CD44 and its implication in neoplastic diseases
Abstract
CD44, a nonkinase single span transmembrane glycoprotein, is a major cell surface receptor for many other extracellular matrix components as well as classic markers of cancer stem cells and immune cells. Through alternative splicing of CD44 gene, CD44 is divided into two isoforms, the standard isoform of CD44 (CD44s) and the variant isoform of CD44 (CD44v). Different isoforms of CD44 participate in regulating various signaling pathways, modulating cancer proliferation, invasion, metastasis, and drug resistance, with its aberrant expression and dysregulation contributing to tumor initiation and progression. However, CD44s and CD44v play overlapping or contradictory roles in tumor initiation and progression, which is not fully understood. Herein, we discuss the present understanding of the functional and structural roles of CD44 in the pathogenic mechanism of multiple cancers. The regulation functions of CD44 in cancers-associated signaling pathways is summarized. Moreover, we provide an overview of the anticancer therapeutic strategies that targeting CD44 and preclinical and clinical trials evaluating the pharmacokinetics, efficacy, and drug-related toxicity about CD44-targeted therapies. This review provides up-to-date information about the roles of CD44 in neoplastic diseases, which may open new perspectives in the field of cancer treatment through targeting CD44.
1 INTRODUCTION
CD44, a nonkinase transmembrane glycoprotein,1 is expressed in various human cell types, such as immune cells, differentiated cells, cancer cells, and so on.2, 3 CD44 has numerous ligands, such as hyaluronic acid (HA),4 osteopontin (OPN),5 and serglycin.6 The extracellular region of CD44 binding to ligands has been found to involve various of signaling pathways associated with physiological and pathological processes,7, 8 in particular, pathways related to carcinogenesis and tumor progression including proliferation and migration of cells, drug resistance, as well as epithelial–mesenchymal transition (EMT).9-11
Alternative splicing of the CD44 gene produces two splice isoforms, CD44s and CD44v. Notably, CD44s and CD44v play an overlapping or distinct role in cancers. In most cases, CD44s is associated with tumor growth12 and progression,13 while CD44v, such as CD44v3 and CD44v6, is associated with invasiveness14 and chemoresistance.15
Accumulating evidence indicates that CD44 can regulate numerous cancer-associated signaling pathways to influence cancer cell motility, EMT, and stemness.16-18 In recent years, a number of novel functions of CD44 and its associations with cancers have been revealed. Recent advances in understanding the complex interactions of CD44 with ligands have led to the development of CD44 not only as an important cancer stem cells (CSCs) marker but also as a potential cancer therapeutic target.3, 19, 20 In neoplastic diseases therapeutic areas, it is very crucial to clarify the mechanism of CD44 in the signaling pathways, thereby delaying, treating, and preventing the development of tumors through targeting CD44. However, there are relatively few clinical studies about CD44-targeted therapies in cancer treatment. Hence, an updated and comprehensive understanding of CD44 is very crucial, which contributes to the research and development of innovative CD44-targeted therapeutic strategies.
In this review, we discuss the structure and ligands of CD44 briefly. And we focus on the biological functions of CD44 in different cancers and cancer-related signaling pathways regulated by CD44 and provide critical assessment of therapeutic strategies and clinical studies through targeting CD44.
2 THE STRUCTURE OF CD44
CD44 is a nonkinase cell surface transmembrane proteoglycan that includes an ectodomain, a stem region, composed of standard region and variable region, a transmembrane region and an intracellular tail.21 In human, the gene encoding CD44 protein is on chromosome 11, while in mice, the gene is on chromosome 2, which comprises 19 exons and 20 exons.22 Comparing with mice, CD44v1, includes homolog exon 6, is not expressed in humans.23 For the reason that the variable exon 6 of the human gene has a stop codon that is normally not expressed in human CD44,24 CD44s is the most common and smallest CD44 isoform, which includes the constant exons 1−5 and 16−20. Based on the structure of CD44s, CD44v consists of variable exons 6−15 located in the exon 1−5 and 16−20 regions by alternate splicing or insertion.25 CD44v1–v10 correspond to alternative splicing or insertion of variable exons 6–15, respectively,26 which have different functions in neoplastic diseases.27 Moreover, a CD44v isoform could have more than one variable exon. For example, CD44v8–10 is based on CD44s structure with the insertion of v8, v9, and v10, three variable exons.28 The correspondence between structure and gene arrangement of CD44 in mice is shown in Figure 1.
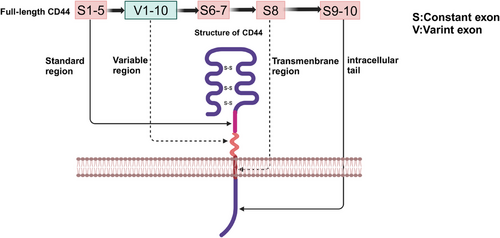
3 CD44 IN NEOPLASTIC DISEASES
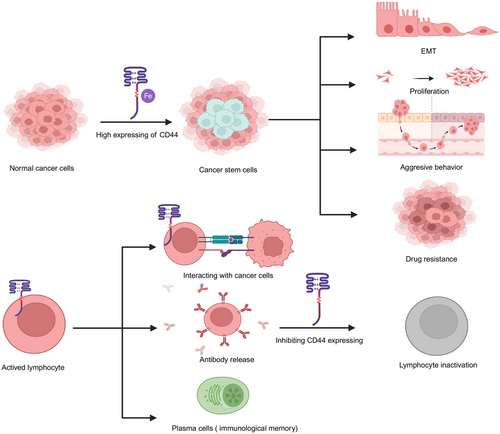
CD44 has been reported as a classic marker of CSCs, which is mainly associated with of iron endocytosis-mediated cellular plasticity.29 A recent study has found that in cancer cell lines, through the endocytosis of iron-bound HA regulated by CD44, the EMT was enhanced.30 The effects of the expressing of CD44 on immune cells and cancer cells in neoplastic diseases are summarized in Figure 2. Moreover, previous studies have found that the expression of CD44 isoforms in tumors plays crucial roles.31 Some researchers have demonstrated that part of CD44v is associated with aggressive tumor progression and drug resistance,32 whereas CD44s is involved in tumorigenesis and tumor growth.13 However, recent studies have shown the inverse result. For instance, CD44s has been shown to promote migratory, invasive, and lung metastatic potential in breast cancer.33 In 3D cultures, CD44 switching from the standard isoform to the variant 6 isoform was found to be associated with EMT in gastric cells.34 The different or overlapping functions of CD44s and CD44v may depend on the difference in tumor types. In addition, different induction conditions, accompanied by alternative splicing of CD44 isoforms, may lead to different expression of features in tumor cells. The correlation between different cancers and CD44 are summarized in Table 1. Moreover, CD44 could also play its biological roles by interacting with ligands and messenger molecules, such as HA,35 OPN,36 chondroitin sulfate (CS)37 and growth factors38 mainly found in the tumor microenvironment. In this part, we have not only made a discussion in terms of different kinds of cancers but also discussed the effects of CD44 interacting with its different ligands on cancers.
| Cancer type | Role of CD44 in cancer progress | References |
|---|---|---|
| Brain cancer | Poor prognosis, tumor progression, aggressive behavior | 39-41 |
| Head and neck cancer | Stemness, tumor progression | 48, 49 |
| Breast cancer | Aggressive behavior, tumor progression, stemness, poor prognosis | 56-60 |
| Kidney cancer | Poor prognosis, aggressive behavior, tumor growth | 65-67 |
| Liver cancer | Cancer initiation, poor prognosis, stemness | 72-74 |
| Pancreatic cancer | Tumorigenicity, clinicopathological features, aggressive behavior | 80-82 |
| Gallbladder cancer | Stemness, aggressive behavior, tumor progression, poor prognosis | 91-94 |
| Esophageal cancer | Tumor progression, stemness, poor prognosis, aggressive behavior | 97-99, 101, 102 |
| Prostate cancer | Tumor progression, stemness, aggressive behavior | 104-106, 109 |
| Gastrointestinal cancer | Tumor progression, stemness, aggressive behavior, CSCs self-renewal characteristic tumorigenesis | 114-117, 119 |
| Melanoma | Stemness, tumorigenesis, aggressive behavior | 122, 123, 125 |
| Squamous cell carcinoma | Aggressive behavior, stemness, tumorigenesis, tumor progression | 128-130 |
| Sarcoma | Tumor progression, stemness, aggressive behavior, poor prognosis | 133-135, 138, 139 |
3.1 Brain cancer
It has been shown that overexpression of CD44 is linked to poor prognosis,39 tumor progression,40 and aggressive behavior41 of tumors. For instance, CD44 is a possible CSCs marker in meningioma; the high expression of CD44 was associated with a shorter progression-free survival.42 Furthermore, in meningioma, the level of CD44 expression is correlated with the grade of the tumor and its invasiveness.43 Moreover, inhibiting CD44 dimerization via verbascoside has been reported to suppress stem cell-like cell properties and tumor cell growth in glioblastoma.44 Recent studies have found that tumor cells with high expression of CD44 is associated with brain metastases. It has been revealed that in lung, melanoma, and breast cancer patients, the circulating tumor cells expressing CD44 was a prognostic marker for brain metastases.45 Moreover, in lung adenocarcinoma, GPR124-enhanced trans-endothelial migration mediated brain metastases caused by lung CSCs with high expression of CD44.46 Furthermore, in breast cancer brain metastases patients, a retrospective transversal study revealed that CD44 was associated with worse overall survival.47 In general, CD44 is a promising marker associated with brain metastases in cancer patients, which provides us with the new insight into stratification of patients and therapy clinically.
3.2 Head and neck cancer
It is found that CD44 seems to be a classic CSCs marker,48 which is also associated with tumorigenesis in head and neck cancer.49 In head and neck squamous cell carcinoma (HNSCC) CSCs mouse models, targeting CD44 was shown to inhibit PI3K–4EBP1–SOX2 signaling and tumor growth and decrease the number of CSCs.50 Moreover, it is also suggested that the switch from CD44s to CD44v8–10 was associated with tumorigenic phenotypes.52 In addition, CD44+ cells were reported to stimulate tumor angiogenesis in HNSCC.53 In the invasion zone of HNSCC, Odenthal et al.54 found that CD44v6 could express constitutively. This suggests that targeting CD44v6 could be used to trustworthy near-infrared detection. Furthermore, Choi et al.55 has found that the interaction of COL1A1 and CD44 between fibroblasts and malignant cells was associated with HNSCC progression. Therefore, CD44 could be a promising biomarker for clinical diagnosis, and targeting CD44 could be a treatment strategy for drug resistance and tumor metastasis.
3.3 Breast cancer
In breast cancer, CD44 plays key roles in aggressive tumor behavior,56 tumor progression,57 CSCs trait induction,58, 59 and prognosis.60 Rokana et al.51 found that aggregated tumor cells with highly expressed CD44 could promote tumorigenesis and polyclonal metastasis. Moreover, depletion of CD44 has been found to effectively prevent the aggregation of tumor cell and decrease the levels of PAK2.51 Interestingly, CD44s was reported to inhibit breast cancer stemness, while the cleaved product of CD44 was reported to contribute to breast cancer stemness.61 However, bioinformatics analysis of breast cancer patients showed the reverse result. Notably, multiple studies have reported inconsistent results. For instance, CD44s being positively associated with CSCs gene signatures, while CD44v exhibited an inverse association.62 Compelling evidence further suggests that the splicing switch of CD44 may play inverse roles in breast cancer. For instance, Yang et al.63 found that enhanced transformation of CD44high to CD44low cancer cells induced migration and invasion behavior. Additionally, CD44 isoform switching from CD44s to CD44v resulted in an increase in the stemness of triple-negative breast cancer.64 Targeting different CD44 isoforms or inducing CD44 alternative splicing may be a favorable treatment strategy for breast cancer. The specific effects of CD44v and CD44s need to be further studied.
3.4 Kidney cancer
It is well documented that CD44 could serve as biomarkers that reflect poor prognosis65 and cancer risk in kidney cancer.66, 67 In transformed cells within multilayered epithelia, CD44 and collagen XVII were reported to play a key role in the clonal expansion.68 Moreover, the ferroptosis-related gene CHAC1 was shown to contribute to poor prognosis in kidney renal clear cell carcinoma associated with the expression of the checkpoint gene CD44.69 Notably, it was reported that silencing CD44, PLOD1, and PLOD2 genes could inhibit the proliferative and invasive potential of renal cancer cells, which suggested that these genes may serve as renal cell carcinoma oncogenes.70 Interestingly, unlike the role of CD44 in other cancer, it was found to display the opposite effect. In renal carcinoma, CD44− cancer cells display stem-like properties and show a higher level of invasiveness.71 All the above results show that targeting CD44 or inhabiting CD44 expression could be a promising therapeutic strategy to suppress kidney progression.
3.5 Liver cancer
CD44 is clearly associated with liver cancer initiation,72 poor prognosis,73 and cancer cells stemness.74 CD44 is expressed in carcinogen-exposed hepatocytes in a STAT3-dependent manner.75 Accordingly, CD44v6 was also found to be a promising biomarker. In patients with grade 1 intrahepatic carcinomas and grade 1 hepatocellular carcinomas (HCCs), TIPRL/LC3/CD133/CD44 also play a key role in prognosis.76 Furthermore, compelling evidence suggests that CD44 is related to stemness in liver cancer. CD44-positive HCC patient-derived organoids were shown to be obviously resistant to sorafenib via Hedgehog signaling.77 Besides, in CD24+/CD44+ cells, knocking down the signature gene CTSE was found to significantly inhibit the self-renewal potential of HCC cells.78 CD44 surface markers were found in cancer cells with stemness properties, which indicate that, in HCC, targeting CD44 expressed in tumorigenic cells through JAK/STAT pathway is a promising therapeutic strategy79
3.6 Pancreatic cancer
In pancreatic cancer, CD44 is involved in tumorigenicity,80 clinicopathological features,81 and invasiveness.82 In pancreatic ductal adenocarcinoma (PDAC) patients with liver metastasis or poor prognosis, the expression of CD44v6 and complement C1q binding protein was higher.83 Moreover, CD44+ PDAC cells were reduced with nimbolide treatment.84 A meta-analysis showed that CD44 overexpression contribute to a poor 5-year overall survival rate and lymph node invasion.85 On top of that, CD44+ stem cells from the Panc-1 cell line have been found to be involved in multiresistance and metastasis.86 In addition, more recent research has demonstrated that the interaction of HA and CD44 could be used for drug delivery in pancreatic cancer. HA-based carriers have been shown to target tumors via interaction with CD44 in pancreatic cell lines.87 HA-based nanomicelles loaded with 3,4-difluorobenzylidene curcumin could also kill CD44+ stem-like pancreatic cancer cells.88 Additionally, HA-conjugated polyamidoamine dendrimers were revealed to deliver 3,4-difluorobenzylidene curcumin to pancreatic cancer cells overexpressed CD44.89 Furthermore, Kang et al.90 reported a nanoparticle loaded with anticancer drug consists of HA, which could target CD44 in pancreatic cancer cells to eliminating tumor-resident intracellular bacteria. Hence, targeting CD44 drug delivery systems in cancer cells provide an avenue for pancreatic cancer treatment. Moreover, decreasing the expression of CD44 in pancreatic cancer is also a promising therapeutic strategy.
3.7 Gallbladder cancer
There are few studies on CD44 in gallbladder cancer (GBC), which mainly focus on the different functions of CD44 isoforms and the stemness of cancer cells expressing CD44.91, 92 CD44v9 and CD44s cells were found to play key parts in the progression and metastasis of GBC respectively, and the isoform switch triggered EMT.93 Interestingly, a research suggested that CD44v8–10 expression was closely associated with perineural invasion, venous invasion, and lymph node metastasis, and in the clinic, the patients with CD44v8–10+ tumors showed poor prognosis.94 In addition, the high expression of CD44 as a stem cell marker in sphere clones of the human GBC cell line GBC-SD was further explored.95 Interestingly, in general, the stemness of cancer cells is positively correlated with drug resistance, but the opposite effect is shown in GBC.96 All the studies indicate that the characteristics of CD44 in GBC provide some new thought in diagnosis and treatment of GBC.
3.8 Esophageal cancer
High expression of CD44 plays roles in tumor progression and stemness in esophageal squamous cell carcinoma (ESCC).97-99 CD44 is a novel stem cell marker in ESCC that has been reported to be eliminated by inhibiting the canonical NOTCH pathway.100 Moreover, CD44v9 was shown to be strongly associated with EMT and poor prognosis in patients with ESCC.101 Furthermore, microRNA (miR)-34a suppressed invasion and metastasis in ESCC by regulating CD44.102 In addition, the latest research has found that the alternative splicing of CD44 isoform from CD44s to CD44v8–10 is associated with ESCC metastasis and poor prognosis in clinic.103 The CD44v isoforms has similar functions in ESCC, which provide useful insights for the development of ESCC treatment and prognostic biomarkers.
3.9 Prostate cancer
CD44 is involved in prostate cancer, and different CD44 isoforms play different roles in tumor progression and stemness.104-106 A recent study showed that TGF-β1-mediated alternative splicing could switch CD44v to CD44s, which enhanced EMT and stemness in human prostate cancer cells.107 In DU145 and PC3 prostate cancer cells, the high expression of CD44v4, v5, and v7 mediated by sulforaphane contributed to tumor cell growth and proliferative activity.108 Furthermore, CD44high stem cell-like cells have been found to be involved in drug resistance and invasive phenotypes in DU145 and PC3 cell populations.109 In addition, high expression of CD44 was reported to be associated with prostate cancer cell migration and proliferation.110 Accumulating evidence suggests that CD44 is a stem cell marker in prostate cancer. Translationally controlled tumor protein (TCTP) was closely associated with survival factor of stem cells, and the TCTP inhibitor sertraline highly downregulated the expression of CD44.111 Enzalutamide has been found to induce stem-like characteristics to acquire resistance, and CD44 has been found in enzalutamide-resistant cells.112 Additionally, STAT3 also contributes to the cell stemness and activation of the CSCs marker CD44 in PC cells.113 In general, CD44v isoform is associated with cancer stemness. However, only some studies have identified the specific CD44v isoform. The specific CD44v isoforms in other studies are unclear, which need further study.
3.10 Gastrointestinal cancer
Abundant evidence indicates that CD44 contributes to tumor progression114 and stemness115 in gastrointestinal cancer. It was revealed that MUC5AC interacting with CD44 promoted cell invasive and migrative potential and decreased apoptosis of colorectal cancer (CRC) cells via Src signaling.116 In addition, PD-L1 was found to increase the population sizes and the tumorspheres forming ability in CD133+CD44+ cell, which resulted in colorectal CSCs self-renewal.117 A previous study revealed that CD44, a stem cell marker in gastric cancer (GC) lines, could be suppressed by DAXX.118 Interestingly, CD44 was also found to play either different or overlapping roles in different gastrointestinal cancers, which may be related to the species of CD44 and its isoforms. A previous study revealed that CD44v8–10, but not CD44s, expressing could restore the tumor-initiating potential of GC cells reduced by silencing total CD44.119 Moreover, CD44v8–10 also plays an important part in regulation of ROS defense and tumor growth by p38 (MAPK) and p21 (CIP1/WAF1) in human gastrointestinal cancer cells.120 CD44v6 was found to be a stem cell marker, and its overexpression promoted migration and metastasis in colorectal CSCs by activating Wnt/β-catenin.14 In addition, accumulating evidence suggests that miRs binding to CD44 plays key roles in drug resistance, tumor growth, and stemness. For instance, miR-302a binding to CD44 was shown to suppress CSCs-like properties and restore cetuximab (CTX) responsiveness in CRC.121
3.11 Melanoma
CD44 is a CSCs marker122 in melanoma cancer and participates in tumor initiation progression.123 Wei et al.124 found that downregulated RNF128-activated Wnt signaling induced cellular EMT and stemness by ubiquitinating and degrading CD44/cortactin. Additionally, the depalmitoylation of the prometastatic cell adhesion molecule CD44 was shown to result in increased melanoma invasion via Wnt5a.125 Compelling evidence suggests that drugs targeting CD44 in melanoma is a promising strategy for melanoma treatment. For instance, expressing BMP4/7-dependent Id1/3 protein was reported to decrease the survival rate in melanoma patients promoted by HA–CD44 interactions with BMPR.126 Moreover, nanodrug based on CS could target CD44 to treat melanoma by inducing mitochondrial apoptosis.127
3.12 Squamous cell carcinoma
CD44 plays a role in various squamous cell carcinomas and has been shown to be mainly involved in EMT128 and cancer stemness.129 In HNSCC, HA binding to CD44 was found to increase CSCs numbers by PI3K–4EBP1–SOX2, whereas CD44 binding to VCAM-1 contribute to invasiveness by ezrin/PI3K. Moreover, the switch from CD44v8–10 to CD44s was reported to promote EMT and participate in tumor invasion,52 which is contrary to the results in ESCC of Yu et al.102 In mouse and human squamous cell carcinoma, it has been demonstrated that induction of a hybrid EMT state contributes to tumor initiation, progression, invasiveness, stemness, and metastasis by activating the CAMK2–CD44–SRC axis.130 Moreover, in ESCC patients, SOCS6 has been shown to significantly decrease the population of CSCs expressing the surface biomarker CD24low/CD44high to overcome radioresistance.131 Furthermore, in CD44high OSCC cells, TGF-β1 was found to induce amoeboid-to-mesenchymal transition (AMT) via activating ERK and phosphorylating Cofilin-1.132 In summary, the role of CD44 in squamous cell carcinoma could serve as a vital area for drug development and tumor marker.
3.13 Sarcoma
Compelling evidence suggests that the overexpression of CD44 in most sarcomas participates in tumor progression,133, 134 stemness,135 and dissemination.134 CD44 was found to increase the resistance of osteosarcoma cells to doxorubicin by upregulating multidrug resistance 1 protein expression.136 The human osteosarcoma cell lines MNNG/HOS and 143B were both highly metastatic, and CD44 was reported to be knocked out by CRISPR/Cas9. Additionally, inhibiting cell proliferation and tumor sphere formation cultured in 3D environment was found to depend on CD44 inactivation.137 Furthermore, in a mouse fibrosarcoma model, the increased expression of human CD44s was shown to promote micrometastasis events.138 Moreover, in osteosarcoma, CD44v6 is an important prognostic factor of patient prognosis.139 In sarcomas, research on the roles of CD44 is conductive to understanding the pathogenesis of these rare cancers.
3.14 Effects of CD44 interacting with HA on cancers
HA, a linear glycosaminoglycan (GAG),140 binds to the N-terminus of the extracellular domain of all CD44 isoforms.141 Aruffo et al.142 first proposed the relationship between CD44 and HA. Through endocytosis mediated by iron,143, 144 the interaction between CD44 and HA plays crucial role in the progression,145 invasion,146 and chemoresistance147 of cancer cells. As reported, patients with CD44s-positive tumors may gain a survival benefit from HA–irinotecan, which is a formulation of HA and irinotecan.148 In tumors with increased expression of CD44, the using of HA-based nanocarriers was reported to have the benefits in the enhancement of drug delivery, the increase therapeutic efficacy with low cytotoxicity, the inhibition of tumor growth, as well as the high potential for targeted chemotherapy.149 Moreover, kynureninase associated with the onset and development of breast cancer was found to be upregulated by CD44, which was induced and activated by HA.141 In addition, CD44 isoforms also regulate the uptake and expression of HA. It has been revealed that the expression of cancer cells with CD44v can negatively influence the uptake of HA, whereas cells expressing CD44s has positive correlations with the uptake and expression of HA. Moreover, the ability of HA uptaking varies across the cell lines. CD44shigh cancer cells could uptake HA more efficiently compared with CD44slow human dermal fibroblasts.87 In summary, the interaction of CD44 with HA and different CD44 isoforms regulates the ability of HA uptaking and expression have effect on cancer, which provides important advances with respect to cancer treatment strategies based on HA and the of selection of different CD44 isoforms as a target.
3.15 Effects of CD44 interacting with OPN on cancers
OPN, a phosphorylated glycoprotein, which expressed in the mineralized extracellular matrix (ECM) of bone in normal tissues cells such as fibroblasts, osteoblasts, and osteocytes.150, 151 As one of the classical and important ligands of CD44, OPN affects the proliferation and invasion of tumor cells as well as inflammation in normal cells, by regulating related signaling pathways.150, 152 Activation of the JUN N-terminal kinase pathway has been shown to contribute to the promotion of clonogenicity and tumor growth in a xenograft model via OPN secreted by tumor-associated macrophages.153 In PC3 cells, OPN binds to CD44 receptors that could inhibit the c-RaF and ERK1/2 by activating the AKT pathway, thereby inhibiting cell cycle arrest.154 Additionally, Yang et al.155 found that the interaction of CD44 with OPN could promote tumor-associated mesenchymal stem cells formation, leading to lung cancer cells invasion and migration. Moreover, decreased expression of interferon regulatory factor 8 in colon carcinoma was reported to increase the expression of OPN, which is associated with lower patient survival rate. This phenomenon is associated with the interaction of OPN with CD44 leading to immune escape.156 Taking together, high expression of OPN could promote immune escape of tumor cells, which may be related to regulate T cells activation through OPN interacting with its receptor CD44 and compensating for the function of PD-L1.157
3.16 Effects of CD44 interacting with serglycin on cancers
Serglycin has been shown to be a ligand for CD44. Previous studies have found that the binding of CD44 to serglycin is implicated in tumorigenesis and prognosis.158 The attachment of GAGs to serglycin could facilitate this binding.159 For instance, the proteoglycan serglycin was reported to promote the migration of non-small cell lung cancer cells via the binding of its GAG motif to CD44.160 Moreover, serglycin binding to CD44 was shown to regulate the expression of CD44 in a β catenin-dependent manner, a finding that might improve the poor prognosis in triple-negative breast cancer.161 In addition, the interaction of serglycin with CD44 was revealed to promote tumorigenesis in giant cell tumors of bone via activating focal adhesion kinase.162 There are few studies on serglycin and CD44 in tumors, but for the important role of serglycin binding to CD44 in tumorigenesis, it is believed that there will be more therapeutic strategies based on the regulating the binding of the two in future studies.
3.17 Effects of CD44 interacting with CS on cancers
In recent years, the studies on CD44 and CS mainly focused on the nanodelivery systems based on the specific binding between CD44 and CS.163 Accumulating evidence suggests that CS modifies the drug delivery of nanoparticles that target CD44 into tumor cells with low cytotoxicity.164 Nanoparticles composed of CS, doxorubicin, and bovine serum albumin targeting CD44 were reported to suppress 4T1 tumor growth.165 Additionally, a nanosystem targeting CD44, composed of d-α-tocopherol polyethylene 1000 glycol succinate and CS dual-modified lipid-albumin, was found to deliver paclitaxel into multidrug-resistant tumor cells, thereby overcoming drug resistance.166 Moreover, Chu et al.167 found that suppressing the expression of CD44 and integrin β1 could reduce the invasiveness of glioma cells by suppressing CS synthase 1. All the evidence shows that CD44 plays an important role in cancer treatments by nanoparticles modified by CS and other drugs.
3.18 Effects of CD44 interacting with matrix metalloproteinases on cancers
Matrix metalloproteinases (MMPs) is a large family of zinc endopeptidases whose members can interact with CD44. The binding of CD44 to MMPs has also been reported to mediate tumor growth, stemness, as well as the aggressive behavior.168, 169 It has been shown that hybrid nanoparticles targeting CD44 or MMPs have antitumor efficacy in 4T1 breast tumor,170 MCF-7, and MDA-MB-231 cells171 by inhibiting the expression and activity of MMPs. Also, polyphenols extracted from Artemisia annua L. showed anticancer effects by suppressing CD44 and MMP9 in radio-resistant MDA-MB-231 human breast cancer cells.172 Interestingly, CD44 has been shown to be both positively and negatively correlated with the expression of MMPs.173 For instance, the overexpression of CD44 was reported to reduce the levels of MMPs for the maintenance of ECM homeostasis.174 In Papadopoulou's study,175 the increase in MMP1 and MMP13 expression followed by the decreased expression levels of CD44 was found to promote tumor growth. Another study also showed that MMP2 activation contributed to decrease the number of CD44+/CD24− cells in MDA-MB-231 cells after normothermic microwave treatment.176 In contrast, inhibiting the expression of MMPs also inhibited the expression of CD44, for instance, DSPP/MMP20 gene silencing resulted in downregulation of CD44, a marker of oral squamous cell carcinoma (OSCC), and increased sensitivity to cisplatin.177 Moreover, CD44 was shown to promote lung ADC cell invasion by regulating MMP-2 expression.178 Also, in PC3 cells and breast carcinoma cells, the intracellular domain of CD44 was found to form a complex with the RUNX2 protein, which mediated cancer metastasis, migration, and progression through activation of MMP-9.179 In encapsulated papillary carcinoma (EPC), the high expression of CD44s induced high expression of MMP2 associated with invasion.180 Overall, the complex relationship between CD44 and MMPs expression provides us with more ideas for selecting different targets in cancer therapeutic strategies.
3.19 Effects of CD44 interacting with fibronectin on cancers
It was revealed that fibronectin (FN) in the ECM could interact with CD44, which is involved in cell metastasis,181 migration,182 adhesion,183 and survival.184 In endothelial cells, space microgravity was found to modulate the expression of CD44 and restrain collagen I and FN deposition, which involved cellular adhesion.185 Additionally, by using a CD44 antibody to partially inhibit extracellular FN signaling, a significantly decrease in adhesion and survival of melanoma cells was obversed.186 Furthermore, FN type III domain-containing protein 3B circular RNA increased migration and invasion in GC with a decline in CD44.187 Although there are few studies on regulating the interaction of CD44 with FN to treat cancer, owing to the key role of FN in cell migration and adhesion, this aspect may be a promising therapeutic strategy in cancer metastasis.
The relationship in neoplastic diseases between CD44 and its ligands is complex, and the expression of the two may be positively or negatively correlated. The main studies is about CD44 rather than a specific CD44 isoform interacts with its ligands. Together, CD44 interacts with its ligands plays key roles in cancer progression. All the abovementioned studies provide new insights into cancer therapy by interfering the interaction of CD44 with its ligands.
4 CANCER-ASSOCIATED PATHWAYS REGULATED BY CD44
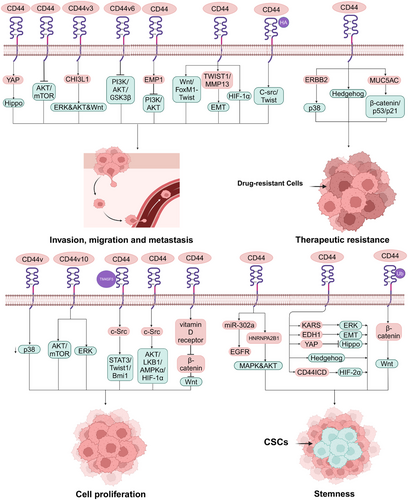
Activation and deactivation of CD44 isoforms regulate the activities of the components of signaling pathways, including enzymes,188 protein kinase pathways,189, 190 and transcription factors,191 which have been found to be associated with tumor initiation progression and aggressive behavior.192, 193 Moreover, CD44 is also found to be a component in some cancer-associated signaling pathways.194 Some signaling pathways associated other cancers regulated by CD44 are summarized in Figure 3. Moreover, the biological functions of different CD44 isoforms in cancer are summarized in Table 2
| CD44 isoforms | Biological functions | References |
|---|---|---|
| CD44s | Tumorigenesis, tumor growth, aggressive behavior, micrometastasis events, inhabiting stemness, CSCs characteristics, EMT | 13, 52, 61, 62, 93, 107, 138, 180 |
| CD44v | Invasiveness, stemness, tumor growth | 62, 64, 120 |
| CD44v3 | Metastasis, EMT | 221 |
| CD44v4, v5, and v7 | Growth and proliferative activity | 108 |
| CD44v6 | EMT, aggressive behavior, poor prognosis, | 34, 54, 139, 223 |
| CD44v8–v10 | Tumorigenic phenotypes, aggressive behavior, poor prognosis, restoration of tumor-initiating potential, tumor growth | 52, 76, 83, 94, 103, 119, 120 |
| CD44v9 | Progression and metastasis, poor prognosis, EMT | 14, 93, 101 |
| CD44v10 | Cell proliferation | 199 |
4.1 MAPK signaling pathway
The activity of the MAPK signaling pathway by CD44 has been associated with the growth of cancer cells.195 In CRC patients, miR-302a binding to CD44 was shown to restore CTX responsiveness by suppressing CSCs-like properties via EGFR-mediated MAPK and AKT signaling.121 Furthermore, MCF-7-A2B1 cells with a high proportion of CD44+/CD24−/low CSCs were reported to activate the AKT and MAPK pathways via the expression of HNRNPA2B1.196 Moreover, although there are few studies on the regulation of CD44 on p38 in cancer, it is well documented that CD44 regulates cancer progression via activation of p38.197, 198 In a transgenic mouse model of GC, ablation of CD44v was reported to suppress tumor growth via activation of p38 signaling.120 In prostate cancer cells, CD44 collaborates with ERBB2 to contribute to the radioresistance of cancer cells via p38 phosphorylation. In breast cancer cells, CD44v10 was found to facilitate cell proliferation via activating ERK/p38 MAPK and AKT/mTOR signaling.199 Cell cycle arrest and inhibition of the survival, proliferation, and migration of PC3 cells expressing CD44 and CD133 isolated from prostate cancer by inhibiting p-p38, p-ERK, NF-κB, and PARP.200 Furthermore, CD44-regulated ERK signaling is mostly described as stemness and aggressive behavior of tumors.201, 202 Yokoyama et al.132 found that OSCC cells highly expressing CD44 had been shown to be associated with AMT via activation of ERK. In glioblastoma multiforme cells, high expression of CD44 was found to induce cancer stemness and EMT features via KRAS/ERK signaling activation.203 In addition, the high expression of CD44 was capable of inducing ERK phosphorylation, which affects the migratory and invasive potential of lung cancer cells.204
4.2 Hippo signaling pathway
In the Hippo pathway, CD44 plays key role in cancer stemness and aggressive behavior.205-207 In lung adenocarcinoma, EDH1 interaction with CD44 was shown to promote CSCs-like traits, EMT, and metastasis via inhibition of the Hippo pathway.208 CD44 upregulation can also confer CSCs-like properties to malignant mesothelioma cells by activating YAP via Hippo signaling pathway inactivation.209 In addition, in docetaxel-resistant prostate cancer cells, CD44 was reported to promote cell migration and invasion via induction of Hippo–YAP pathways.210 Moreover, knockdown YAP partially abolished the stemness of CD44+ retinoblastoma stem-like cells.211
4.3 Hedgehog signaling pathway
CD44+ CSCs is a more appreciated subset which plays key roles in conventional stemness-related Hedgehog signaling activation.212, 213 In CD44+ HCC, suppressing Hedgehog signaling was reported to reverse sorafenib resistance.77 On top of that, in patients with CRC, CD44-high tumors have been shown to be enriched for the Wnt/β-catenin and Hedgehog signaling pathways.214 Additionally, a bladder CSCs subpopulation with the stem cell-like marker CD44 was responsive to inhibition of Sonic Hedgehog signaling.215 Overall, regulation of the Hedgehog signaling pathway is a promising therapeutic target in CD44+ CSCs. CD44+ CSCs is associated with activation of Hedgehog signaling.216
4.4 PI3K-AKT signaling pathway
A number of studies have also highlighted the effect of CD44 on AKT in relation to cancer cell growth, motility, invasion and stemness.217-219 Thanee et al.220 found that silencing CD44 inhibited cholangiocarcinoma progression and aggressiveness, as well as AKT and mTOR phosphorylation. As demonstrated by GC cell lines and an experimental animal model, chitinase-like protein CHI3L1 binding to CD44v3 was a specific inducer of activation of ERK, AKT, and β-catenin signaling and enhancement of GC transfer.221 Moreover, studies of in vitro and in vivo found that CD44 promoted HCC migration and extrahepatic metastases mediated by the AKT/ERK signaling CXCR4 axis.222 In addition, more recent researches have demonstrated that activation of the PI3K signaling pathway is positively associated with tumor growth and aggressive behavior. In HNSCC, targeting CD44 was found to decrease tumor growth and CSCs by inhibiting PI3K–4EBP1–SOX2 signaling.52 Furthermore, in OSCC, silencing CD44v6 diminished invasion/metastasis potential by blocking the PI3K/AKT/GSK3β pathway.223 Finally, inhibiting the PI3K–AKT signaling pathway contribute to inhabit invasion and proliferation of glioma cell mediated by epithelial membrane protein 1 (EMP1), which was affected by the expression of CD44.224
4.5 Twist signaling pathway
Twist is mainly related to EMT in cancer cells,225 and CD44 is found to play a key part in the adaptive plasticity of cancer cells by regulating Twist signaling.226 CD44 was reported to promote lung CSCs metastasis through Wnt–FoxM1–Twist signaling.227 In addition, in colon CSCs with CD133+ and CD44+ markers, triptolide inhibited cell death, apoptosis and altered cell cycle distribution by inhibiting snail slug and Twist expression, which has been reported to be associated with EMT.228 Moreover, in ESCC, the TWIST1–CD44–MMP13 axis has been shown to be involved in tumor aggressiveness and EMT.229 On top of that, T mutant allele of CD44 (rs8193C>T) could trigger PKC–Twist, PKC–Nanog, and Nanog–Stat signaling pathways through binding to PUM 2, which is related to prediction of prostate neoplasms and prognosis factor in prostate neoplasms.230
4.6 HIF signaling pathway
Both HIF-1α and HIF-2α play roles in tumor stemness,231, 232 progression,233 and metabolism.234 Researches found the expression of CD44 could affect HIF signaling pathway. In glioma, CD44ICD binding to HIF-2α (but not HIF-1α) induced stemness via activation of HIF-targeting genes.235 Interestingly, Yang et al.236 found HIF-1α is also associated with MDA-MB-231 and 468 cells stemness through Coenzyme Q0 treatment to decrease the expression of CD44. Moreover, in TE10 and TE11 cells, the expression of SET domain-containing 5 could increase the expressing level of cancer stemness-related protein HIF-1α and CD68 expression, which is associated with the coexpression of CD44 and SET domain-containing 5.237 The multiple effects of HIF may associated with the type of cancers, which expressing different interacting factors affected by CD44. In the human gastric cell lines SGC-7901 and BGC-823, hypoxia-increased expression of CD44 was found to increase cell viability and invasion associated with high expression of HIF-1α.238 Additionally, in human breast cancer cells, silencing CD44 was also shown to decrease the glycolytic phenotype of cancer cells via regulating c-Src/AKT/LKB1/AMPKα/HIF-1α signaling.239
4.7 c-Src signaling pathway
In recent years, although few studies have investigated the c-Src signaling pathway in cancer regulated by CD44, accumulating evidence suggests that activation of c-Src plays a key part in tumor progression.240, 241 In human breast cancer cells, CD44 knockdown suppressed both the mRNA and protein expressing of c-Src and its downstream MAPK signal.242 Moreover, in breast cancer cells, HA-induced CD44 interacting with c-Src-activated Twist resulted in downregulation of tumor suppressor protein, Rho GTPase ROK activation and tumor cell invasion, which are critical prerequisite steps for obtaining metastasis.243 Additionally, the TM4SF5/CD44 interaction of metastatic HCC cells activated c-Src/STAT3/Twist1/Bmi1 signaling, which caused spheroid formation.244
4.8 Wnt signaling pathway
CD44 is a target gene in the Wnt signaling pathway245 and plays a key part in activation of the Wnt pathway mediating chemoresistance,246 EMT,247 and tumor progression.248 In CRC cells, upregulation of CD44, positively associated with the secretory mucin MUC5AC, was reported to confer chemoresistance via β-catenin/p53/p21.116 Moreover, downregulation of RNF128 led to the ubiquitination and degradation of CD44/cortactin, inducing cellular EMT and stemness via activating Wnt/β-catenin signaling.124 Furthermore, in human GC cells MKN45 and KATO III, vitamin D receptor-induced suppression of CD44 was shown to suppress cell growth, probably via inhibition of Wnt/β-catenin signaling.249
5 ANTICANCER THERAPEUTIC STRATEGIES BASED ON TARGETING CD44
5.1 Antibodies, peptides, and aptamers
It has been shown that suppressing the expression of CD44250 or blocking its interaction with other ligands251, 252 is a promising strategy in cancer therapy. Antibody against CD44 has been reported to be used in this way in CD44-positive cancer. For instance, in the human bladder cancer cell line EJ, a novel mouse monoclonal antibody (mAb) KMP1 inhibited proliferation, migration, and adhesion as well as suppressed the xenograft tumor growth in nude mice by blocking CD44.253 In OSCC, a defucosylated anti-CD44 mab 5-mG2a-f was found to have antitumor effects both in vitro and in vivo.254 Apart from antibodies, another antitumor strategy is to take advantage of synthetic peptides to selectively bind CD44 to inhibit its functions or block the interaction of CD44 and its ligands. In mice harboring tumors, intravenously administered CNLNTIDTC and CNEWQLKSC peptides targeted tumors and inhibited metastasis by binding to CD44v6.255 Moreover, it has been demonstrated that a synthetic IGFBP-3 peptide (215-KKGFYKKKQCRPSKGRKR-232) can inhibit the viability of A549 cells as a result of CD44 competing with the HA receptor.256 Indeed, a research suggested that CD44 aptamers exert antitumor effects by targeting CD44. In ovarian cancer, an RNA-based bispecific CD44–EpCAM aptamer was shown to inhibit cell growth and to induce apoptosis by blocking CD44 and EpCAM simultaneously.257 Moreover, follow-up experiments identified the inhibition of orthotopic glioma growth by AS1411 aptamer coloading shikonin and docetaxel targeting CD44-overexpressing glioma.258 Of note, the anticancer effect of antibodies, peptides, and aptamers depends on their binding affinity and specificity to CD44. This suggests a direction for targeted therapy targeting CD44 isoforms and highlights the different roles of the stroma in tumors.
5.2 Pharmacological inhibition
Other than directly targeting CD44, several natural compounds and chemotherapeutic agents are also important anticancer agents in cancer cells and CSCs expressing CD44.259-261 In glioma cells, galangin was found to inhibit EMT and angiogenesis by suppressing the expression of CD44.262 Similar results are further supported by Jobani et al.,263 who demonstrated that combination treatment with allicin and all-trans retinoic acid significantly reduced the IC50 value in CD44 expressing melanoma cells CD44. Additionally, in the breast cancer cell line MDA-MB-231, ivermectin was shown to preferentially inhibit the CD44+/CD24− CSCs subpopulation. Overall, these data suggest that pharmacological therapy that suppresses CD44 expressing in cancer cells is a promising strategy.
5.3 Gene therapies
MiRNAs can directly bind to CD44 to silence gene expression at the RNA level, which has been shown to regulate CSCs characteristics and tumor progression.264-266 For example, in colon cancer cells, miR-145 and antagomir-21 were found to suppress CSCs proliferation by inhibiting the expression of CD44.267 In addition, another study found that in CRC cells, CTX responsiveness could restored by miR-302a by inhibiting CD44-induced CSCs-like properties.121 Moreover, Liu et al.268 found miR-34a could directly target CD44 and miR-34a inhibited prostate CSCs and metastasis by negatively regulating the expression of CD44. On top of that, Feng et al.269 found that miR-373 and miR-520s could affect the growth and invasiveness of glioblastoma cells through interacting with CD44 to decrease its expression. In papillary thyroid cancer, miR-205-5p/GGCT was found to inhabit its growth and metastasis through regulating the expression of CD44.270
On the other hand, it is worth noting that short interfering RNAs (siRNAs) have been shown to knockdown the expression of CD44 via repression of translation to suppress EMT, drug resistance, and growth in tumors.271-273 In colorectal CSCs, siRNA inhibited EMT-induced proliferation and invasion by silencing the expression of CD44.274 In addition, in the human breast cancer cell line MDA-MB468, siRNA-mediated silencing of CD44 was shown to enhance doxorubicin chemosensitivity.275 In addition, in the EGFR wild-type non-small cell lung cancer cell line H460, knocking down CD44 by siRNA has been found to reduce cell growth and induce cell apoptosis.276
Overall, recent developments support the implementation of gene therapy as a new component in therapeutic agents targeting CD44.
5.4 Cell therapies
Recently, CD44 isoforms have been found as an promising target for chimeric antigen receptor T cells (CAR T cells) to eliminate CD44 expressing cancer, which is a novel and specific treatment.277, 278 CD44v6 is found to be associated with tumor progression and aggressive behavior.279 Hence, CD44v6 CAR T cells is an attractive therapy to control tumor growth. For instance, CD44v6–CAR T cells were found to specifically lyse CD44v6+ acute myeloid leukemia cells associated with cytokine release.280 Moreover, Haist et al.281 showed a positive link between CD44v6 expression levels and the cytotoxicity of CAR T cells and found that CD44v6–CAR T cells specifically eliminated CD44v6+ HNSCC. Furthermore, minicircle DNA-mediated CD44–CAR T cells were shown to suppress HCC.282 Token together, little research has been done on CAR T cells targeting CD44 expressing cancers cells, but it is being rolled out and is a novel therapeutic strategy. CAR T cells targeting CD44s or other CD44v expressing cancer cells require further study, for the reason that highly customized CAR T treatments could focus on the differences of CD44 isoforms expressing in cancer cells to improve their specificity and off-target effect.
5.5 Biological materials
Robust evidence supports that antitumor agents conjugated by biomaterials show good antitumor effects and targeting activity through a ligand–receptor-mediated targeting mechanism.283-285 For instance, CS-conjugated doxorubicin poly(lactic-co-glycolic acid) (PLGA) nanoparticles were found to directly target CD44 with low cardiac toxicity and strong antitumor ability.286 Moreover, an HA-labeled PLGA nanoparticle encapsulating both paclitaxel and focal adhesion kinase siRNA was reported to bind to CD44-positive epithelial ovarian cancer cells to overcome chemoresistance.287 In addition, novel HA cross-linked zein nanogels were developed to deliver curcumin into CT26 cells expressing CD44, which showed high anticancer activity.288 In conclusion, biomaterial coupling of antitumor agents shows good target activity and low toxicity, which is a promising therapeutic strategy in cancer treatment. Moreover, a recent research has found a self-crosslinkable chitosan–HA dialdehyde nanoparticles could target the delivery of siRNA to T24 bladder cancer cells, which could affect bladder cancer through the interaction of CD44 and HA.289
6 CLINICAL STUDIES
Although accumulating evidence suggests that CD44 plays a key role in the clinicopathological features of numerous cancers,290, 291 there are relatively few clinical studies designed to evaluate the efficacy of CD44-targeted therapies in cancer treatment.
To date, 16 clinical studies have been registered on ClinicalTrials. Gov to assess the anticancer effects of targeting CD44. The summary content is shown in Table 3. Among them, three trials are designed to evaluate CD44v6-specific CAR T-cell therapies; two trials are using humanized mAb drugs for the treatment of CD44+ cancer; two trials are aimed at inhibiting the expression of CD44 in cancer; and one trial is about a drug designed to bind to CD44 for the treatment of chronic lymphocytic leukemia and small lymphocytic lymphoma. However, a phase I trial of an anti-CD44 humanized antibody, RG7356, in CD44 expressing solid tumor patients was failed.292 The study was terminated early because RG7356 does not have a relationship with clinical and/or pharmacodynamic dose–response. On the one hand, in plasma, the RG7536 was converted to a binding-impaired molecule that cannot result in sufficient antibody levels due to the deamidation of asparaginases in the complementarity determining region of intact antibody. One the other hand, the expression of CD44s or CD44v in patient tumor is crucial. Some CD44s expressing tumor patients (four out of nine) showed tumor shrinkage which was not observed in CD44v expressing tumor patients. This phenomenon may be associated with the iron endocytosis is recompensated by transferrin receptor, resulting in the reducing of drug specific uptake of tumor cells.293, 294, 30
| Form of drug | Cancer applications | Interventions | Phase | NCT No. |
|---|---|---|---|---|
| Gene-engineered T cells | CD44v6+ cancer | CD44v6 CAR T cells | I/II | NCT04427449 |
| Breast cancer | Her2, GD2, and CD44v6 CAR T cells | I/II | NCT04430595 | |
| CD44v6+ acute myeloid leukemia and multiple myeloma | Drug: MLM-CAR44.1 T cells | I/II | NCT04097301 | |
| Humanized mAb | CD44v6+ breast neoplasms | Drug: Bivatuzumab mertansine | I |
NCT02254005 NCT02254031 |
| CD44+ malignant solid tumors | Drug: RO5429083 | I | NCT01358903 | |
| CD44v6 inhibitor | Malignant solid tumor | Drug: AMC303 | I | NCT03009214 |
| CD44+ ovarian epithelial cancer | Drug: SPL-108 | I | NCT03078400 | |
| CD44 binding peptide | Chronic lymphocytic leukemia and small lymphocytic lymphoma | Drug: A6 | I | NCT02046928 |
- Data sources: ClinicalTrials. Gov.
Together, treatments targeting CD44 are promising, and it is expected that more translational studies will be concentrated on targeting different kinds of CD44 isoforms. Moreover, due to the dominant advantage of combination regimens,295, 296 more clinic trials about combination regimens should be considered to overcome potential tumor escape or drug resistance.
7 CONCLUSIONS
In recent years, there has been an increasing awareness of the complex functions of CD44. A systematic review of landmark CD44-related studies could facilitate a better understanding of the functional role of CD44 in cancer development and progression and thus will contribute to the development of novel strategies to circumvent their disadvantage and acquire optimal clinical efficacy. Here, we encapsulate the present understanding of the structural and functional roles of CD44 in neoplastic diseases as well as CD44-regulated signaling pathways. We also discuss current targeting CD44 therapeutic strategies as well as prospective directions for future expansion and highlight existing clinical data supporting its use.
Overall, high expression of CD44, regardless of isoforms, is mainly positively associated with the development of neoplastic disease. Extensive studies have revealed that CD44 mediates cancer initiation and progression through interactions with its ligands, including but not limited to HA, OPN, serglycin, CS, the MMPs family, and FN. As one of the important functions of CD44, its regulation of cancer-related pathways is pleiotropic, including cancer initiation, aggressive behavior, and stemness. CD44 promotes activation of MAPK, p38, and so on, which is associated with cancer cell stemness, growth, radiation resistance, and cell proliferation. Moreover, CD44 interacting with proteins such as YAP in the Hippo signaling pathway can regulate the stemness and aggressive behavior of cancer. On top of that, as a classical CSCs marker, CD44 regulates cell plasticity through iron mediation.
Expansive evidence indicates that anticancer therapeutic strategies based on targeting CD44 are effective methods, including antibodies, peptides, aptamers, natural and synthetic inhibitors, gene and cell therapy and biomaterials, depending on the interaction of CD44 with its ligands. Among these methods, targeting CD44–CAR T cells is promising. Although it has few applied clinically, its good targeting and durability in CD44 expressing patients show CAR T treatment is worthy of further study. However, clinical trials on CD44 in the treatment of cancer are limited, concentrating on targeting CD44v6, an isoform associated with metastasis and invasiveness. Further studies may focus on other isoforms. On top of that, it is expectable that more translational studies will be conducted to focus on chelating iron or regulate iron concentration to depleting CSCs through regulating the expression of CD44. In addition, clinical evidence suggests that highly expressing CD44 is a potential biomarker, including poor prognosis, tumor grade, and potential malignancy. However, the expression of CD44 was also found to be negatively associated with tumor invasiveness and Gleason grades, which is mainly related to the switch between CD44s and CD44v on the cancer cells through alternative splicing of CD44 isoforms. In conclusion, the conflicting data reports indicate that further study calls for exploring the specific functions of different CD44 isoforms in different kinds of cancers.
AUTHOR CONTRIBUTIONS
Chenying Fu and Ping Cheng designed this study. Yiming Xu drafted the manuscript. Yiming Xu, Ziyi Bai, and Tianxia Lan revised the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This study was supported by grant from the National Science and Technology Major Projects of New Drugs (2018ZX09201018−013).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interests.
ETHICS STATEMENT
No ethics approval was required for this review that did not involve patients or patient data.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




