by enhancing the abundance of Prevotella falsenii and P. distasonis in the gut. Subsequent single-bacterium gavage experiments showed that P. distasonis aggravated DSS-induced colitis in mice.119 In CD, P. distasonis was the most typical growth, while Faecalibacterium prausnitzii and B. fragilis were obviously lessened.120 A total of 80 bacterial strains were isolated from the small intestinal mucosa of CD patients after anaerobic culture, and nine strains were identified on the basis of their link with CD, which included P. distasonis.121 A strain of P. distasonis named CAVFT-Har46 was isolated from intestinal cavernous fistulous tract (CavFT) microlesions of a CD patient and was tested for its complete genome sequence.122 P. distasonis has promising applications in the treatment of IBD, but its possible pathogenic effects and interference with the nervous system still need to be vigilant in clinical practice.
4.2 P. distasonis and colorectal cancer
Colorectal cancer (CRC) is one of the most ordinary and fatal cancer of the alimentary tract. Tumor localization of CRC is in direct contact with trillions of gut microbes. Altered microbial profiles induce dysbiosis and CRC.123 The gut microbiota has been shown to be disturbed in the early stages of colorectal neoplasia and aggravated during disease progression. Gut microbiota is critical in HFD-associated colorectal tumorigenesis.124 In germ-free mice treated with azoxymethane (AOM) under HFD, Alistipessp.Marseille-P5997 and Alistipessp.5CPEGH6 accumulated in the gut of the CRC model.124 P. distasonis was depleted, and intestinal barrier function was impaired, indicating that HFD-modulated gut microbiota promoted the occurrence of colorectal tumors.124 Gut microbiota plays a central role in CRC. Many different kinds of microbes are of the relationships of generation and restriction in the gut. Fusobacterium nucleatus promoted colorectal neoplasia in Villin-Cre/KrasG12D+/−mice, while P. distasonis could antagonize it and reduce the number and size of tumors.125 Smoke exposure is one of the main inducer of CRC. Significant differences of gut bacteria abundance were monitored in the smoking mice, including the enrichment of Eggerthella lenta and loss of P. distasonis.126 Compared with wild-type mice, the amount of Clostridium septicum in the stool of TGFB signaling deficient mice was increased, while the abundance of P. distasonis was decreased, which was strongly associated with colon tumor development.127 The depletion of P. distasonis was correlated with increased tumor burden.128 Loss of P. distasonis contributes to CRC progression. Dietary supplementation of P. distasonis appears to be a potential therapeutic strategy for CRC. P. distasonis diet mice have a lower toll-like receptor 4 (Tlr4), Il-4, and tumor necrosis factor αlpha (Tnf-α) expression in colon and a higher colonic interleukin 10 (Il-10) and transforming growth factor beta (Tgf-β) expression. P. distasonis could increase colonic concentration of the proteins Zonula occludens-1 (Zo-1) and Occludin.129 In addition to directly supplementing live P. distasonis, its outer membrane vesicles inhibited the proliferation of CT26 colon cancer cells in vitro and suppressed tumor growth in CT26 tumor-bearing mice.130
Nevertheless, one study reported that an increased abundance of P. distasonis was negatively associated with Kupffer cells and positively associated with liver metastasis of CRC after vancomycin supplementation. Here is a lack of direct evidence that P. distasonis is the trigger for liver metastasis in CRC. A lot of research and further evidence are needed to clarify the confusion.131 In summary, P. distasonis is showing a significant protective effect on the progression of CRC.
4.3 P. distasonis and other bowel diseases
Many factors may induce intestinal injury. P. distasonis has also demonstrated a broad spectrum of protective effects against other enteropathies. Gastrointestinal dysfunction is a common complication in diabetic patients. The abnormal proliferation of gram-negative bacteria in patients with type 2 diabetes resulted in increased intestinal permeability and impaired intestinal barrier by producing large amounts of LPS.132 Supplementation of P. distasonis ATCC 8503 for 12 weeks decreased the concentration of LPS in serum, inhibited the expression of Il-6, Tnf-α, and Il-1β, stimulated the excretion of IL-10, and promoted the expression of colon Occludin, Claudin-1, Claudin-2, and Zo-1.80 The protective effects of some natural products are closely associated with altered gut microbiota. Poria cocos polysaccharide (PCP) promoted the expression of intestinal tight junction protein Zo-1 and regulated seven characteristic flora in cecum of antibiotic-associated diarrhea mice, which involve P. distasonis.133 After ileocecal excision, the supplementation of tributyrin lessened the concentration of TNF-α and IL-6, augmented the abundance of Bacteroides thetaiotomicorn and P. distasonis, and alleviated colon inflammation.134 Some patients with irritable bowel syndrome (IBS) have colonic hypersensitivity, which is characterized by urgent bowel movements, bloating, and abdominal pain. Although the mechanism of chronic visceral hypersensitivity in IBS patients is still unclear, it does not prevent researchers from looking for a solution. P. distasonis F1-2 isolated from a healthy donor has neuroinhibitory properties.135 Oral administration of F1-2 reduced intracolonic pressure and intestinal permeability, significantly improving colon hypersensitivity induced by 0.5% DSS and Citrobacter rodentium postinfectious, respectively.135 However, the efficacy of P. distasonis on IBS was worth pondering in another study. Transformation in gut microbiota were present in loperamide-induced constipation and constipation-dominated IBS featured by the increased Bacteroides ovatus and P. distasonis in both models.136 The causal relationship between P. distasonis and the two models is unknown, and a lot of work is still needed to explore in the future.
4.4 P. distasonis and cholestasis
Bile secretion and excretion disorders lead to excessive accumulation of bile in the liver and systemic circulation, resulting in abnormal liver function and skin status, which called cholestasis. Recently, the changes of P. distasonis in cholestasis have been reported. Initially, it was noted that the relative abundance of P. distasonis was higher in healthy infants than in cholestatic infants.137 Some researchers conducted an initial analysis of data from the BIG Data Center (CRA001920) and found a significant correlation between clinical cholestasis and decreased levels of Bacteroidetes and Parabacteroides.66 The changes in P. distasonis were observed in the feces of 13 cholestasis patients and found that its abundance in patients was significantly lower than that of healthy individuals (n = 10).66 It suggested that the level of P. distasonis could be inhibited in cholestasis.
BAs are the main components of bile and regulating the pathways related to BA synthesis and excretion is a method to improve cholestasis. Cholesterol 7 alpha-hydroxylase (Cyp7a1) and cholesterol 27 alpha-hydroxylase (Cyp27a1) are the two pathways that initiate BA synthesis.138 The bile salt export pump (Bsep) is the microvilli efflux transporter for BAs, while Na+-taurocholate cotransporting polypeptide (Ntcp) is the basolateral absorptive transporter for BAs.139 A recent report indicated that P. distasonis inhibited the expression levels of Cyp7a1 and Cyp27a1, promoted the expression of Bsep and Ntcp, and regulated the synthesis and secretion of BAs in the liver.140 Therefore, P. distasonis may be a potential approach for clinical cholestasis treatment.
4.5 P. distasonis and liver fibrosis
Hepatic fibrosis is a phenomenon of abnormal hyperplasia of connective tissue caused by multiple causative factors-induced liver injury. Long-term cholestasis may be developed into liver fibrosis, and liver fibrosis may further develop into cirrhosis on a long-term basis. Patients with hepatic fibrosis in Rio de Janeiro, Brazil, and Spain were reported to reduce P. distasonis in the gut.141, 142 It was also found that the feces of 17 patients with liver fibrosis in Kunming, China, showed a significant decrease in P. distasonis.66 The abundance of P. distasonis was decreased in both senile sepsis related liver injury rats and thioacetamide-induced liver fibrosis mice.66, 143 It suggests that P. distasonis is negatively correlated with liver fibrosis. BSHs are highly conserved in major classes of intestinal microbial phyla, and they differ among bacteria due to their preference for binding to glycine or taurine-containing BAs.144 Clinical data showed that P. distasonis is positively correlated with BSH activity in patients with hepatic fibrosis.66 Multiple strains of P. distasonis regulated BA metabolism through BSH, including P. distasonis DSM 20701, P. distasonis CGMCC 1.30169, and P. distasonis ATCC 8503.19, 66, 104 Supplementation of P. distasonis may be a therapeutic strategy. Transplantation of P. distasonis ATCC 8503 improved liver histology in mice with hepatic fibrosis and reduced alanine aminotransferase (ALT) and the gene and protein expression of proinflammatory factors and liver fibrosis genes, including IL-1β, IL6, TIMP1, and TGF-β.66 In general, P. distasonis has an antifibrosis effect in liver.
4.6 P. distasonis and fatty liver disease
Fatty liver is characterized by hepatocyte steatosis, which is common in nonalcoholic liver disease (NAFLD). P. distasonis is associated with the development of fatty liver. Compared with the control group, the abundance of P. distasonis in mice with high-fat-diet induced hepatic steatosis decreased from 2.28 ± 0.43 to 0.39 ± 0.12%.145 Some researchers found that P. distasonis fluctuated during the course of the disease, with the highest abundance on the 10th week induced by high-fat and high-cholesterol diets. With the development of hyperlipidemia, the abundance of P. distasonis was decreased until the 30th day.146 A clinical investigation also found that the abundance of P. distasonis was significantly higher in fatty liver than in NAFLD.147 NAFLD was officially renamed as metabolic dysfunction-associated fatty liver disease (MAFLD) in 2020 due to unreasonable exclusivism. As MAFLD progresses, it may lead to liver inflammation and eventually develop into nonalcoholic steatohepatitis (NASH). Metagenomic sequencing revealed that P. distasonis in the intestines of mice fed a HFD lacking choline for 16 weeks was depleted, while the liver steatosis and necroinflammatory area in mice fed a liquid diet containing P. distasonis were significantly lower.148 The HFD is one of the triggers of diabetes. Clinical data showed that P. distasonis is significantly lower in type 2 diabetic patients (n = 14) than in healthy subjects (n = 91).80 Liver dyslipidemia is also considered to be associated with diabetes. It was found that P. distasonis significantly improved insulin resistance (IR) index, lowered triglycerides (TG), total cholesterol (TC), and low-density lipoprotein (LDL-C) levels, indicating that it could improve lipid metabolic disorders in T2D rats.80 It was also found that transplantation of P. distasonis could decrease the serum TC of ob/ob mice by 20% and reduced the serum concentrations of LDL-C, serum free fatty acids, and serum TG, contributing to alleviate hepatic steatosis.19 In conclusion, the lipid-lowering function of P. distasonis supports its therapeutic potential in fatty liver disease.
The growing evidences suggested that sarcopenia was associated with IR.149 Fat accumulation leads to a condition calling muscle-wasting obesity, which is common among older adults.150 The ultimate outcome of fatty liver disease is cirrhosis, while sarcopenia is one of the frequent complications during advanced liver disease.149 Accumulating evidences suggested that gut microbiota was involved in the pathophysiology of musculoskeletal diseases through the gut muscle axis.151, 152 Gut microbiome imbalance is a major contributing factor to sarcopenia.153 A clinical investigation was conducted in 62 patients with metastatic renal cell carcinoma, among which patients 25 had sarcopenia. It was found that P. distasonis and Dialister species were strongly associated with sarcopenia, and the gluconeogenesis contributed to sarcopenia.154 Another study indicated that P. distasonis effectively improved the fasted blood glucose, glycated serum protein, and oral glucose tolerance test in T2D rats, indicating that P. distasonis has excellent glucose control ability.80 These results pointed out a beneficial effect of P. distasonis on sarcopenia, and supplementation of P. distasonis contributes to alleviate or delay its progression.
Individuals with excessive alcohol use are prone to develop alcoholic liver disease (ALD), and liver steatosis is considered to be the earliest symptom.155 Alcohol intake increases the levels of TC and TG in the serum and LDL-C in the liver, leading to liver fat accumulation.140 After 14 weeks of alcohol liquid diet intake, liver fat droplets, inflammation, and macrophage accumulation were observed in the alcohol mice, while the administration of P. distasonis led to a significant decrease in the levels of AST, AST, TG, and mRNA levels of endoplasmic reticulum (ER) stress-related genes in alcohol mice.156 P. distasonis has great potential to alleviate ALD and make a significant contribution to ALD intervention measures in the future. In term of MAFLD, obesity, diabetes, sarcopenia, and alcohol-related liver disease, metabolic dysregulation is the common characteristic. Therefore, it is predicted that P. distasonis may be a potential therapeutic option for these metabolic syndromes.
The abundance changes of P. distasonis were summarized in three types of intestinal diseases and three types of liver diseases. It was demonstrated that P. distasonis tended to have a protective role in liver diseases, while is dual nature in intestinal diseases (Figure 3). The possible negative effect of P. distasonis still needs further study to perform its greater functional value.
5 ACTION MECHANISM OF P. DISTASONIS
Gut microbiota are able to influence host health through different pathways. Here, we searched the recent reports about the relationship between P. distasonis and various diseases. Five mechanisms of action were summarized, including immune regulation, improving intestinal barrier function, interfering with BA receptors, glucose homeostat. A more precise understanding of the interactions between P. distasonis and biomolecules may be achieved through these mechanisms research, which is beneficial for drug design and development for P. distasonis in the future.
5.1 Immunomodulation
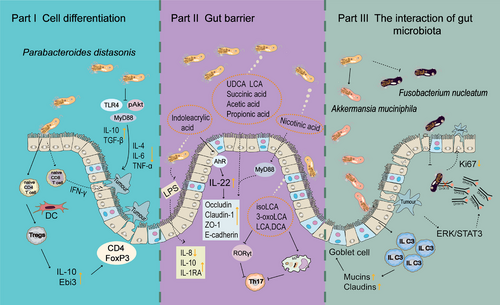
Bacteria generally colonize in the gut and direct contact with the mucosal surface of the bowel. The intestinal epithelium was mixed with certain lymphocytes, DCs, microfold cells, and goblet cells. In the lamina propria, there are lymphoid follicles, effector T and B cells, macrophages, and NK cells.
Intestinal immunity is essential for mucosal homeostasis. Receptor retinoic acid-related orphan receptor gamma t (RORγt) type 3 innate lymphoid cells (ILC3) regulated intestinal homeostasis by producing granulocyte-macrophage colony-stimulating factors, which in turn acted on macrophages and DCs.157 Mucosal homeostasis provides a habitat for intestinal flora. The gut microbiota supported the development of host metabolism by providing beneficial nutrients (such as BAs and SCFAs), in addition to regulating the maturation of the gut immune population158 (such as ILC3,159 CD8 T cells,160 CD4 T cells,161 DC,162 T helper 1163) by producing signals. P. distasonis facilitated the intestinal colonization of A. muciniphila and amplified the protective effect of A. muciniphila on acute colitis by further promoting intestinal ILC3, enhancing the expression of mucins and claudins proteins, and increasing the number of goblet cells.116 When p.G12D was mutated, ERK/STAT3 signal was activated and DHX15 was upregulated. P. distasonis inhibited the invasion of F. nucleatum, prevented it from binding to DHX15, reduced the expression of Ki67 in colonic organoids, inhibited intestinal epithelial over proliferation, and alleviated the progression of CRC with KRAS p.G12D mutation.125
P. distasonis stimulates mature immune cells to secrete antibodies, mobilize the systemic immune system and killer system, and eliminate these aging lesions, mutated tissues (such as tumor cells), and exotic pathogenic microorganisms. Initially, some scholars found that Bacteroides enhanced its antitumor effect and reduced gastrointestinal toxicity in melanoma patients who received CTLA-4 blocking therapy.164, 165 It was further demonstrated that P. distasonis enhances anticancer immunity by inducing the production of IFN-γcd8 T cells.127 P. distasonis blocked the activation of Tlr4, decreased the expression of Il-4, Il-6, and Tnf-α, increased the expression of IL-10 and TGF- β, and inhibited the expression of MyD88 and pAkt in colon of CRC mice.129 P. distasonis stimulated anti-inflammatory IL-10-expressing human CD4CD25 T cells and IL-10FoxP3 Tregs in mice.166
Bacteria is able to induce the differentiation of immune cells. It was reported that two strains of P. distasonis PF-BaE5 and PF-BaE11 induced the conversion of DCs from naive CD4 T cells to regulate T cells, resulting in increased levels of IL-10 producing CD4+ FoxP3+ T cells and enhanced expression of the gene encoding Ebi3.22 Supplementation of P. distasonis augmented the infiltration of CD4T and CD8T cells into the tumor, significantly upregulated key immune response pathways, including the PI3K/Akt signaling pathway and PPAR signaling pathway, and potentiated the antitumor efficacy of α-PD-1 mAb.167 P. distasonis also has the same significant anti-inflammatory effect in vitro. The results showed in vitro experiments that P. distasonis CS15 and CS17 could stimulate peripheral blood mononuclear cells induced by LPS to increase the production of IL-1RA and IL-10.168 P. distasonis MRx0005 significantly decreased the IL-6 level of U373 cells induced by LPS.73 P. distasonis exerts its anti-inflammatory effect by inhibiting LPS-induced IL-8 release from HT-29 cells.169
In addition to the direct effect of P. distasonis, its derivatives have immunoregulatory effects. P. distasonis-derived BAs promoted M2 polarization of macrophages, which inhibited the differentiation of Th17 cells and reversed the elevation of inflammatory factors in arthritis mice.113 3-OxoLCA and isoLCA also inhibit the differentiation of Th17 cells in vitro through suppressing the expression of RORγt, while it had no effect on the differentiation of Treg cells.113 The mPd prevented the increase of several proinflammatory cytokines (IFN-γ, IL12, IL-17, and IL-6) induced by DSS, increased mPd-specific serum antibodies IgA and IgG, and stabilized the gut microbial ecology.20 In conclusion, P. distasonis induces the differentiation of immune cells and secrete antibodies, which has significant anti-inflammatory effects in vitro and in vivo (Figure 4).
5.2 Improves intestinal barrier
The microorganism living in the gut is the main force of intestinal homeostasis, and its change directly affects the intestinal barrier function. The effect may also be positive or negative. For example, Akkmensia muciniphila and Bacillus subtilis preserve the intestinal barrier, while Desulfovibrio and Salmonella Typhimurium destroy it. Intestinal inflammation altered intestinal barrier function. C-X-C chemokine receptor type 4 (CXCR4) expressed by intestinal plasma cells was considered to be an indicator of gut inflammation in humans.170 Metformin downregulated the upregulated CXCR4 gene expression in HFD-fed mice, reversed the nuclear factor kappa B (NF-κB) signaling pathway, and positively correlates Rel and Rela gene expression levels with abundances of P. distasonis.171 As mentioned earlier, P. distasonis is able to regulate immunity. It reduced the expression of proinflammatory factors in colitis mice, and restored colonic tight junction protein expression.22 In CRC, P. distasonis improved the intestinal epithelial barrier in colon of mice and decrease the level of LPS in plasma.129 Interestingly, the membrane proteins of P. distasonis also increase transepithelial electrical resistance and the ZO-1 protein in Caco-2 cell treated with LPS.129
The growth and metabolism of bacteria occur in the intestinal cavity, which has attracted many researchers to focus on the metabolites of P. distasonis. The results of animal experiments showed that P. distasonis F1-28 alleviated UC and reduced mucosal damage in DSS-induced mice.118 P. distasonis F1-28 produces acetic acid, propionic acid, and succinic acid in vitro, which have been reported to be beneficial for gut barrier integrity, and contribute another reason to understanding the mechanism of P. distasonis.118 Recent studies have shown that the action of P. distasonis is partially mediated by its active metabolites. Live P. distasonis and its production (UDCA, LCA and succinate) improve the Claudin-1 and Zo-1 in the ileum of obese mice.19 The researchers also found that P. distasonis treatment was effective in reversing the characteristics of T2D and alleviated the expression of Occludin, Claudin-1, and Zo-1 in SD rats.80 As mentioned earlier, P. distasonis produces indoleacrylic acid; the serum and fecal samples of experimental animals were detected by targeted liquid chromatography-tandem mass spectrometry, and the treatment of P. distasonis increased indoleacrylic acid. Indoleacrylic acid activates AhR in colon and promotes the secretion of Il22,80 which has been reported to maintain the intestinal barrier.172 P. distasonis and its product NA enhance the integrity of the intestinal barrier by activating G-protein-coupled receptor 109a in intestine, thereby improving IR, and reducing systemic inflammation in mice.82
It was found that P. distasonis DSM 108218 could reduce serum LPS, promote the expression of tight junction proteins E-cadherin, restore intestinal barrier function to suppress NASH, and inhibit hepatic proinflammatory cytokine and chemokine signaling. Additionally, P. distasonis is also able to metabolize inulin to produce pentadecanoic acid, which has a therapeutic effect on intestinal barrier. These discovery also indicated that P. distasonis was able to metabolize dietary fiber to connect its effects on diseases.148 In summary, P. distasonis and its metabolites are beneficial for intestinal barrier maintenance.
5.3 Interacts with BA receptors
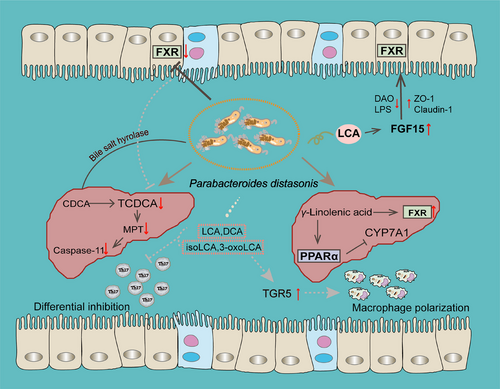
The liver synthesizes BAs from cholesterol and releases them into the intestine, where enzymes produced by microbiota convert primary BAs into secondary BAs, such as DCA and LCA. Disturbances in the BA profiles, especially changes of secondary BAs, are associated with various diseases. The modulatory effects of BAs on metabolic homeostasis primarily occur through their combination with various intracellular nuclear receptors, such as farnesoid X receptor (FXR) and cell surface G protein-coupled receptors 5 (TGR5).
In one study, P. distasonis was the most nodal species in the differential gut flora species-BA network, which may play a role in the acute aggravation of chronic liver failure by reestablishing intestinal homeostasis through the production of more secondary BAs.173 Chaihu-Shugan-San (CSS) was reported to increase circulating levels of hyocholic acid and 7-ketodeoxycholic acid in blood and alter the expression of hippocampal genes involved in BA transport (Bsep and Fxr) and neurotrophic signaling.174 Fecal transplanting experiments showed that the efficacy of CSS was associated with increased intestinal P. distasonis abundance.174 As mentioned earlier, P. distasonis has genes encoding the synthesis of multiple secondary BAs. It has been reported that treatment with P. distasonis was able to modify the BA profiles of mice. Increased LCA and UDCA reduce hyperlipidemia by activating the FXR pathway, and increase fibroblast growth factor 15 (Fgf15) in ileum and serum to restore gut barrier integrity.19 γ-Linolenic acid, a fatty acid metabolite of P. distasonis ATCC-8503, enhances lipid catabolism, promotes hepatic FXR, and inhibits the expression of Cyp7a1 in the liver through peroxisome proliferator-activated receptor alpha (Pparα) signaling pathway, which changes the BA synthesis pathway in NAFLD.99 P. distasonis increased the mRNA level of Oatp4 and Bsep, which may contribute to reverse liver BAs in mice with hepatic fibrosis. P. distasonis promoted the BSH activity in cecum and reduce the level of TCDCA, which was increased in hepatic fibrosis and induced pyroptosis to damage hepatocytes and HSCs. In addition, P. distasonis inhibited ileal Fxr and decreased the expression of Fxr and organic solute transporter belta protein, which reduce the reabsorption and promote the excretion of BAs.66
Recently, researchers have provided solid evidence that P. distasonis could generate LCA, DCA, isoLCA, and 3-oxoLCA.113 LCA and DCA are the known ligands of TGR5. Molecular docking and molecular dynamics simulations revealed that isoLCA and 3-oxoLCA are also the potential ligands for TGR5. It was observed that LCA, DCA, isoLCA, and 3-oxoLCA reversed the inhibitory effect of LPS on the TGR5 in macrophages and required TGR5 to mediate M2 macrophages polarization, which contribute to alleviating inflammatory arthritis.113 Overall, these reports highlight the potential role of P. distasonis in host health closely related to BA receptors (Figure 5).
5.4 Maintains glucose homeostasis
Glucose meets the energy needs of vital organs and maintains personal health. Various pathways regulate glucose metabolism, including glycogenesis, glycogenolysis, glycolysis, and gluconeogenesis (IGN). Interfering with one of these processes was able to affect the normal metabolism of glucose. P. distasonis is linked with the glucose modulatory effects by metformin.171 Two P. distasonis strains were isolated from human feces, which induce the secretion of the glucagon-like peptide 1 (GLP-1) in STC-1 cells and improve glucose homeostasis in obese mice by reducing plasmatic leptin and increasing the insulin receptor substrate 2.175 The regulation of P. distasonis on host metabolism is not only itself, but also closely related to its metabolites. A previous report also confirmed that P. distasonis activated intestinal IGN and reduced obesity and metabolic dysfunction. P. distasonis increases succinic acid to activate fructose-1, 6- bisphosphatase (FBPase) and promote IGN in jejunum.19 The administration of P. distasonis resulted in enhanced glucose and energy metabolism, decreased fasting blood glucose levels, improved glucose tolerance and insulin sensitivity, as well as increased thermogenesis to facilitate weight gain following a period of fasting.109 Live P. distasonis promotes the synthesis of UDCA and LCA to induce the secretion of GLP-1 and increases the level of uncoupling protein 1 (UCP1), which increase glucose homeostasis and energy consumption.109 In conclusion, P. distasonis has a regulatory effect on glucose metabolism.
5.5 Other potential mechanisms
The metabolic regulation of P. distasonis is mainly shown in two aspects. One is to regulate host metabolism, and the other is to regulate the secreted metabolites. Depletion of P. distasonis causes changes in urea cycle, l-citrulline biosynthesis, and creatinine degradation pathway, as well as B vitamins and lipoic acid synthesis in patients with hepatic fibrosis in Spain.142 P. distasonis is also closely related to tryptophan metabolism. Single bacterial colonization experiments have demonstrated that P. distasonis induced the decrease of indole-formaldehyde and the increase of indole-3-lactic acid, and this metabolic imbalance directly regulates depression-like behavior.79 Mice with psoriatic skin phenotype showed an intestinal microbiome imbalance, that is, the increase of Prevotella and decrease of P. distasonis. With the recovery of gut flora, host metabolism in mice was regulated, and the reduction of oleic acid and stearic acid reduces Th17 and monocyte-derived DCs infiltration in the skin lesion area in vivo, as well as reduced the secretion of IL-23 by avoiding the stimulation of DCs in vitro.176 A recent study was the first to provide positive evidence suggesting that P. distasonis has the potential to enhance propionic acid production. Specifically, propionic acid derived from P. distasonis activated MUP1 expression in the liver, improved ER stress, and significantly reduced macrophage infiltration in mice fed with alcohol.156 Spermine is one of the most prevalent and important polyamines, and has wide functions such as antioxidation, inhibition of lipid synthesis, anabolism, maintenance of intestinal homeostasis, and promotion of immune system maturation.177 P. distasonis ameliorated triptolide-induced testicular dysfunction and increased spermine that upregulate heat shock protein 70s (Hsp70s) and improve oxidative stress.95 The brain–gut axis has received increasing attention. The microbe released signals that triggered inflammatory mediators to act directly on the nervous system, producing pain signals that traveled to the periphery.178 P. distasonis F1-2 relieved visceral pain associated with leaky gut by reducing nociceptor response to capsaicin, inflammatory soup, and bradykinin stimulation.135 These works suggest that P. distasonis has an independent mechanism in different diseases. Much more research is needed to try to more fully understand the complex relationship between P. distasonis and human health.
In conclusion, these action mechanisms provide the support for the potential therapeutic value of P. distasonis. A further investigation about these mechanisms will clarify the effective metabolites of P. distasonis and administration formulation to achieve the enhanced therapeutic efficacy and minimized side effects.
6 MANIPULATING P. DISTASONIS TO PROMOTE BODY HEALTH
The crosstalk between gut microbiota and natural components is a complicated but captivating field and attracts growing attention. On the one hand, as an integral part of the human body, gut microbiota plays the crucial roles in bioavailability and bioactivity of natural components and their biotransformation process. On the other hand, natural components were able to modulate gut microbiota, affecting the composition and its metabolites, signal transduction, or genetic expression. Numerous studies provide evidence of the linkage between pharmacological efficacy of natural components and the structural shift and metabolic activity of gut microbiota, but only few investigate certain species. Natural components especially prebiotics from food sources, as well as various traditional Chinese medicines (TCMs) and some natural products are well known for improving host metabolism and immune system via nourishing gut microbes.180, 181 Here, we summarize the literatures on the role of natural components regulating the specific gut bacteria P. distasonis to provide scientific support for potential novel prevention or therapeutic strategies for gastrointestinal diseases.
6.1 Dietary fibers
Dietary fibers mainly include plant carbohydrates that are not recovered by alcohol precipitation, nonstarch polysaccharides, lignin, chitin, pectin, and resistant starch (RS)182 and are the most widely studied plant-derived natural components that modulating gut microbiota.
6.1.1 Plant carbohydrates
Certain natural fibers such as inulin, oligosaccharides, and fructans act as prebiotics. Chicory inulin supplement is currently the most widely used and studied prebiotic. In a study, the prebiotic activity of inulin was tested in an in vitro model of human colon. For the first time, it was reported mainly used by the Parabacteroides genus, selectively leading to a significant increase of P. distasonis.183 Recently, it was reported inulin protects NASH development by enriching potential beneficial bacteria P. distasonis, which was depleted in choline-deficient high-fat diet-induced mice NASH model. The underlying mechanism is the inulin-derived P. distasonis and its metabolite pentadecanoic acid alleviated NASH by improving gut barrier and suppressing inflammation.148
6.1.2 Nonstarch polysaccharides
Non-starch polysaccharides (NSPs) are one kind of dietary fiber commonly found in many plants and include a variety of different types of polysaccharides, such as cellulose, pectins, gums, and glucans. Numerous studies have shown many plant polysaccharides are able to repair colon injury and improve gastrointestinal disease by modifying gut bacteria. The fermentation of Chlorella pyrenoidosa polysaccharides (CPP) altered the structure of fecal microbiota in vitro, remarkably decreasing the harmful bacteria and increasing the probiotic bacteria. CPP intervention increased the abundance of P. distasonis and SCFAs production, such as acetic acid, propionic acid, and n-butyric acid. Thus, it may contribute to promote gut health and prevent diseases.184 The effect of edible brown algae, containing fermentable dietary fiber polysaccharides alginate and laminaran on rat cecal microbiota was investigated. The result suggested that these two dietary fibers were able to change the microbial composition of cecum in rat and affect the gut microenvironment. Only laminaran increased the abundance of P. distasonis, and this difference may be related to the ability of P. distasonis to utilize polysaccharides.185 A study revealed gut microbiota communities from different donors utilized the sulfated polysaccharides of sea cucumber Stichopus japonicas (SCSPsj) to different degrees in vitro fermentation, demonstrating that SCSPsj benefits the host health by effectively modulating gut microbiota composition. Those gut microbiota communities with P. distasonis enriched showed the strongest capability to utilize SCSPsj.186 SCSPsj is able to be used by P. distasonis to produce small molecules such as organic acids and lipids. In addition, those molecules were significantly consumed by bacteroidales members Bacteroidales stercoris, Bacteroidales vulgatus, and Parabacteroides johnsonii, consequently, promoted the growth of these species through cross-feeding.187 Rice bran has been shown the suppressive effect for colitis and colon cancer in multiple animal models via modifying human gut microbiota. Mice transplanted with rice bran-modified microbiota displayed improved CRC and distinctly depleted P. distasonis is correlated with increased tumor burden.128
6.1.3 Resistant dextrins and resistant starch
Resistant dextrins (RD) and resistant starch (RS) are not digested by human enzymes in the small intestine but are fermented in the colon by intestinal bacteria, contributing to produce SCFAs, which have health benefits. Diet supplement with RD NUTRIOSE, a soluble fiber, did not alter the overall composition of human gut microbiota but dramatically increased the abundance of bacterium P. distasonis. The response to RD depends on the gene susCD, a starch-binding membrane protein complex, carried in P. distasonis strains.188 Interestingly, the structures of RD and RS play an important role in microbiota regulating. For example, a study compared different effects of two RS, high-amylose maize starch (HAMS) and butyrylated HAMS (HAMSB), on fecal microbiota composition of rats treated with carcinogen AOM. HAMS was found only increased concentration of propionate and developed R. bromii-like bacteria in rats, whereas HAMSB, a chemically modified RS, increased both the propionate and butyrate levels, and was related to the appearance of two nonbutyrate-producing bacteria, P. distasonis and Lactobacillus gasseri. Additionally, P. distasonis-related phenotypes were only detected in butyrylated RS group, suggesting that these bacteria may be adaptable and able to deacylate and utilize the starch backbone for growth.189 According to a clinical trial, ingestion of HAMSB has the potential to improve human gut health probably because it effectively released esterified butyrate to the gastrointestinal tract, and therefore caused significant change in gut microbial diversity. The release was likely facilitated primarily by P. distasonis, of which the increased abundance is positively correlated to HAMSB but not HAMS.190
6.2 Natural products
Many types of natural compounds such as saponins, flavonoids, alkaloids, and polysaccharides have been proven to possess beneficial effects for intestinal health. Among them, dietary polyphenols have attracted the most attention. However, their extremely low bioavailability limited the mechanistic understanding of their function. Recent research revealed their effects were closely related to the gut microbiota and therefore drove the breakthrough strategy of targeting gut microbiota for gastrointestinal disease by natural products. Polyphenols exert various biological activities such as antioxidation, anti-inflammation, and antiobesity by adjusting gut microbiota composition.191 Liu et al. performed a comparative study of green tea phenolics (GTP) and oxidized tea phenolics (OTP) on antiobesity and their gut microbiota modulating effects. The supplementation of GTP or OTP both restored P. distasonis reduction caused by HFD, and GTP was demonstrated better effect.192 Compared the fecal samples of patients with or without precancerous lesions, the anaerobic bacteroides P. distasonis and equol were only detected in patients without precancerous lesions. The presence of P. distasonis in the colon could contribute to the metabolism of soy.193 The pomegranate fruit pulp polyphenols (PFP) exhibited significant antiobesity effect in mice fed a HFD. Remarkably, the increased abundance of P. distasonis by PFP supplement was negatively correlated with clinical indicators of obesity such as body weights, ALT, TC.194 Propolis is another natural product of which the main bioactive substances are polyphenols such as flavonoids. Ethanol extract of propolis (EEP) significantly increased the abundance of P. distasonis in HFD-fed mice to a much higher level than that in Chow mice, which was correlated with the improvement of inflammation, IR, obesity, glucose tolerance, and lipid profile.191
The potential protecting effect of Scytosiphon lomentaria fucoidan (SLF) on ALD was assessed in a study. It was demonstrated that SLF reduced the levels of ALT, AST, TG, and TC in ALD mice and its benefits was partly attributed to P. distasonis regulating gut–liver axis. SLF increased alcohol intake and reduced levels of Bacteroides and Parabacteroides, particularly P. distasonis. P. distasonis administration alleviated liver inflammation by suppressing the NF-кB/MAPK pathway and mitigated liver oxidative stress by activating Nrf2 pathway. The underlying mechanism is closely associated with modulation of gut microbe composition and improved profiles of microbiota metabolites, especially amino acid and BA metabolism.140 Lycium barbarum fruit is a widely used food supplement that is rich in plant polysaccharides. Lycium barbarum glycopeptide (LbGP) is a glycopeptide isolated and purified from crude lycium barbarum polysaccharide, which significantly alleviated acute colitis by inhibiting the colonization of harmful bacteria P. distasonis. It dominated the intestinal bacterial community during the acute phase of colitis.195 Oregano essential oils (OEO) improved rumen digestive ability of cattle. P. distasonis was identified among the top five significantly increased species after OEO administration. OEO diets changed rumen content metabolites in beef cattle, and a positive correlation was found between P. distasonis and β-glucosidase, cellulase, propionate, and valerate.196
6.3 TCMs formulas and active ingredients of TCMs
TCMs such as Si Miao formula, Shenling Baizhu Powder, and Gegen Qinlian Decoction has been used for centuries to treat various gastrointestinal disorders. These TCM formulas and active ingredients of them have been demonstrated exerted therapeutic effects by directly or indirectly interacting with gut microbiota.
The protective effects of P. distasonis on metabolic syndrome, such as obesity, diabetes mellitus and NAFLD, have been long and widely investigated. It was reported that Rhizoma Coptidis (RC) alkaloids, including berberine, coptisine, palmatine, and epiberberine, obviously suppressed the richness of P. distasonis and alleviated hyperlipidemia. Respectively, the inhibition rates of berberine, coptisine, palmatine, and the total Rhizoma Coptidis alkaloids (TRCA) are 33.3, 47.6, 47.3, and 61.9%.146 In another study, RC classically paired with Dolomiaea souliei (VR) was used for gastrointestinal diseases treatment. RC alkaloids gavage decreased gut microbiota diversity, whereas combination of VR and RC reversed the perturbation. However, both RC and VR alone promoted the proportion of P. distasonis.197
PCP demonstrated the potential prebiotic and therapeutic efficacy in the treatment of antibiotic associated diarrhea in mice via regulating intestinal mucosal barrier and the homeostasis of gut microbiota, specifically restoring seven characteristic species of intestinal flora, including P. distasonis.133
The commercial herb product Panax notoginseng saponins (PNS) includes ginsenosides Rb1, Rd, Re, Rf, Rg1, and notoginsenoside R1 directly shifted the gut microbial community in HFD-fed obesity mice prior to body weight change, particularly increased the level of P. distasonis within a short time. The crosstalk of microbiota and fat via leptin-AMPK/STAT3 signaling pathway plays an essential role on the antiobesity effects of PNS.198 Celastrol from the roots of Tripterygium Wilfordii was reported possessing the antiobesity effect and ameliorated hepatic fibrosis via inhibition of intestinal Fxr signaling and decreased TCDCA in liver by promoting the growth of P. distasonis.66
Natural components have been long widely used in human daily life and clinical practice, and have been proven to possess benefits for maintaining intestinal homeostasis and symbiosis. Recent research revealed that the specific strain P. distasonis was involved in the regulatory effects of many natural components on host health (Table 2). Natural components addressed gut dysbiosis by restored the abundance of P. distasonis, which contributed to the diversity of microbial composition. Also, it produced metabolites with different physiological functions, such as SCFAs, amino acids, BAs, and lipids. Meanwhile, P. distasonis enriched gut microbiota communities could better utilize and transform natural components, making them more easily absorbed and harnessed by the host. This interaction is inseparable for host health, but molecular mechanism studies are few at present, and further exploration is needed.
| Natural components type | Name | Gut bacterial alteration | Potential effects and possible mechanism | References |
|---|---|---|---|---|
| Dietary fiber | Inulin | P. distasonis↑ | P. distasonis and its metabolite pentadecanoic acid alleviated NASH by restoring gut barrier and inhibiting proinflammatory signaling. | 148 |
| Natural products | SLF | P. distasonis ↑ | P. distasonis alleviated liver inflammation by suppressing the NF-кB/MAPK pathway and mitigated liver oxidative stress by activating Nrf2 pathway in ALD mice. | 140 |
| EEP | P. distasonis ↑ | P. distasonis was positively correlated with improvement of proinflammatory mediators, insulin resistance, obesity, glucose tolerance, and lipid profile. | 191 | |
| PFP | P. distasonis ↑ | P. distasonis was negatively correlated with indicators of obesity such as body weights, glucose, ALT, AST, TG, TC, LDL-C, NEFA, and insulin. | 194 | |
| LbGP | P. distasonis ↓ | LbGP inhibited the colonization of P. distasonis which dominated in acute colitis. | 195 | |
| TCM formulas and active ingredients | Celastrol | P. distasonis ↑ | P. distasonis possessed the antiobesity effect and ameliorated hepatic fibrosis via inhibiting intestinal Fxr signaling and decreased TCDCA in liver. | 66 |
| PNS | P. distasonis ↓ | The crosstalk of P. distasonis and fat was through leptin–AMPK/STAT3 signaling pathway. | 198 |
7 CURRENT RESEARCH IN CLINICAL TRIALS
Regulating dysbiotic intestinal microbiota is a novel strategy for treating diseases, especially those that are difficult to be cured by conventional therapies.199, 200 Fecal microbiota transplantation (FMT), prebiotics, probiotics, synbiotics, and dietary intervention possess the potential to become measures for treating or preventing diseases.201, 202 In addition, it may also mitigate adverse effects in patients, improve their quality of life, and support disease treatment.203 At present, these methods have been exhibited therapeutic effects in the treatment of Clostridium difficile infection,204, 205 UC,206 NAFLD,207 and IBS.208
In current clinical trials, prebiotics and dietary intervention alter P. distasonis and exert modulatory effect. The clinical trials related to P. distasonis are summarized in Table 3. Continuous supplementation of HAMSB at a dose of 40 g/day for 28 days resulted in the most significant increase of P. distasonis in feces, as well as elevated levels of IL-10 and TNF-α in plasma samples, which may contribute to the ability to resist infections.209 A high intake of red meat enriched diet is a risk factor for CRC due to its stimulation of a toxic substance O(6)-methyl-2-deoxyguanosine (O(6)MeG). In a clinical trial involving 23 healthy volunteers, the HAMSB diet significantly increased the abundance of P. distasonis and the level of stool SCFAs, thus reducing O(6)MeG adducts in rectum.210 Inulin increased the abundance of P. distasonis and could be transformed by P. distasonis in animal experiments.148 Consistent with the previous results, supplementation with inulin resulted in an increase in P. distasonis in aged individuals. However, the content of SCFAs in feces did not show significant changes in this trial.211
| Method | Numbers of subjects | Main effects | References |
|---|---|---|---|
| HAMSB |
Male (n = 23) Female (n = 18) |
HAMSB increased P. distasonis and F. prausnitzii in feces. HAMSB elevated the levels of IL-10 and TNF-α in plasma. |
209 |
| HAMSB | 23 healthy volunteers |
HAMSB increased P. distasonis in feces. HAMSB diet resisted the increase of O(6)MeG adducts caused by red meat. |
210 |
| Inulin | 24 healthy volunteers | Inulin increased the abundance of P. distasonis. | 211 |
| Intermittent fasting |
Male (n = 33) Female (n = 39) |
Intermittent fasting decrease weight, BMI, and atherosclerosis index. P. distasonis and B. thetaiotaomicron increased in feces. |
212 |
| Intermittent energy restriction | 25 obese volunteers |
Intermittent energy restriction reduced weight and TC, ALT, AST, high-density lipoprotein, and low-density lipoprotein in serum. Intermittent energy restriction reduced Escherichia coli and increased P. distasonis. |
213 |
Obesity is a risk factor for fatty liver, hepatic fibrosis, and IBD. Intermittent fasting is an effective strategy for weight loss. After fasting, both normal and obese participants were able to reduce weight and body mass index (BMI), accompanied by a significant increase in P. distasonis.212 In another clinical trial, intermittent energy restriction also increased P. distasonis and reduced obesity-related parameters.213 The relationship and ameliorative effects of P. distasonis on obesity have been demonstrated in animal studies.19, 175 Alterations in gut microbiota caused by dietary interventions may contribute to these improvements.
Previous animal studies have shown the role of P. distasonis in gastrointestinal and hepatic disease. The effects of dietary intervention and prebiotics on P. distasonis have also been well validated in clinical trials. However, the direct role of P. distasonis in clinical disease has not been verified yet, more research is required to determine the safety and efficacy of P. distasonis.
8 CONCLUSIONS AND PERSPECTIVES
P. distasonis is one of the popularly studied and potential next-generation probiotics candidates due to its properties of anti-inflammation, antiobesity, and gut barrier protection,21 which has shown protective effects against various gastrointestinal and hepatic diseases. It should be noted that the mechanisms of P. distasonis and its impact on diseases are not well understood, and several aspects require further research. P. distasonis exhibits dual effects in some diseases. It displays both protective and damaging effects in animal models of IBD.22, 119 The variability in outcomes may be caused by differences in strains, modeling methods, and experiments conditions. The role of P. distasonis requires further validation in multiple models to draw more definitive conclusions. It is also important to consider the variations among strains from different sources. For instance, P. distasonis isolated from healthy human feces demonstrated different abilities to exert anti-inflammatory effects and protect against intestinal barrier.22 Additionally, the invasion and antibiotic resistance are also related to strain differences. To ensure safe use of P. distasonis, selecting effective and safe strains for trials or employing genetic manipulation is necessary.214
The impact of gut microbiota on active metabolites is currently a widely studied mechanism of action. P. distasonis exerts its effects by influencing the production of active metabolites, such as produce indoleacrylic acid that is able to protect intestinal barrier.80 In other experiments, components of P. distasonis such as its membranous fraction and outer membrane vesicles regulate immune system.20, 130 These studies indicate that the role of P. distasonis is linked to its own structure, metabolites, and secreted products. The proteins and metabolites of bacteria as potential postbiotics could avoid the side effects and uncertainties of bacterial transplantation.215 The unique metabolic enzymes of bacteria also demonstrate the therapeutic value in degrading harmful substances to the host and protecting against diseases.216 Therefore, utilizing isotope labeling, genetic engineering, high-throughput sequencing, and other technologies are able to enhance our understanding of the regulatory mechanism of P. distasonis and facilitate the development of reliable alternatives.
Natural components with thousands of years enriched clinical experience is a rich source of prebiotics and were proven to possess advantages and effectiveness by research evidence and modern clinical practice. Therefore, in addition to FMT and live biotherapeutic products, targeting P. distasonis by natural components provides a promising microbiome-based intervention strategy or therapeutic option for intestinal and liver disease. To standardize treatment protocols and robust therapeutic effects, better understanding of the mechanism how natural components interact with P. distasonis on host health is essential. More scientific attention and research efforts should be paid to these future perspectives. First, natural products, whether extracts or single phytochemicals, regulate gut microbiota by targeting multiple strains, but may not specifically P. distasonis, implying potential cross interactions between the microbial communities that require further mechanistic investigation. Second, the different structural characteristics of natural compounds also lead to variations in impacting microbial strains.217 It may be possible to selectively enrich or downregulate certain species such as P. distasonis for better disease intervention. In addition, despite prospects of natural components, some concerns are innegligible. For example, algal polysaccharides including carrageenan, agar, fucoidan, and ulvan are reported to increase H2S, which could affect colonic mitochondrial function. Meanwhile, their thickening/emulsifying properties are also emerged concerns.218 Therefore, either using natural components as prebiotic or P. distasonis to treat gastrointestinal and hepatic disease requires more clinical trials to confirm.
P. distasonis has shown potential in the protection of gastrointestinal and hepatic disease. The complexity of the intestinal environment and the diversity of bacterial functions present challenges for its research. Thoroughly studying its mechanism of action and regulatory methods will contribute to a better understanding of how P. distasonis is able to be effectively utilized for health benefits while ensuring its safety in clinical application.
AUTHOR CONTRIBUTIONS
Jingyi Duan, Qinmei Li, and Yan Cheng wrote and revised the manuscript. Hongning Liu and Weifeng Zhu supervised the manuscript and edit the writing. Fei Li conceived the framework of the article, edited the writing, and provided the funding. All authors read and approved the manuscript.
ACKNOWLEDGMENTS
This study was supported by National Natural Science Foundation of China (82473999), the National Key Research and Development Program of China (2021YFF0702003), and 1.3.5 Project for Disciplines of Excellence from West China Hospital (ZYYC21003). All figures in manuscript are created in Adobe Illustrator.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




