New insights into immune cells in cancer immunotherapy: from epigenetic modification, metabolic modulation to cell communication
Abstract
Cancer is one of the leading causes of death worldwide, and more effective ways of attacking cancer are being sought. Cancer immunotherapy is a new and effective therapeutic method after surgery, radiotherapy, chemotherapy, and targeted therapy. Cancer immunotherapy aims to kill tumor cells by stimulating or rebuilding the body's immune system, with specific efficiency and high safety. However, only few tumor patients respond to immunotherapy and due to the complex and variable characters of cancer immune escape, the behavior and regulatory mechanisms of immune cells need to be deeply explored from more dimensions. Epigenetic modifications, metabolic modulation, and cell-to-cell communication are key factors in immune cell adaptation and response to the complex tumor microenvironment. They collectively determine the state and function of immune cells through modulating gene expression, changing in energy and nutrient demands. In addition, immune cells engage in complex communication networks with other immune components, which are mediated by exosomes, cytokines, and chemokines, and are pivotal in shaping the tumor progression and therapeutic response. Understanding the interactions and combined effects of such multidimensions mechanisms in immune cell modulation is important for revealing the mechanisms of immunotherapy failure and developing new therapeutic targets and strategies.
1 INTRODUCTION
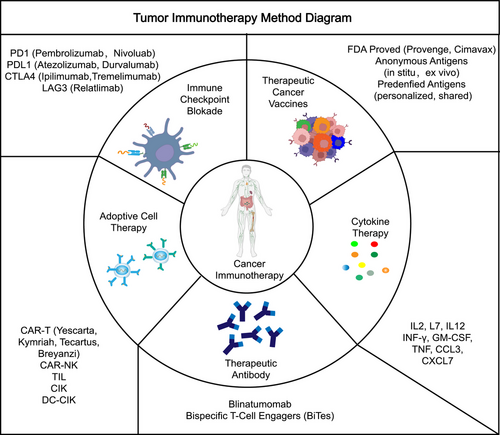
Cancers are a challenge that has never been conquered, and they are a serious threat to human health. With regard to the treatment of cancers, surgery remains the first-line treatment for many cancer types, and the clinical use of chemotherapy, radiation, neoadjuvant, and combination therapies have also improved patient survival, while the emergence of targeted therapies and immunotherapies may have the potential to revolutionize the treatment of cancer.1 Cancer immunotherapies (Figure 1), mainly including cytokine therapies, immune checkpoint blockades, therapeutic cancer vaccines, adoptive cell therapies, therapeutic antibodies such as bispecific T-cell engagers, and so on, which usually trigger T-cell-based immune responses against cancer antigens through the body's immune system, are now emerging as a unique pillar of cancer treatment.2 However, the efficacy of cancer immunotherapy in a large part of patients is not satisfactory. The complexity of immune cells in recognizing and destroying cancer cells remains at the forefront of research, and the mechanisms are not well understood.
Epigenetic modifications are dynamically variable and they can alter the functions of nucleic acid and proteins on chromosomes through chemical modifications thus modulating genes expression at multiple levels, including splicing, transcription, translation, stability, and chromatin structure.3 Typical epigenetic modifications are DNA/RNA methylation, noncoding RNAs, histone modifications, and chromatin remodeling. Currently, there is evidence that epigenetic modifications play an essential role in the cancer immune response. For example, epigenetic regulation including DNA methylation, histone methylation, histone acetylation, and histone ubiquitination were proved to target programmed cell death protein 1 (PD1)/programmed cell death lidand 1 (PDL1), and these epigenetic regulations were found to be critical in the immunotherapy of malignant melanoma and hepatocellular carcinoma (HCC).4, 5 Other epigenetic modifications such as noncoding RNAs can also exert a regulatory role in the immune response by affecting immune checkpoints, epithelial–mesenchymal transition, the tumor immune microenvironment (TIME) including modulating the activation of immune cells such as T cells, dendritic cells (DC), myeloid-derived suppressor cells (MDSC), and B cells.6-8 These evidences indicated that epigenetic mechanisms are critical in immune cell differentiation and function, ensuring that immune cells can exhibit specific gene expression patterns in different TIME conditions, thereby influencing the efficacy of cancer immunotherapy.
Tumor cells can modify metabolic pathways to enable them to adapt to the adverse microenvironment. In the tumor environment, the body often suffers from intensified glycolysis, enhanced lipid synthesis, altered amino acid metabolism, and higher production of lactic acid (LA), and these metabolic changes provide tumor cells with an advantage for proliferation and survival. And the metabolic competition that exists between the tumor and the immune cells hinders the function of the later.9 For example, prostate cancer cells release 1-pyrroline-5 carboxylate and repress T cell function to produce inflammatory cytokines through triggering of the shp 1/cytochrome c oxidoreductase/ROS axis.10 Furthermore, it has been shown that amino acid metabolism could strongly influence the tumor therapy resistance by controlling the destiny of diverse immune cells.11 That is to say, metabolism process in cancer cells and immune cells might be different, and the metabolism pathways in TIME are complexed and need to perform further researches.
Cell-to-cell communication within the TIME provides an important signaling for tumorigenesis and tumor development, and the investigation of message exchanges between different immune cells is crucial for the development of tumor immunotherapy and will help improve immune response rates.12 For example, DC-based vaccines have proven to be one of the most promising therapeutic agents for cancer treatment. Researchers have found that enhancing the interaction between DCs and T cells by engineering DCs can facilitate tumor-specific T-cell activation and enhance immune response effects.13 So, targeting immune cell communication also is a great strategy for cancer immunotherapy.
Immunotherapy is based on the activation and functional execution of immune cells, and this process is regulated at multiple levels. The success of cancer immunotherapy not only relies on the direct activation of immune cells, but also on the comprehensive understanding and precise regulation of the complex behaviors and interactions of immune cells in the tumor microenvironment. Epigenetic modification, as an important mechanism for determining gene expression status, directly affects the differentiation and function of immune cells. The metabolic state of immune cells not only determines their energy supply, but also affects their response capacity and survival, especially in the immunosuppressed tumor microenvironment. Finally, cellular communication is critical for coordinating immune cell behavior and tumor cell interactions, affecting the efficiency of immune surveillance and tumor cell clearance. Taken together, the latest findings in these areas can provide a comprehensive perspective on the mechanisms of immune cell action in cancer therapy and offer important directions for overcoming the limitations of existing therapies and improving the efficacy of immunotherapy. We provide a relatively comprehensive review on the impact of cancer immunotherapy by focusing on immune cells from the perspective of their epigenetic modifications, metabolic reprogramming, and cell-to-cell communication. In particular, we provided some clinical trial results for cancer immunotherapy based on immune cell therapy and illustrated the relationship between immune cells and clinical prognosis, also summarized the currently identified biomarkers that can be used to assess and predict the cancer immunotherapy efficacy and briefly characterize frequent mouse models for investigation of cancer immunotherapy, which may provide new intensive research ideas and opportunities for further refinement of immunotherapies.
2 RELATIONSHIP BETWEEN IMMUNE CELL AND CANCER IMMUNOTHERAPY
The relationship between immune cells and the efficacy of cancer immunotherapy is complicated and multifaceted.14 The efficacy of cancer immunotherapy is influenced by a variety of elements, among which the number, activity, and functional status of immune cells and their interaction with tumor cells are crucial.15 Immune cells such as T cells, NK cells, and B cells are essential components of the body involved in the immune response, and their status and function directly affect the response of the immune system as well as the efficacy of immunotherapy.16-18
2.1 Effector T cells and cancer immunotherapy
The main purpose of immunotherapy is to enhance the body's immune response and one of the most common ways is to enhance T cell function, especially CD8+ T cells, so that they can more effectively recognize and eliminate cancer cells. There are many studies targeting effector T cells to enhance immune efficacy. CD8+ T cell phenotype is markedly increased after receptor activator of nuclear factor-κB deletion, which enhances the efficacy of breast cancer immunotherapy.19 Metformin was found to improve the survival and the effector function of CD8+ T cells under hypoxic conditions as well as reduce hypoxia-induced apoptosis, while inhibiting PD1 and LAG3, thus improving the efficacy of cancer immunotherapy.20 The stress response states (STRs), which is characterized as expressing heat shock genes, were significantly upregulated in immune checkpoint blockade treated unresponsive tumors, and T cells could link STR to immunotherapy resistance, suggesting that T cells may take a role in immunotherapeutic efficiency.21 Studies have demonstrated that the utilization of EP4 antagonist, YY001, enhances the proliferation and anticancer function of T cells while inhibiting the differentiation and maturation of MDSCs, thereby reversing the level of infiltration of MDSCs and T cells in the tumor microenvironment, and can be used for enhancing the prostate cancer immunotherapy efficiency.22 In conclusion, in many cases, effector T cells are a reflection of cancer immunotherapy efficiency. The number, function, and activation of effector T cells, represented by CD8+T, can often be used as an observable indicator for the efficacy of cancer immunotherapy.
2.2 Other T cells and cancer immunotherapy
In addition to effector T cells, other T cells are also critical for cancer immunotherapy efficacy. So far it has been discovered that tissue-resident memory T cells are prognostic markers for a variety of cancers, including breast and lung cancers, and potential biomarkers for predicting the efficacy of immunotherapies such as immune checkpoint blockade and cancer vaccines.23 In addition, the tissue-resident memory T cells are regulated by TGF-β and they interact with tumor cells, DC cells, and other T cells in the TIME to ensure a positive T cell effector state.24 In addition, it has been demonstrated that neoantigen-specific T cells have high affinity and perform an essential role in adoptive cell therapy (ACT).25, 26 Recent studies have revealed that T cells associated with recognizing cancer-independent antigens (“bystander T cells”) also perform important functions in cancer immunotherapy and they differ in specificity, activation state, and effector function.27 Meanwhile, the exhausted T cells and immunosuppressive macrophages exhibited enhanced interactions in urothelial carcinoma of the bladder and performed significant functions in antitumor immunotherapy.28 Deletion of sorting nexin 9 (SNX9) in CD8+ T cells reduces the exhaustion of CD8+ T cells and enhances both T memory cell differentiation and interferon-γ (IFNγ) secretion of adoptively transferred T cells, thereby enabling effective CAR-T cells immunotherapy.29 In addition, the differentiation and modulation of T(H) subpopulations as well as γδ T cells in the TIME is critical for cancer immunotherapy efficacy.30, 31 Cellular senescence of tumor infiltrating lymphocytes (TIL) has recently been identified as an important state of T cell dysfunction in malignancy. Cancer cells and Treg cells are capable to induce senescence of responsive T cells through MAPK signaling, thus suppressing antitumor immunity and immunotherapy.32 In a word, besides effector T cells, tissue-resident memory T cells, neoantigen-specific T cells, “bystander T cells”, exhausted T cells, T memory cells, Th subpopulations, γδ T cells, senescence of TILs, and Treg cells also are significant index of cancer immunotherapy efficiency and worthy to be further studied.
2.3 Other immune cells and cancer immunotherapy
It is generally accepted that the eventual aim of cancer therapy is to induce a prolonged memory T cell response. So, the degree and function of T cell infiltration exerts an essential role in the response to tumor therapy. However, Chen et al.33 predicted the efficacy of anti-PD1/PD-L1 immunotherapy and survival rates in gastric cancer patients by multidimensional tumor-infiltrating immune cells (TIICs) labeling. Also, Jiang et al.34 constructed a TIICs model for immune cell-targeted therapy of gastric cancer and predicted the immunotherapy effect, which revealing that other immune cells in the TIME, such as B cells, macrophage cells, DC, and myeloid cells, also positively or negatively modulate the efficacy of antitumor immunity.
Here are some specific examples. B cells have been found to trigger antitumor immune responses either by direct tumor killing or by interacting with T cells and follicular DCs in tertiary lymphoid structures (TLSs). However, B cells can also acquire a suppressive function under certain conditions, becoming transformed into regulatory B cells that inhibit the tumor immune response.35 Depletion of tumor-infiltrating B cells (TIBs) to enhance antitumor immunity is a novel and promising immunotherapeutic approach. Remodeling the TIME by decreasing the proportion of TIBs, enhancing the infiltration of CD8+/CD4+ T cells, and inhibiting the proliferation of Treg cells, as well as increasing the secretion of IL-2 and IFN-γ and decreasing the secretion of IL-10, IL-4, and TGF-β, could improve cancer immunotherapy efficiency.36 Bufalin, as an antitumor immunomodulator, activates antitumor immune response by promoting the polarization of tumor-infiltrating macrophages (TIMs) toward the M1 phenotype, and meanwhile facilitates the production of immunostimulatory cytokines by regulating the NF-κB signaling pathway, thus effectively improving the therapeutic efficacy of HCC immunotherapy.37 Poly(I:C) and resiquimod combination therapy provides a synergistic activation of macrophage-mediated antitumor immune response.38 DC vaccines induces specific CD8+/CD4+ T cell responses, and the DC-cell-based cancer immunotherapy possesses promising future perspectives.39 In addition, tumor-infiltrating plasmacytoid DCs (pDCs) are known to have critical roles in the triggering and maintenance of antitumor immunity. The activated pDCs (IRF7+) were identified as a key regulator of adaptive antitumor immunity in CRC.40 In addition, TIL cells can also be used as markers to predict immunotherapy responsiveness and survival outcomes in melanoma,26, 41, 42 breast cancer,43-45 osteosarcoma,46 and other cancers.47, 48 All in all, current researches indicated that large amounts of immune cells possess the potential to affect cancer immunotherapy efficiency. And some of these immune cells such as different subset of B cells and T cells might exert extremely different effect (to be better or worser indicator for cancer immunotherapy efficiency), and these different isoforms can transform into each other under certain conditions.
Based on the important role of immune cells in tumor immunotherapy, we will next explore the function and mechanism of cancer immunomodulation in terms of epigenetics modification, metabolism modulation, and cell-to-cell communication in immune cells to provide new possible directions for tumor immunotherapy.
3 EPIGENETIC MODIFICATION
Epigenetics explores changes in the expression of genetic information that do not involve alterations in the DNA sequence, but rather chemical modifications that control the turning on and off of genes. The aim of this chapter is to review recent advances in epigenetic modifications in tumor cells and immune cells, focusing mainly on aspects related to DNA/RNA, histone, and chromatin modification.
3.1 Epigenetic modifications in tumors
Cancer is known to be a disease triggered and driven by genetic abnormalities, but epigenetic pathways also play an important role in tumorigenesis. And many features of cancer, including differentiation blockade, self-renewal, cell death evasion, and invasiveness, are deeply affected by epigenomic changes.49 Epigenetic modifications have attracted increasing attention as anticancer strategies in recent years, which is largely based on their direct impact on cancer cells.
The epigenetic modification such as DNA and RNA methylation modifications have been shown to play an important role in tumorigenesis and diagnosis. For example, the researchers analyzed DNA methylation profiles in colon cancer and discovered that the levels of 5-hmdC and 5-mdC modifications were low, 5-fdC modifications were the lowest, and 5-cadC modifications were high, suggesting a relationship between aberrant patterns of DNA epigenetic modifications and cancer development.50 Liang et al.51 conducted DNA methylation analysis using high-throughput DNA bisulfite sequencing of early-stage lung cancer tissue samples and found that DNA methylation patterns were differentially expressed in tumors and benign lesions in circulating tumor DNA (ctDNA). In addition, deficiency of ALKBH5-mediated m6A modification in osteosarcoma causes an increase expression of histone deubiquitinase USP22 and ubiquitin ligase RNF40, which inhibits histone H2AK199 monoubiquitination, induces the expression of genes related to DNA repair, and promotes the progression of osteosarcoma.52 The m6A RNA methylation in LINC01559 was found to inhibit the progression of colorectal cancer (CRC) by modulating the miR-106b-5p/PTEN axis.53 The m6A, m5C, m7G, and m1A-related genes have also been demonstrated to have a correlation with immunotherapy response in cervical cancer,54 HCC,55 and glioma.56 However, the mechanisms and exact functions of these modifications in cancer still need further investigation.
In addition, histone modifications are another common epigenetic mark that involves the maintenance of stability of chromatin, as well as dynamic cellular processes like transcription and DNA repair.57 A recent research demonstrated that the lactonization of H3 histone modification sites H3K9la and H3K56la promoted the progression of HCC.58 In addition, H3K14, H4K5, and H4K12 histone acetylation promote non-small cell lung carcinoma (NSCLC) cell growth.59 Different modifications of histones can be mutually modulated. For example, the deacetylation of AKAP12 at K531 by HDAC6 can increase its level of ubiquitination, promotes the proteasome-dependent degradation of AKAP12 and facilitates colon cancer metastasis.60 H3K27 acetylation-activated COL6A1 inhibits STAT1 by interacting with SOCS5 in a ubiquitinated and proteasomal degradation manner thereby promoting osteosarcoma progression.61 Inhibition of histone deacetylase protein 6 (HDAC6) decreases ERK phosphorylation levels and inhibits cancer proliferation by upregulating microtubule protein acetylation.62 Histone methyltransferase DOT1L affects prostate cancer progression by increasing the methylation at H3K79 of MYC and decreasing expression of E3 ubiquitin ligases HECTD4 and MYCBP2.63 The methyltransferase SMYD3 mediates H3K4 trimethylation at the cold shock structural domain-containing protein E1 (CSDE1) and contributes to STAT1 dephosphorylation through stabilization of the T cell protein tyrosine phosphatase, which alters the antigenicity of tumor cells.64 The methyltransferases SMYD3 promotes RNF113A K20me3 methylation modification while impairing the interaction with phosphatase PP4, which downregulates its phosphorylation level, promotes and maintains RNF113A E3 ligase activity, and inhibits chemo-sensitivity in small cell lung cancer.65 In a word, histone modifications act as a part of epigenetic area, they could participate in cancer progress alone or together with several different histone modifications form. Also, some epigenetic modifications among cancer seem to be involved in immune cells or immunotherapy. As the epigenetic modifications and immunotherapy are believed to be hot pot in recent cancer researches. So, it is urgent to figure out the specific mechanism of epigenetic modifications in immune modulation.
3.2 Epigenetic modifications of immune cells in the TIME
In recent years, the contribution of the immune system in restraining the development and progression of cancer has been largely acknowledged. New evidence is emerging that tumors usually hijack diverse epigenetic mechanisms to evade immune system constraints, and the immune cells engaged in antitumor response also may be influenced by altered epigenomes.66
3.2.1 DNA/RNA methylation modifications of immune cells in TIME
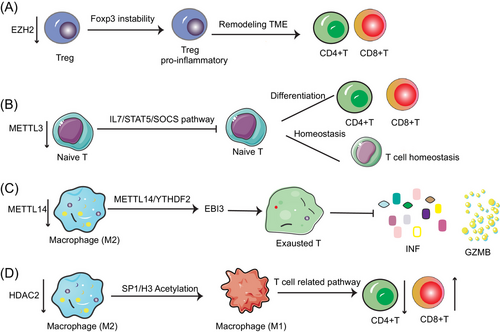
Epigenomic profiles of immune cells can serve as potential predictors for the evaluation of tumor immunotherapy. In addition, since epigenetic modifications are dynamic, they are expected to be targets for modulating immunotherapy.67 DNA methylation, as a kind of typical epigenetic modifications, has been shown to mold the epigenetic landscape of CD8+ T cells transfer to exhausted T cells or T memory cells, which can be persistent over time.68 It has been shown that treatment with demethylating agents recovered the immunogenicity of cancer cells, while enhancing the capacity of CD8+ T cells to kill tumors.69, 70 Additionally, RNA methylation can exert influence on immune cell maturation and function to modulate tumor immunity in the TIME. For instance, the deficiency of METTL3 (m6A “writer” protein) in T cells destroys T cell homeostasis and differentiation by targeting the IL-7/STAT5/SOCS pathway. That is because deficiency m6A modification in T cells inhibits SOCS mRNA degradation, which in turn inhibits IL-7-mediated STAT5 activation and also inhibits the T cell homeostasis and differentiation.71 In addition, it was found that macrophage-specific knockdown of the m6A methyltransferase Mettl14 resulted in elevated expression levels of the cytokine subunit EBI3, which could lead to dysfunction of CD8+ T cells, thereby impairing its tumor elimination ability.72 In conclusion, methylation modifications had a potential to control immune cell phenotype (such as T cells), immune cell maturation and function, and modulating immune-related pathways.
3.2.2 Histone modifications of immune cells in TIME
The effect of epigenetic modifications of histones in TIME should not be ignored. Epigenetic modulators have also been proven to be critical in controlling macrophage fate. And the histone-modifying enzymes usually work together with some transcription factors to regulate gene activity and perform essential roles in the development of antitumor immunity. For example, HDAC2 modulates the M2-like TAM phenotype through acetylation of the transcription factor SP1 and histone H3. The deletion of HDAC2 induced the transition from M2 to M1-like TAM, activated T-cell activation-related signaling pathways, reduced CD4+ T cells and increased CD8+ T-cell infiltration, and inhibited the lung cancer progression.73 Furthermore, downregulation of HDAC8 increased H3K27 acetylation, increased CD8+ T cell expression, and reactivated T cells to produce chemokines, thereby enhancing the immunotherapeutic efficacy of HCC.74 In addition, ketolysis can also alter the CD8+ T cell function by modulating histone acetylation.75 Inhibition of HDAC1 and HDAC2 also induced the expression of natural killer (NK) G2D (NKG2D) ligand and enhanced NK cell-mediated anticancer immunity.76 HDAC3 can modify H3K27ac of Cxcl10 promoter. And HDAC3-deficient HCC cells recruited CXCR3+ T cells into the tumor microenvironment to inhibit tumor growth.77 In addition, it was demonstrated that reduced epigenetic histone modification (such as H3K4me1, H3K9me2, H3S10p, and H2BK16ac) are associated with the death of mast cell HMC-1, providing new ideas for the pathogenesis of mast cell leukemia.78 In conclusion, histone modifications, especially HDAC-based deacetylation modifications, regulate immune cell differentiation and function. Notably, KAT6A acetylation of SMAD3 regulates MDSC recruitment and immunotherapy in triple-negative breast cancer (TNBC), providing new insights into the targeting of epigenetic factors in immunotherapy to enhance the therapeutic efficacy.79 This indicates that some nonhistone acetylation modifications also regulate immune cell fate.
3.2.3 Other chromosome modifications of immune cells in TIME
Polycomb family
The Polycomb family (PcG) is a group of highly conserved chromatin modifiers, mainly comprising Polycomb repressive complex 2 (PRC2), a methyltransferase for histone H3K27 methylation, and PRC1, which has E3 ubiquitin ligase activity.80 Members of PRC1 include the CBX family and the PHC family, while members of PRC2 include the EZH family, SUZ12, and EED. This family mainly works by modifying chromatin structure in order to maintain the silenced state of genes.
The PcG has been discovered to participate in modulating immune cell fate. CBX7 can activate CD4+ T cell polarization and apoptosis by regulating FasL expression.81 CBX4 deficiency leads to reduced accumulation of inhibitory histone modifications such as H2AK119ub1 and H3K27me3 of the PDCD1 promoter in T cells, and thus inhibiting antitumor immunity.82 In addition, the role of PRC2 member in immune cells also been researched. Inhibition of EZH1 can epigenetically remodel iPSCs to efficiently generate mature T cells for immunotherapy.83 Inhibition of EZH2 reduces antitumor immune efficiency by reprogramming CD8+ T cells and reducing their survival and effector functions.84 In bladder cancer, inhibition of EZH2 induces NK cell-mediated immune responses leading to tumor cell differentiation and death.85 Inhibition of EZH2 stimulated the production of chemokines such as CCL2 and CXCL9/10, leading to NK cell and T cell infiltration in pancreatic ductal adenocarcinoma.86 EZH2 maintains silencing on the FOXP3 gene through H3K27me3 labeling, which prevents overactivation of T cells and helps maintain immune tolerance.87 Similarly, EZH2 has been identified to inhibit NK cell proliferation and promote the progress of cancer cells through modulating the H3K27 methylation and downregulate NKG2D ligands.88 Also, EZH2 is involved in the regulation of epigenetic reprogramming in Treg cells, which resulted in a proinflammatory function and maintains the survival and function of effector CD4+/CD8 T cells through remodeling TIME.89 Currently, most studies on the effects of PcG members on immune cells have focused on PRC2, especially EZH2, while studies on PRC1 members such as the PHC family are almost absent.
CTCF
In addition, some transcription factors bind to DNA to ensure precise regulation of gene expression and maintenance of structural stability of chromatin. They are critical for biological processes such as cell development and differentiation. CTCF, a DNA-binding protein involved in the formation of chromatin circuits, which has two main functions, one is to act as a chromatin terminator, preventing chromatin regions from interacting, and the other is to act as a medium of chromatin interconnection, facilitating chromatin regions to interact.90-92 It has been shown that Tcf1 recruits CTCF and promotes chromatin interactions to regulate the genomic structure and homeostatic proliferation of CD8+ T cells.93 CTCF regulates CD8+ T cell heterogeneity and promotes terminal differentiation of CD8+ T cells by altering the transcription factor landscape and transcriptome interactions.94 CTCF is required to establish NF-κB-dependent chromatin interactions in DCs to promote inflammatory response.95 However, another research declaims that CTCF is dispensable for immune cell trans-differentiation although it affects the topologically associating domains but rather facilitates rapid responses to external stimuli.96 All in all, CTCF is involved in the regulation of gene expression in immune cells by modulating chromatin structure to ensure the differentiation and function of immune cells. Investigating the function of CTCF can help reveal the epigenetic regulatory mechanisms of immune cells and provide new targets and strategies for the cancer immunotherapy treatment. In conclusion, epigenetic modifications can modulate tumor immunity by regulating the function of multiple immune cell subtypes and can also reshape the immune response by regulating the expression of immune-related genes (Figure 2). Consequently, specific reprogramming through epigenetic modification of immune cells holds great promise for remodeling aspects of tumor microenvironment. Epigenetic modifications are largely dependent on various enzymes, and specific functions of epigenetic regulation are investigated by knocking down these enzymes. A number of drugs targeting these enzymes have also been developed, such as targeting DNA methyltransferases (e.g., 5-azacytidine, decitabine, and guadecitabine), EZH2 inhibitors (e.g., Tazemetostat and GSK126), HDAC inhibitors (e.g., vorinostat, romidepsin, and panobinostat) making research more convenient and laying the foundation for clinical translation of epigenetic modifications.
4 METABOLIC MODULATION
Metabolic processes are fundamental to life activities and are involved in all chemical reactions throughout an organism, supporting cell growth, division, and energy production. Modulating the activity and direction of metabolic pathways can affect the state and function of cells and organisms. The aim of this section is to explore the recent discoveries in metabolic modifications and their role in tumor and immune cell function and tumor progression.
4.1 Metabolic reprogramming in tumors
With a more sophisticated understanding of tumor biology and the complexity of tumor metabolism, it is now believed that the metabolic reprogramming is one of the hallmarks of cancer.97 There is no doubt that cancer cells have different metabolic states compared to normal cells, and cancer cells can show great metabolic diversity and plasticity in different tumors.98 Studies have shown that metabolic phenotypes vary at different stages of cancer development. For example, tumors need more nutrients to grow in the early stages, while during metastasis stage, new metabolic phenotypic dependencies often emerge due to the treatment resistance in tumors.99 The metabolic resources within local tumor tissues are limited, creating a tumor microenvironment of nutrient depletion and metabolic waste accumulation. In order to maintain growth under various conditions of nutrient distribution in the body, cancer cells utilize different metabolic adaptations. And the metabolites of cancer cells not only can be used as substrates for a source of energy, but also can adjust the expression of genes and proteins in normal cells so as to influence their behaviors.100 Luo et al.101 analyzed the metabolic phenotype of HCC cells and found that the expression of CD36 was positively correlated with the degree of glycolysis. Overexpression of CD36 induced an increase in mammalian target of rapamycin (mTOR)-mediated glycolytic flux and lactate generation through activation of the Src/PI3K/AKT signaling pathway, thereby promoting the progression of HCC.101 In non-small cell lung cancer, LINE-1 is activated by L1 antisense promoter (L1- asp) or reverse transcription to form L1 gene chimeric transcript, which participates in the metabolic process of tumors and the regulation of mitochondrial function, facilitating lung cancer progression.102 In addition, a strong link exists between tumor metabolism and immune cell metabolism, as metabolic changes in the TIME can affect immune cell function and response.
4.2 Metabolic modulation of immune cells
4.2.1 Influence of metabolism and its products on immune cell phenotype in the TIME
Cellular metabolism governs the differentiation of immune cells and regulates their function, which is one of the fundamental processes that sustains cellular life and is a critical driving force in determining cell fate.103 Since the tumor is a highly heterogeneous environment, each cell in the tumor exists in a different microenvironment, and each cell could also have its own metabolic state. The current understanding of the metabolic characteristics of immune cells has been inspired by the metabolism of cancer cells, though there are obvious discrepancies between cancer cell and immune cell metabolic reprogramming. Studies have found that the overall activity of metabolic pathways in tumor cells is higher and more plastic than in nontumor cells such as immune cells, which is more likely to lead to cell-specific metabolic reprogramming in the tumor microenvironment.104, 105 And the variation of mitochondrial activity is the leading cause of metabolic heterogeneity between tumor cells and nontumor cells. Moreover, these differences may provide opportunities to target immune cell metabolism as a tool to enhance the efficacy of immunotherapies.106 Interactions during metabolic reprogramming of cancer cells and immune cells are thought to be key determinants of antitumor immune responses to cancer. Among these, tumor metabolism can modulate antitumor immune responses by releasing metabolites and influencing the expression of immune molecules (e.g., lactate.), and in turn, the metabolic reprogramming of various types of immune cells is also essential in maintaining their own and the organism's homeostasis.9 Immune cells are able to recognize diverse signals of tissue metabolism (e.g., nutrient and oxygen consumption, and reactive nitrogen production) in the microenvironment and initiate specific immune functions.107, 108 Metabolites also contribute to the phenotypic transformation of immune cells. It has been shown that B-cell-derived metabolites and the neurotransmitter GABA promote the differentiation of monocytes into anti-inflammatory macrophages, which produce IL-10 and suppress CD8+ T-cell killing function, thereby inhibiting the antitumor response.109 Imbalances in the distribution of applicable nutrients, substrates, or other sources in immune cells can affect the metabolism of immune cells, and hence the function and destiny of the cells. What is more important is that whether metabolic changes are responsive or instructive throughout functional changes in immune cells is now still required more detailed researches.
4.2.2 Glucose metabolism
Glucose metabolism has always been the core of the cancer metabolism field, yet recent studies have demonstrated that glucose in the TIME is preferentially assigned to infiltrating immune cells rather than to cancer cells, which reveals an important function of immune cell glucose metabolism.110 Studies have shown that the LA in TIME suppresses immune cell function and leads to immune escape. And the high glucose induces a Warburg effect in γδ T cells, leading to lactate accumulation, which in turn leads to the loss of antitumor capacity in patients by inhibiting the activation of the AMPK pathway.111 In addition, recent research has assumed that the alterations in the metabolic landscape of TIME are associated with increased activity of Treg cells. Treg cells have been found to upregulate the metabolic pathway of LA to produce intermediates required for proliferation and maintain an inhibitory identity by exploiting metabolites in the TIME.112 In highly glycolytic tumors (e.g., HCCs), Treg cells take up LA via monocarboxylate transporter 1 (MCT1), which facilitates the entry of NFAT1 into the nucleus and thus increases PD1 expression, whereas effector T cells inhibit PD1 expression, resulting in a higher level of PD1 expression in Treg cells than in effector T cells.113 MCT leads to the establishment of an extracellular acidic TIME that facilitates the selection of more invasive tumor cells and suppresses T cell mediated antitumor immune responses to promote tumor progression.112 In addition, metabolic reprogramming of NK cells maintains their metabolic adaptation in the tumor state and also enhances their tumor-killing ability in the tumor microenvironment. In the TIME, the dysfunction of NK cells is mainly due to the inhibition of glucose metabolism by lipid peroxidation-related oxidative stress, whereas the function of NK cells can be recovered by activation of the Nrf2 antioxidant pathway and leads to stronger antitumor activity.114 Yet another study showed that LA was able to enhance antitumor immunity by suppressing histone deacetylase activity, leading to an increase in acetylation of H3K27 at the Tcf7 super enhancer site, thereby increasing the stemness of CD8+ T cells. Meanwhile, CD8+ T cells pretreated with LA in vitro were capable of effectively suppressing tumor growth.115 These researches revealed that glucose metabolism especially LA affect the immune cell function through MCT, signaling pathways or altering the epigenetic modification to modulating cancer progression.
4.2.3 Lipid metabolism
Fatty acids provide immune cells with cell membranes and other key lipid cell structures required for proliferation, and they can also be directly involved in signaling modulation within immune cells. For example, researchers have found that pentanoate and butanoate (short-chain fatty acids) regulated the metabolic (mTOR pathway) and epigenetic reprogramming (modulation of histone deacetylase activity), leading to elevated production of effector molecules such as CD25, IFN-γ, and TNF-α to enhance the antitumor activity of CAR-T cells.116 In addition, PGE2, a major metabolite produced from arachidonic acid, exerts an anti-inflammatory effect on natural immune cells such as NK cells, neutrophils, and monocytes. It can also participate in immune responses as an immunosuppressive factor.117-119 For instance, PGE2-EP2/EP4 signaling was proved to promote active inflammation by inducing NF-κB gene expression in myeloid cells and to trigger immunosuppression by activating mregDC (mature DCs enriched in immunoregulatory molecules) and Treg cells.120 In addition, PGE2 was reported to promote the transformation of M1 macrophages into M2 macrophages through cAMP/cAMP-responsive element binding signaling pathway.121 Cheng et al.122 revealed that there was aberrant lipid metabolism in HCC tissues by lipidomic analysis. And accumulation of long-chain acylcarnitines, such as palmitoylcarnitine and stearoylcarnitine, suppressed NKT cell expansion and facilitated their senescence, which weakened their immune surveillance ability against tumors.122 Research demonstrated that T cell functional status is a critical determinant of effective antitumor immunity and immunotherapy. And the lipid metabolism has been reported to govern T cell differentiation and effector functions. For example, suppression of lipid metabolizing enzymes (IVA phospholipase A2) reprogrammed effector T cells and thus blocking CD8+ T-cell senescence and boosts antitumor immunity and immunotherapeutic effects in melanoma and breast cancer.123 In conclusion, the effect of lipid metabolism on immune cells in tumors is a complex and dynamic process. Lipids maintain the energy balance of immune cells, modulate their polarization state and function, and lead to aberrant activation or inhibition of immune cell signaling pathways, thereby affecting their response to tumors. An in-depth understanding of these interactions can help develop more targeted immunotherapy.
4.2.4 Amino acid metabolism
Competition for amino acid metabolism also occurs in the tumor microenvironment. It was believed that increasing the level of amino acids in TIME was beneficial in enhancing the killing effect on immune cells. For example, glutamine is competitively consumed by effector T cells and TNBC cells, whereas specific blockade of glutamine uptake by TNBC cells using V-9302 (glutamine transporter protein inhibitor) drives glutathione synthesis and improves effector function of CD8+ T cells.124 In addition, obstruction of glutamine metabolism has been shown to enhance anticancer immunity. Huang et al.125 demonstrated that JHU083 (a glutamine antagonist) increased the infiltration of CD8+ T cells and CD4+ Th1 cells and decreased immunosuppressive cells, such as MDSC, Treg cells, resulting in strengthened immunoprevention of lung cancer. Additionally, blocking glutamine induced different metabolic processes could prevent tumor cells to immune escape. Powell et al.126 discovered that glutamine blockade inhibits oxidative and glycolytic metabolism in cancer cells, which leads to hypoxia, acidosis, and decreased nutrient consumption, while with the use of glutamine antagonists, effector T cell oxidative metabolism was significantly upregulated with a significantly activated T cell phenotype. Additionally, arginine metabolism plays a crucial role in T cell activation and regulation of immune responses. It sensed by BAZ1B, PSIP1, and TSN and could modulate metabolic processes from glycolysis to oxidative phosphorylation in activated T cells and promote the production of T memory cells.127 So, arginine supplementation and prevention of arginine degradation in TIME are promising approaches to activate T cells to trigger positive immune response. Similarly, the activation of T cells is critically sensitive to the concentration of tryptophan in the TIME, which is consumed by the metabolism process of tumor cells, triggering T cell apoptosis and inhibiting the tumor-killing function of T cells.128 Metabolic reprogramming of tumor methionine metabolism has been shown to be a potentially viable therapeutic strategy to enhance immunity in HCC. Hung et al.129 used the metabolomics analyses of HCC to demonstrate that elevation of 5-methylthioadenosine and s-adenosylmethionine induced T-cell exhaustion by reducing chromatin accessibility and thus promoted the HCC progression. In conclusion, amino acid metabolism plays a key role in tumor immunity. Arginine and glutamine are important substrates that influence the activity of macrophages, MDSC, NK cells, and T cells, whereas tumor cells may influence immune cell function by competing for and limiting amino acid supply.
4.2.5 Microbiome
The contribution of microbes in cancer development, diagnosis, prognosis, and therapy has received much attention in recent years. According to recent studies, microbes may be a key factor in cancer immunotherapy. The microbes in cancer cells and immune cells may be of the same origin. The microbes and their bioactive metabolites can be involved in immune regulatory processes in the development of tumors. Thus, there may be a common evolutionary dynamic between the host immune system, the microbiota, and tumor development.130 The researchers discovered that DCs from germ-free animals and those without IFN-I signaling had low levels of gene expression for genes involved in the mitochondrial respiratory chain. And further analysis demonstrated that the metabolism of the DCs cells from germ-free animals was abnormal and they were unable to initiate immune responses, which suggests that the microbiome plays a crucial role in the function of DCs.131 Studies have demonstrated a link between bile acid metabolism controlled by the gut microbes and liver antitumor immune surveillance. The intestinal microbes promote TLR7–MyD88 signaling in pDCs through secondary bile acid metabolism, and promote IFN production.132 In addition, Ma et al.133 found that gut microbes regulate CXCL16 expression through bile acid metabolism, which in turn modulates an increase in NKT cells, leading to an increase in IFN-γ production. Exposure to helicobacter hepaticus in a mouse model of CRC induced upregulation of specific T follicular helper cells (Tfh) and led to the maturation of tertiary lymphoid-like structures adjacent to the tumor, which inhibited tumor growth. This study demonstrates that antitumor immunity is not dependent on CD8+ T cells but on CD4+ T cells, NK cells, as well as B cells.134 Microbiome deficiency slowed melanoma-induced migration of intestinal NK cells and Th1 cells from the gut to the bone, thereby accelerating melanoma metastasis and intraosseous tumor growth.135 It was found that butyrate, a gut microbial metabolite, could contribute to the therapeutic efficacy of oxaliplatin by promoting the IL-12 signaling pathway, which enhanced the response of antitumor cytotoxic CD8+ T cells in an ID2-E47 dependent way.136 Peng et al.137 performed an analysis of the tumor microbiome of 53 gastric cancer patients and the gastric mucosal tissue microbiota of 30 chronic gastritis patients, revealing that Methylobacterium could reduce the expression of TGFβ and CD8+ T memory cells in tumors, and significantly correlate with the adverse prognosis of gastric cancer. It was found that deletion of the TAK1, an innate and adaptive immune molecule in myeloid cells, promotes the IL-1β and IL-6 signaling pathways (necessary for induction of Th17 cells) through the microbiome (Odoribacter splanchnicus), thus resulting in anti-CRC properties.138 Thus, the microbiome is also critical for the functional modulation of immune cells, and targeting the microbiome is a potential direction for cancer immunotherapy.
4.2.6 Relationship between mitochondrial function and the phenotype of immune cells
The membrane of mitochondria contains a large number of signaling molecules, which are important hubs for determining the fate of cells, and they serve important regulatory functions in cell signaling pathways and metabolism. It has been well established that mitochondria exert an essential driving role in the development and function of immune cells. For example, mitochondrial metabolism supports T cell anabolism by modulating key metabolic pathway cGAS–STING signal that control the fate and function of Treg cells.139 It has been shown that alterations in mitochondrial metabolism (involving oxidative metabolism, and the tricarboxylic acid cycle, mitochondrial membrane potential, reactive oxygen species (mtROS), DNA, and ultrastructure) may be the basis for macrophage activation, and that mitochondria may act as dynamic signals to modulate the immune response through modulation of macrophage biology.140 It was demonstrated that nanotubes transferred mitochondria from immune cells to cancer cells, which enhanced cancer cell metabolism and exhausted a large number of immune cells, while inhibiting the assembly of nanotubes greatly reduced the transfer of mitochondria and minimized the exhaustion of immune cells.141 Additionally, inhibition of the mitochondrial membrane protein FAM73b promotes Toll-like receptor-regulated (TLR)-mediated mitochondrial fission and induces IL12 production through stabilizing IRF1 in macrophages, thereby promoting CD8+ T cell activation to antitumor immunity.142 As an important hub for determining cell fate, mitochondria possess modulatory functions in cell signaling pathways and energy metabolism. The role of mitochondrial function in tumor cell and immune cell metabolism and dysfunction is a challenge in cancer immunometabolism research and might be a promising research area for cancer immunotherapy in the future.
4.2.7 Relationship between ions metabolic pathways and immune cells
In recent years, multiple ion metabolisms have been found to be involved in modulating cancer immunity. For example, magnesium ions could induce conformational changes of LFA-1 by binding to β1-MIDAS and α1-MIDAS on the membrane of CD8+ T cells, thus altering their cytotoxicity and promoting tumor cell killing functions.143 OTUD1 promotes the deubiquitination of IREB2, which inhibits the degradation of IREB2 protein and activates the expression of TFRC, thereby increasing the concentration of iron ions in tumor cells and enhancing the sensitivity to ferroptosis as well as the infiltration of CD8+ T cells, improving antitumor immune response.144 In addition, high potassium ions concentrations in tumors disrupt glutamine uptake and reprogram TAM metabolism from oxidative phosphorylation to glycolysis, thereby inhibiting the antitumor capacity of TAM.145 Studies have revealed that Mn2+ can effectively activate the cGAS–STING pathway, which significantly promotes the function of DC and enhances the survival, proliferation, and function of cytotoxic T cells and memory T cells and NK cells, thus promoting the tumor immunosurveillance effect.146 The researchers found that zinc ions released from ZnS@BSA nanoclusters under acidic TIM significantly enhanced cGAS/STING signaling and promoted the function of CD8+ T cells and DCs, which improved the immunotherapy effect of HCC147 (Figure 3).

Immunogenic cell death (ICD) is a novel tumor cell death manner that could promote the antitumor immune responses. Recent studies have indicated that some ions may modulate tumor immunity by triggering the ICD. For example, researchers used nanomaterials to construct a cocarrier with Cu2+ and DSF, which generate the cytotoxic metabolite DTC-Copper complex for tumor therapy. One of the active metabolites, CuET, can effectively induce ICD to modulate the TIME.148 In addition, other ions such as calcium was also reported to induce ICD in cancer.149 Therefore, ion metabolism targeting immune cells is also significant part in cancer immune therapy, and their roles and mechanisms for tumor immunotherapy are worth exploring. With the development of nano-based therapeutic biomolecule immunotherapies, targeted ion metabolism will have a broader future.
Cancer cells produce metabolites that can have profound effects on immune cells in the TIME. Targeting metabolic pathways in tumors facilitates alteration of metabolic competition between tumors and immune cells that increases the immunogenicity of tumors. The metabolic products produced by tumor cells are various and have different effects on immune cells. Immune cells can employ a variety of programs to respond to different incentives in the microenvironment, and they need to adapt to the cellular metabolism reprogramming in order to match the biochemical demands for each different functional state. For example, different states of T cell activation need metabolic programs appropriate to their functional requirements. Transitions among states are associated with active cellular metabolic reprogramming. And this requires the coordinated adaptation by a network of signaling and transcription factors for each transition.150 The shift from a resting to an activated state of immune cells involves the distribution of nutrients into different pathways, and therefore studying how metabolic pathways are modulated to directly change the immune cell function is critical to the immunotherapy of tumors.
5 COMMUNICATION AMONG IMMUNE CELLS IN CANCER
The immune system is a highly complex and dynamic network that relies on sophisticated regulation and communication between immune cells to maintain the health of the organism and combat disease. This communication mechanism involves a series of complex signaling pathways, including exosomes, and cytokine release and surface molecule interactions in the TIME. The aim of this section is to explore recent research advances in immune cell communication, focusing on the role of these communication mechanisms in cancer immunity.
5.1 Exosome
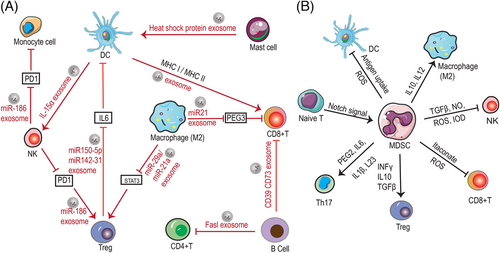
Exosomes, as an emerging part of tumor–host interactions, which are key mediators of signal transfer between secretory cells and target cells, are now increasingly considered as message carriers and key molecules in TIME. Exosomes derived from immune cells have immunomodulatory properties and promising therapeutic properties that providing an important role in cancer therapy. For example, M2 macrophage-derived exosomes miR21 interacts with CD8+ T cells through inhibiting the expression of PEG3, which results in a decrease amount of CD8+T cells and inhibits immune response in glioma.151 In addition, M1 macrophage-derived exosomes can facilitate T-cell production of IFN-γ and thus promoting immune response.152 DC-derived exosomes directly interact with specific cytotoxic T lymphocytes (CTL) and promote the activation of CD8+ and CD4+ T cells to inhibit tumor growth.153 DC-derived exosomes express IL-15Rα, leading to amplification of NKs and secretion of IFN-γ, which induces tumor regression.154 B cell-derived exosomes with CD73, CD19, CD39, C3, FasL proteins, and integrins on their surface can regulate T cells function and thus influence tumor progression through some specific factors.155 MHCII(+) FasL(+) exosomes secreted by B cells that induce the apoptosis of CD4+ T cell.156 Treg-derived exosomes containing miR-150-5p and miR-142-3p can be delivered to DCs to produce more IL-10 and less IL-6 thereby suppressing immune cell activity.157 TAM-secreted exosomes containing miR-21-5p and miR-29-3p promote the progression of epithelial ovarian cancer by suppressing STAT3 expression and inducing an imbalance of Treg/T helper cell (Th)17.158 In addition, mast cell-derived exosomes contained heat shock proteins have been reported to induce DC maturation.159 NK cells secrete exosomes contained miR-186 that stimulate other immune cell (such as T cells and monocytes) functions and also attenuate the immunosuppressive effects of tumor cells by reducing PD1 expression.160 As mentioned above, a variety of immune cells possess the function of secreting exosomes, which contain complex biological signals that interact with other immune cells as well as tumor cells, thereby killing tumor cells through a variety of mechanisms. Exosomes carry biomolecules with the capacity to regulate the immune response, such as cytokines, miRNAs, and other regulatory substances. These molecules, delivered through exosomes, can influence the immune status of recipient cells and thus regulate the activity and function of immune cells. Exosomes transmit information between different types of immune cells, prompting them to collaborate with each other to perform specific immune tasks. This intercellular communication helps to create synergies and increase the efficiency of the immune response.
5.2 TIME
The TIME, which offers conditions for the tumor cells to grow, is a deeply immunosuppressive environment. In this section, we will focus on immune cell types that are directly engaged in regulating tumor-killing activity, such as T cells, NK cells, and MDSC. Among them, MDSC are considered to be the immune cells that make the greatest contribution to the development of the TIME.161 The MDSC usually including tumor-associated macrophages (TAM) and tumor-associated neutrophils. MDSC can not only act as a source of TAM in tumors, it can also influence macrophage polarization, activation and function.162 In the tumor environment, MDSC produce IL10 to regulate IL12 production in macrophages, thus playing an important role in macrophages’ polarization toward the M2 phenotype.163 In addition, MDSC have also been reported to affect MHC II expression in macrophages possibly.164 The interactions that occur between MDSC and macrophages aggravate the suppression of immune cells in TIME through changing the production of cytokines and the expression of some key molecules. However, there is very few literatures to introduce the interaction between neutrophil and MDSC, and these field remains to be investigated.
As reported, researchers cocultured MDSCs with NK cells and found that the MDSCs can inhibit the tumor cytotoxic activity of NK cells by regulating TGFβ and resulted in immune tolerance.165 Previous studies have suggested that the ability of MDSC to mediate NK cell unresponsiveness may be related to the MDSCs-mediated downregulation of CD247 expression on the surface of NK cells.166 Furthermore, MDSCs can also excrete diverse soluble elements, such as nitric oxide-inducible nitrogen oxygenase and ROS in order to enhance immunosuppression and suppress NK cell activation.167 In addition, MDSCs also generate IDO to reduce the expression of DNAM1, NKG2D, and NCR, thereby blocking NK cell activation.168
Recently, it was demonstrated that MDSCs could secrete itaconate, which could be absorbed by CD8+ T cells and inhibit the properties of CD8+ T cells proliferation and also inhibit the production and activity of cytokine, thus promoting the growth of melanoma.169 Another study demonstrated that dysregulation of notch signaling in immature T cells facilitated the formation of CD11b+Gr-1+ MDSCs, while deficiency of anti-Gr-1-mediated MDSCs reduces the malignant T-cell proliferation and expansion in acute lymphoblastic leukemia (ALL).170 In oral squamous cell carcinoma, MDSCs induced the generation of Treg cells by inhibiting TGF-β and promoted the differentiation of Th17 cells by secreting PGE2, IL-1β, IL-6, and IL-23.171 In addition, MDSC facilitate natural Treg cell expansion and promote the development of induced Treg cells by producing IFN-γ, TGFβ, and IL-10.172 MDSC suppresses T cell activation by generating ROS and RNS and depleting arginine and cysteine, which are both required for the activation and proliferation of T cells.173 In addition, mutual crosstalk between T follicular helper cells and B cells in TLSs plays an important function in antitumor immunotherapy.174
MDSC can increase immunosuppressive function through interactions with DC. The impaired cross-presentation of DC in cancer may be one of the barriers to successful cancer immunotherapy. In melanoma, MDSC reduces antigen uptake and prevents DC maturation, while simultaneously obstructing the capacity of DCs to activate T cells.175 It has been reported that MDSC prevents cross-presentation of DC while not interfering the direct presentation of DC to antigen, which process was linked to lipid peroxidation and requires no direct cell-to-cell contact.176 The MDSCs have been shown to inhibit not only DC function, but also their maturation and progression. A recent study examined the expression of CD11b and CD33 (MDSC markers) as well as CD303 and IDO1 (DC markers) in patients with OSCC and analyzed their relationship with clinicopathological parameters, and the results showed a positive correlation between these biomarkers. In addition, MDSC and DC were highly expressed in OSCC patients, suggesting that MDSCs and DCs infiltration was significantly correlated with the progression of OSCC.177
In addition, apart from immune cells, TIME contains fibroblasts, endothelial cells, epithelial cells, and so on; these nonhematopoietic cells have the ability to generate immunomodulatory factors and also participate in the interactions between the tumor and the immune system.178 Some components of TIME can produce cytokines, chemokines, and so on, which modulate the function of immune cells through a variety of signaling mechanisms. For instance, cancer-associated fibroblasts modulates the tumor immune response by secreting cytokines, chemokines, and so on, which regulate macrophage polarization179, 180 and influence the interaction between immune cells such as DC cells and T cells.181, 182 In addition, TIME also have affected the activity of immune cells and thus impacted their ability to attack cancer cells. Comprehension of the interactions between immune cells in TIME is crucial for the clinical application of tumor immunotherapy (Figure 4).
The communications between immune cells have important implications in cancer immunotherapy. In recent years, some bispecific antibodies have brought different immune cells closer together (e.g., DCs and T cells) and increased mutual communication, which is considered a novel cancer immunotherapy option.183, 184 This novel therapy using bispecific antibodies to increase DC-T cell interactions promotes both T cell activation and function as well as cDC1 maturation.185 On the one hand, communication between immune cells helps modulate and coordinate the immune response. Different types of immune cells can interact with each other through cytokines, chemokines, and other signaling molecules to ensure the timely, intensity, and direction of the immune response. On the other hand, lack of communication or miscommunication between immune cells might result in the inability of immune cells to recognize and attack tumor cells in a prompt manner. Thus, tumor cells could take advantage of the communication between immune cells to establish immune tolerance and thus evade immune attack. In conclusion, understanding and regulating the communication between different immune cells can provide important guidance for the development of more effective immunotherapies.
6 RELATIONSHIPS BETWEEN IMMUNE CELL METABOLISM, EPIGENETICS MODULATION, AND CANCER IMMUNOTHERAPY CLINICAL EFFICIENCY
Available evidence demonstrates that metabolism and epigenetics can collectively regulate immunotherapy efficiency. The metabolic modifications, epigenetic remodeling, and transcriptional modulation influenced the reactivation of exhausted CD8+ T cells in the TIME, which are critical regulators for cancer immunotherapy.186 Immune cells require large amounts of energy and metabolites to maintain their activity in response to tumors. And the immune cell response to cancer is largely relying on the immune cell type and functionally specific metabolic programs. So, an intervention in metabolism can significantly improve the efficacy of cancer immunotherapy.187 A variety of metabolic mechanisms can coordinate with immune cell behaviors in response to reprogramming in the TIME, which modulates the efficacy of antitumor immunity.188 NF-κB-inducible kinase promotes CD8+ T cell metabolism and activation through metabolic reprogramming toward aerobic glycolysis, thereby enhancing the therapeutic efficacy of T cell adoptive therapy (tumor size reduction).189 Studies have shown that reduction of glutamine activates CD8 function, leading to better clinical benefits of neoadjuvant immunotherapies in combination with ICI therapy.190 Nicotinamide phosphoribosyltransferase (NAMPT) was necessary for the activation of T cells, and supplementation with NAD(+) could augment the tumor-killing effect of T cells in CAR-T and ICI treatment by rescuing TUB-mediated NAMPT transcription in T cells.191 In addition, monoamine oxidase A, an important enzyme in the metabolism of monoamines, could lead to T cell dysfunction, which reduces the cancer immunotherapy efficacy of immune checkpoint therapy and is strongly associated with lower survival of various cancers.192 In addition, targeting the metabolism of MDSC contributes to the efficacy of antitumor immunotherapy.193 Tumor-derived immunoglobulin-like transcript 4 (ILT4) and PIR-B (ILT4 homolog) induce effector T cell senescence by activating the MAPK ERK1/2 signaling pathway to increase fatty acid synthesis and lipid accumulation in tumor cells, increased the survival in patients with ICI treatment.194 The researchers discovered that the tumor-infiltrating T cells (especially CD8 T cell exhaustion and Treg suppression) presented the highest active levels of Gln metabolism among immune cells by building a quantitative system of Gln metabolism, which can be used to predict the prognosis and the immunotherapy efficacy including ICI and T cell adoptive therapy in lung cancer.195
The epigenetic gene PR domain zinc finger protein 1 (PRDM1) knockdown in T cells showed an increased chromatin accessibility and promoted the expansion of memory CAR-T cells, which enhanced T cell durability and improved the efficacy of cancer immunotherapy and improved survival rate.196 The ubiquitin ligase MDM2 reduces c-Cbl-mediated STAT5 degradation by competitive binding to STAT5 with c-Cbl, thereby enhancing tumor-infiltrating CD8+ T cell-mediated antitumor immunotherapy (tumor size reduction).197 Studies have shown that DNA-methylated CD8+ TIL cells are reported to assess immune response and prognosis in CRC.198 In addition, the m6A modifiers have also been considered as biomarkers for assessing cancer immunotherapy response. Researchers constructed the m6A regulatory prognostic risk score (MRPRS) to assess the survival and immunotherapy efficacy in clear cell renal cell carcinoma, revealing that MRPRS is a potential hopeful biomarker for immunotherapy response assessment.199 Protein tyrosine phosphatase receptor type O significantly affects CD8+ T cell infiltration in breast cancer and it could be used to predict immunotherapy efficacy and prognosis in breast cancer.200 Furthermore, the epigenetic reader SP140, a regulator of the macrophage transcriptional program, was found to trigger IFN-γ production in TAM by inhibiting STAT1 transcription and phosphorylation. And higher level of SP140 was associated with higher immune scores, infiltration of CD8+ T cells and TAM, which was a potential predictive biomarker of immunotherapeutic response in metastatic melanoma.201
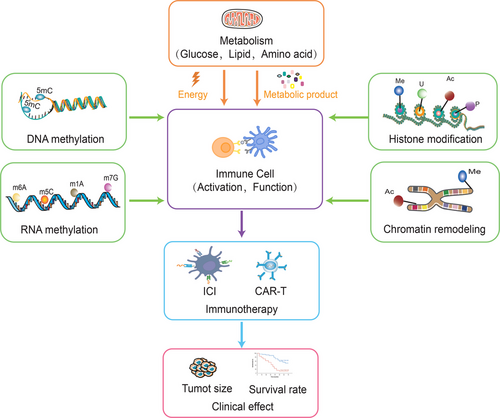
Metabolic reprogramming and epigenetic reprogramming are connected to each other, and to a large extent, metabolic status determines the epigenetics of cancer. Epigenetics modulates the expression of metabolism-related genes and thus exerts an essential role in the metabolism of cancer cells. Epigenetic modifications can also affect the transcriptional regulation of immune cells, thereby influencing the activation and inhibition of their metabolic pathways. Meanwhile, metabolic fluxes are engaged in epigenetic modulation through impact on biosynthesis and energy production of macromolecules such as nucleic acids and proteins. Epigenetic regulation of immune cells can influence the differentiation and activity of immune cells, while metabolic status influences the energy supply and reactivity of immune cells, and they are participated in cancer immunotherapy through immune cells as a bridge (Figure 5). Therefore, exploring the interplay between epigenetic and metabolic regulation in immune cells could provide new ideas for cancer immunotherapy.
7 CANCER IMMUNOTHERAPY: INHIBITOR APPLICATIONS, CLINICAL TRIALS, BIOMARKERS, AND MOUSE MODELS
7.1 Inhibitors in the field of cancer immunotherapy
Different immune cell compositions in TIME can influence the immune checkpoint inhibitor (ICI) response to tumor therapy.202, 203 Immune checkpoint receptors such as CTL-associated protein 4 (CTLA4), PD1, T-cell immunoreceptor with Ig and ITIM structural domains (TIGIT), and lymphocyte activation gene 3 (LAG3) could modulate the activation, differentiation, and function of T cells. For example, PD1 directly modulates TCR signaling to attenuate T cell activity. Additionally, deficiency of CTLA4 in Tregs induces aberrant T cell activation and causes autoimmunity. And deletion of Treg could counteract the expansion of Treg cells induced by CTLA4 blockade, thereby improving the efficacy of anti-CTLA4 therapy.204 Anti-TIGIT targeting Tregs possesses strong potential for cancer immunotherapy,205 and targeting Tregs may also contribute to cancer immunotherapy efficacy when used as monotherapy or in combination with ICB antibodies.206 Recent studies have shown that sialic acid-binding immunoglobulin-like lectin (Siglec) receptors on TIICs have an interplay with glycan-containing sialic acid in the tumor microenvironment, which could be a novel immune checkpoint and a promising potential target for cancer immunotherapy. The Siglec receptor is widely expressed on different immune cells including T cells, DCs, NK cells, neutrophils, and macrophages. And the interaction between Siglec receptors and sialic acid glycan ligands on these immune cells helps to establish an immune suppressive microenvironment and suppress antitumor immunity in the cancer microenvironment.207 More importantly, studies have reported that Siglec-15 can be targeted by the blocking antibody NC318, and it has been shown in early clinical trials that the antibody is responsive to cancer immunotherapy in some patients.208 This suggests that various immune checkpoints can influence tumor immunotherapy by influencing the function and activation of immune cells and modulating the activity and specificity of the immune system.
ICI therapy is one of the most widely utilized cancer immunotherapies at present. Currently, drugs against immune checkpoints focus on targets such as PD1, PDL1, and CTLA4. Among them, PD1 inhibitor nivolumab (trade name Opdivo) used to treat a variety of cancers, including melanoma, NSCLC, renal cell carcinoma, and head and neck cancer.209-212 Pembrolizumab (Keytruda): used to treat melanoma, NSCLC, head and neck cancer, Hodgkin's lymphoma, and so on.213-215 And PDL1 inhibitor, atezolizumab (Tecentriq), is mainly used for the treatment of uroepithelial cancer, HCC, and NSCLC.216-218 Avelumab (trade name Bavencio) is mainly used for the treatment of Merkel cell carcinoma and uroepithelial carcinoma.219, 220 Durvalumab (trade name Imfinzi) is mainly used for the treatment of NSCLC and bladder cancer.221 Moreover, CTLA4 Inhibitors Ipilimumab (trade name Yervoy) initially used to treat advanced melanoma, now also used for other cancer types.222 Notably, the range of indications for these drugs will continue to expand based on new clinical trial results and regulatory approvals. In addition, certain drugs may be more effective in specific patient populations.
While ICIs have achieved remarkable success in cancer treatment, they may also affect noncancer cells, leading to a range of immune-related side effects such as autoimmune reactions, inflammatory responses, and so on.223, 224 Some patients may acquire resistance to ICIs, and further research is needed to address the challenges associated with side effect management, resistance, treatment failure, and cost and accessibility. The combination of ICIs with other treatments, such as radiotherapy, chemotherapy, targeted therapy, or other immunotherapies, is being studied extensively. Such combination therapies may improve treatment efficacy and overcome the limitations of monotherapy.
7.2 Latest clinical trials of immune cell-based cancer immunotherapy
There are two main types of immune cell-based cancer immunotherapies currently on the market or approved, namely DC therapy and CAR-T cell therapy. In 2010, the DC therapy Sipuleucel-T (trade name: Provenge) was approved for the treatment of metastatic desmoplasia-resistant prostate cancer that is asymptomatic or only mildly symptomatic.225, 226 In 2017, the CAR-T therapy Tisagenlecleucel (trade name: Kymriah) was approved for the treatment of ALL,227, 228 and in the same year, the Axicabtagene ciloleucel (trade name: Yescarta) was approved for the treatment of diffuse large B-cell lymphoma.229, 230 In addition, Brexucabtagene Autoleucel (Tecartus) is approved for the treatment of relapsed or refractory B-cell precursor ALL in adults.231 Lisocabtagene Maraleucel (Breyanzi) is approved for the treatment of relapsed or refractory large B-cell lymphoma.232 Additionally, there are also many immune cell therapies in clinical trials.
Clinical trials allow doctors to assess the accuracy and reliability of new diagnostic and therapeutic techniques to provide more appropriate treatment options for patients. Today, immune cell therapies are attracting a lot of attention in cancer immunotherapy, and scientists are continuing to improve therapies and trial methods. Below are some examples of clinical trial results for cancer immunotherapy based on immune cell therapies that have been published in the recent 2 year, providing new discoveries and understanding for fighting cancer (Table 1).
| ClinicalTrials number | Cancer type | Patient amounts | Target | Agent | End points | Phase | Recruitment status | References |
|---|---|---|---|---|---|---|---|---|
| NCT03607539 | NSCLC | 397 | TIL/PD1 | Sintilimab | PFS/OS/ORR | Phase 3 | Completed | 233 |
| NCT02278887 | Melanoma | 168 | TIL/CTLA4 | Ipilimumab | PFS/OS | Phase 3 | Active, not recruiting | 234 |
| NCT02652455 | Metastatic melanoma | 11 | TIL/PD1 | Nivolumab | Safety/feasibility | Phase 1 | Completed | 235 |
| NCT02421640 | Nasopharyngeal carcinoma | 156 | TIL | – | Safety/objectivity | Phase 1 | Unknown states | 236 |
| NCT04555551 | Multiple myeloma | 17 | CAR-T/ GPRC5D | lentiviral vector containing GPRC5D CAR | Safety | Phase 1 | Active, not recruiting | 237 |
| NCT04093596 | Multiple myeloma | 43 | CAR-T/ BCMA | ALLO-715 | Safety/tolerability | Phase 1 | Active, not recruiting | 238 |
| NCT02640209 | Chronic lymphocytic leukemia | 20 | CAR-T/ CD19/Bruton's tyrosine kinase (BTK) | Ibrutinib | Safety/feasibility | Phase 2 | Terminated | 239 |
| NCT04148430 | Lymphoma | 31 | CAR-T/ CD19/IL-1R | Anakinra | Severe (grade≥3) ICANS | Phase 2 | Active, not recruiting | 240 |
Researchers conducted a large-scale randomized controlled phase 3 clinical trial (NCT03607539) to explore the relationship between TIL and the efficacy of immunotherapy in 397 advanced NSCLC patients, where the primary endpoint was progression-free survival (PFS) while OS and objective remission rates (ORR) were secondary endpoints. The results showed that combination therapy of chemotherapy plus sintilimab (anti-PD1) was associated with statistically significant improvements in PFS (HR = 0.12, 95% CI: 0.06–0.25, p < 0.001) and OS (HR = 0.27, 95% CI: 0.13–0.55, p < 0.001) only for subtype II (PDL1+ and TIL+) compared with chemotherapy, which further illustrates the critical role of immune cells in cancer immunotherapy.233 Recently researchers conducted a phase 3 multicenter open-label clinical trial (NCT02278887) in which 168 patients with advanced melanoma were randomly assigned to receive either TIL or ipilimumab, with the results revealing a median PFS of 7.2 months (95% CI, 4.2–13.1) and median overall survival was 25.8 months (95% CI, 18.2 to not reached) in the TIL group while PFS of 3.1 months (95% CI, 3.0–4.3) and 18.9 months (95% CI, 13.8–32.6) in ipilimumab group.234 Researchers reported a phase I clinical trial (NCT02652455) of nivolumab in combination with TIL ACT for the treatment of melanoma which proved to be a safe and feasible treatment.235 A phase I clinical trial (NCT02421640) of 156 nasopharyngeal carcinoma patients using TILs following concurrent chemoradiotherapy was determined to be safe and objective.236 This demonstrates the potential survival benefit of TILs in patients with low levels of circulating CD8+ T cells and PDL1 expression.
Recent results from a Phase I clinical trial (NCT04555551) of CAR-T cell therapy (MCARH109) targeting GPRC5D confirmed that GPRC5D is an important immunotherapeutic target in multiple myeloma.237 Researchers conducted a phase 1 clinical trial (NCT04093596) in 43 patients with multiple myeloma evaluating allogeneic anti-BCMA CAR-T cell therapy (ALLO-715). Overall, 24 patients (55.8%) responded, with 17 (70.8%) in remission, 11 (45.8%) in partial remission or better, and six (25%) in complete remission, demonstrating the viability and safety of allogeneic CAR-T cell therapy.238 In addition, a phase II clinical trial (NCT02640209) of autologous anti-CD19 humanized binding domain T cells (huCART-19) added to Bruton's tyrosine kinase (BTK) inhibitor ibrutinib for 20 patients with B-cell chronic lymphatic leukemia and the results indicated that patients in the huCART-19 group had higher rates of remission with favorable safety and feasibility outcomes.239 Investigators conducted a phase II clinical trial (NCT04148430) of the IL-1 receptor antagonist (IL-1Ra) anakinra in patients with relapsed/refractory large B-cell lymphoma who were treated with anti-CD19 CAR-T cells. The results showed that anakinra treatment reduced immune effector cell-associated neurotoxicity syndrome (ICANS) while not damaging the efficacy of anti-CD19 CAR-T therapy.240
In addition, there are many ongoing clinical trials of immune cell-based cancer immunotherapies, including NKT cell therapy, NK cell therapy, CAR-M, LAK cell therapy, TCR-T cell therapy, DC-CIK cell therapy, and so on. For details on the progress of these clinical trials, please refer to https://clinicaltrials.gov/. Among them, CAR-M and NK cell therapies are already in the late clinical stages, and the results of their clinical trials are extremely promising.241
7.3 Potentially available biomarkers for evaluation of cancer immunotherapy
There is no doubt that the advent of immunotherapy has provided a new and more effective therapy for the prevention and treatment of tumors. However, it is urgent to evaluate and predict the efficacy of immunotherapy and thus improving the effectiveness of immunotherapy, since immunotherapy cannot be suitable for all patients. The Cancer ImmunoMonitoring and Analysis Center and the Cancer ImmunoData Commons have been established and are able to systematically identify biomarkers and correlate them with clinical outcomes, which is essential for enhancing immunotherapeutic efficiency for cancer patients.242
It has been believed that CTLA4, TMB, and SMACA4 mutations are considered as potential predictive biomarkers of ICB efficacy in EBV virus-associated gastric cancer.243 A recent study showed that the expression of PDL1, B7-H3 and VISTA as well as some tumor necrosis factor receptor superfamily such as OX40L, CD27, 4-1BB, CD40, and CD95/Fas were correlated with the head and neck squamous cell carcinoma immunotherapy efficiency, and could serve as potential biomarkers to predict the immunotherapy response.244 Using meta-analysis, Zhou et al.245 discovered that high expression of PDL1 in tumor cells instead of tumor tissues was associated with better prognosis, and PDL1 could be used as a prognostic biomarker of immunotherapy efficacy for HCC.
However, tumor tissue biopsies are invasive and cannot obtain the full spatial heterogeneity of the tumor, as well as being difficult to follow up on treatment efficacy. In recent years, it has been found that cancer immunotherapy efficacy can be reflected by comprehensive analysis of systemic immune events through peripheral blood immune cell subpopulations. Thus, circular immune cell-based biomarkers can assess and provide guidance for cancer immunotherapy.246 Liquid biopsies can be used to assess the progression of tumors and the efficacy of clinical therapies during cancer immunotherapy. Among them, ctDNA could be used as a tumor load biomarker to assess the efficacy of immunotherapies.247 In addition, PDL1 in circulating tumor cells and CD4/CD8+ T cell populations may serve as prognostic biomarkers for immunotherapy in metastatic genitourinary cancer patients.248 GNG4 is expressed typically in exhausted CD4+ T cells, and its high expression is correlated with a higher immune cell infiltration level and a better immunotherapy response, which could be recognized as a biomarker for assessing the immunotherapy efficacy in bladder cancer.249 A recent study has indicated that the tumor mutational load in blood is expected to be a biomarker for the prediction of the efficacy of immunotherapy in NSCLC.250 Researchers found that patients with high expression of LAG-3 in the surface of CD8+ T cells in peripheral blood were less responsive to immunotherapy through analysis of peripheral blood cells from melanoma patients. At the same time, they suggested that LAG-3 protein could serve as a potentially vital biomarker for predicting the efficacy of ICB.251 Study demonstrates that circulating extracellular vesicles expressing CD81 may serve as a possible biomarker of ICI response in patients with low levels of PDL1 expression in advanced NSCLC.252 Serum biomarkers, the CEA and Ca-125, potentially serve as biomarkers for predicting the immunotherapy efficacy in NSCLC treated with PDL1 inhibitors.253 Circulating soluble programmed death ligand 1 (sPDL1) is a prospective dynamic biomarker that negatively regulates the function of T cells, evaluates the efficacy of immunotherapy in pMMR CRCs, and can serve as a predictive prognostic marker in a diverse range of cancers.254
In recent years, with the development of bioinformatics, some novel potential markers to predict cancer immunotherapy efficacy have been identified such as PTTG,255 EXTL3,256 CTNNB1,257 CXCL5,258 APC,259 and TASL.260 Researchers identified 11 immune-related gene prognostic indicators that have the capacity to predict immune cell infiltration in the tumor microenvironment, the efficacy of immunotherapy and can more accurately predict the survival of HCC patients.261 In addition, the researchers compiled a list of 52 candidate genes that could possibly predict the efficacy of ICB therapy, based on data from a cohort of 350 patients with NSCLC. These candidate genes are characterized by compound mutations that might be better predictors than TMB and could serve as new biomarkers for assessing the NSCLC immunotherapy efficacy.262 Researchers developed a computational model of resilient T cells (Tres) using single-cell transcriptomics data to recognize immunosuppressive signatures (e.g., TGFβ1 and PGE2). It is a reliable predictor of clinical response to immunotherapy in a wide range of tumors including melanoma, TNBC, and lung cancer. The Tres revealed that FIBP is the major negative biomarker of tumor resilient T cells and can be used to assess the immunotherapy response in solid tumors.263 An immune infiltration score (IIS) was calculated based on immune cell infiltration in the cancer microenvironment. And patients with higher IIS had significantly higher PDL1 expression and shorter PFS time, which can be used as a novel and reliable biomarker to assess the prognosis of TNBC patients.264 A recent study found that using spatial quantification of CD8 and PDL1 markers to quantify immune scoring is a novel in vitro diagnostic methods, which is capable to predict the efficacy of anti-PD1/PDL1 immunotherapy in NSCLC, and is a potential powerful tool for evaluating the outcomes of cancer immunotherapy.265 The researchers tried a new method to automatically select tissues and calculate the frequency of cell to cell spatial interactions happening in the PD1/PDL1 pathway, which could be used to reveal spatial interactions for immune escape and provide potential prognostic information for oropharyngeal squamous cell carcinoma.266
In addition to the classical immune checkpoints and immune cells, other new areas such as cancer stemness, ferroptosis, pyroptosis, lncRNAs, and radiogenomic traits have also been considered as potential markers for cancer immunotherapy efficiency. Researchers have found that cancer stemness is a key reason for ICI resistance by comprehensively analyzing single-cell and bulk RNA sequencing data, and that cancer stemness gene expression profiles can be used as biomarkers of cancer immunotherapy efficacy.267 In addition, the ferroptosis score is strongly associated with the efficacy of immunotherapy in colon cancer. Where patients with high ferroptosis score (characterized by high TMB and MSI-high subtype) showed poor prognosis using immunotherapy. Among them, ALOX5 is a key ferroptosis gene identified as a predictive marker for immunotherapy efficacy.268 Yet another study found that cancer patients with high ferroptosis scores displayed high CD8+ T cell and TIL infiltration as well as enrichment of immune-related signaling pathways, which could be a good prognostic factor and an assessment of the immunotherapy effect.269 In addition, the researchers also elucidated that tumor-infiltrating B-lymphocyte lncRNA signature can be used as an index of immune cell infiltration in TIME and lncRNA is expected to be used as a potential biomarker for the prediction of immunotherapy response in bladder cancer.270 Also, some pyroptosis-related genes and immune-related lncRNAs have been recognized as potential prognostic markers of cancer immunotherapy.271, 272 In addition, radiogenomic biomarkers to predict therapeutic response to PD1/PDL1 immunotherapy in NSCLC have also been discovered.273
In summary, cancer immunotherapy is a rapidly evolving field, and a growing number of potentially usable biomarkers for predicting and evaluating cancer immunotherapy have emerged. Taken together, these biomarkers allow researchers and clinicians to better understand which patients are most likely to benefit from immunotherapy, thereby personalizing treatment plans and improving outcomes. Currently, there are two major limitations in evaluating potential biomarkers for cancer immunotherapy: first, although many promising markers have been identified, such as PD-L1 expression, tumor mutational load (TMB), and immune cell infiltration, the predictive power of these markers is not always consistent and exhibits variability in different types of cancer. Second, the process of biomarker discovery and validation is complex and time consuming, and there is a lack of uniform standards and methods to assess and validate the clinical application value of new markers, which limits their widespread use in personalized immunotherapy. As more studies are conducted, it is expected that additional biomarkers will be identified and existing assessment methods will be improved (Figure 6).
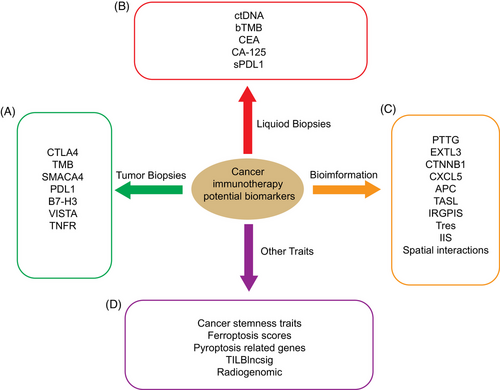
7.4 Mouse tumor models for immunotherapy
Immunotherapy has yielded benefits for some patients with tumors. However, cure rates for most patients remain low, and many patients do not even respond to these treatments. Preclinical in vivo models play a crucial role in the clinical efficacy assessment of tumor immunotherapy. Mouse tumor models are one of the key tools for studying tumor pathogenesis. In recent years, with the advances of tumor immunotherapy, mouse models have been further developed.274 Currently, the main mouse models for tumor immune efficacy evaluation include syngeneic model, genetically engineered mouse models, humanized mouse models with immune system reconstitution.
7.4.1 Syngeneic mouse model
Syngeneic tumor models are the most commonly used preclinical models, and these models are particularly important in the evaluation of immuno-oncology, which have an intact immune system and can be used to investigate the process of antitumor immune response. A recent study performed tumor immunoassays through two syngeneic mouse model (CT-26 and Colon 26), which have similar genetic backgrounds with different sensitivities to anti-PD1 therapy, revealing the underlying mechanisms of anti-PD1 responses. And the results suggest that Wnt pathway is associated with anti-PD1 responses and may be the major factor differentiating these two models.275 A subset of syngeneic resistance models can be used as an analog of the resistance heterogeneity encountered in the clinic and can be used for mechanism exploration.276 Recently, researchers have established a mouse syngeneic model of breast cancer. The model can be successfully used to analyze the efficacy of ICB in combination with predictive biomarkers of breast cancer in immuno-oncology therapy.277 In addition, with a homozygous pancreatic tumor model, the researchers found that combination of treatment with chemotherapy and ICB induced significant delays in tumor regression and regeneration.278 A syngeneic mouse model of glioblastoma revealed the effectiveness of combination therapy and confirmed the power of PARP-targeted alpha therapy in augmenting the potential of PD1 immune checkpoint blockade.279 Notably, although the convenience and availability of syngeneic tumor models have made them the most frequently used preclinical models for assessing immunotherapies, these models still have a fatal drawback: they lack the microenvironmental heterogeneity of cancers.
7.4.2 Genetically engineered mouse models
Using genetic engineering techniques, the expression of tumor-related genes in the mouse genome can be regulated, which could be used to investigate the pathogenesis of diseases. The application of genetically engineered mouse models in tumor immunotherapy is mainly based on the Cre–loxP system. Conditional knockout mice based on the Cre/loxP system use a tissue-specific promoter to specifically control Cre expression in specific tissues to achieve knockout or modification of target genes in specific tissue cells. The Cre recombinase frequently used in the Cre–loxP system to target immune cells include macrophage/monocyte, DCs, mast cells, and T and B cells.280 For example, researchers use Cre/loxP system to discover that the transcriptional coregulator Ess2 controls CD4+ T cell survival through IL-7 signaling pathways.281 With the use of CRISPR/Cas9 and Cre–LoxP knockout methods, the researchers demonstrated that SOX9 inhibited immune cell infiltration and functionally suppressed the infiltration and activity of CD8(+) T cells and NK cells.282 Overall, genetically engineered mouse models in general have TIMEs that are linked to human cancers. However, most genetically engineered mouse models have low tumor mutation loads, which makes it difficult to generate strong antitumor immune responses in these models. In recent years, it has been found that combining the genetically engineered mouse model with UV irradiation could increase the mutational burden of the melanoma model, which undoubtedly provides a favorable direction for the development of the genetically engineered mouse model.283
7.4.3 Humanized mouse models with immune system reconstitution
Since the mouse immune system does not have exactly the same composition as the human immune system, scientists have constructed humanized mouse models that interact with human immune system cells to assess immunotherapeutic approaches more accurately and realistically. Humanized mice models are immunodeficient mice cotransplanted with human tumors and immune components, which mainly indicates patient-derived xenografts (PDX) models with immune system reconstitution.
The methods of immune system reconstitution in humanized mice models mainly include three types below.284 One of the most convenient ways is to infuse mature human immune cells directly into SCID mice, thereby obtaining the SCID mice that produce human peripheral blood leukocytes.285 Another approach is to transplant human CD34+ hspc to immunodeficient mice for generate human SCID regenerative cell mice, which results in a more integrated hematopoietic system that includes compositions such as innate immune cells, adaptive immune cells erythrocytes, and platelets.286 The third method, which is the most complete and sophisticated approach to human immune system transplantation, which consists of transplanting CD34+ hspc from human fetal liver and autologous fetal thymus tissue into SCID mice to produce a kind of model known as BLT (bone marrow, liver, thymus).287 Notably, most of these immune system reconstitution experimental approaches used mismatched tumors and immune systems, which requires a concern for HLA matching before investigate the immune system–tumor interactions.
Currently, humanized mouse models for immunotherapy mainly include treatment with specific antibodies, small molecule inhibitors, and CAR-T/NK therapy.288-290 Recently, a study has shown that mice reconstituted with umbilical cord blood human CD34+ cells were able to induce recession of the response of PDX tumors to anti-PD-1 therapy.291 In addition, using humanized NOD/Shi-scid/IL-2Rnull mice, researchers found that the pattern of immune cell infiltration in a PDX model of non-small cell lung cancer correlated with cytokines, genetic mutations, stromal content and PDL1 status.292 Moreover, PDX models play an important role in immunotherapy of hematology tumors.293, 294
The PDX model with immune system reconstitution accurately simulates genomic heterogeneity and microenvironmental factors in the development of tumors and is essential for tumor immunotherapy evaluation. However, there is no mouse model that is fully compatible with human biology, although humanized mice have been extensively studied in improving the efficacy of clinical tumor immunotherapy. So, further refinement of mouse models is still needed in the future.
8 DISCUSSIONS AND PERSPECTIVES
The response of cancer immunotherapy differs from that of chemotherapeutic agents. In an effort to standardize the evaluation criteria for assessing the efficacy of immunotherapy in clinical studies, the RECIST Working Group released new standards in late 2017. This guideline evaluates the tumor load variations during cancer treatment to provide critical information on disease progression. More importantly, the standard methods of solid tumor measurement and the definition of objective changes in tumor sizes among this guidance will contribute to consistent implementation, explanation, and analysis of immunotherapy trials for researchers and doctors.295
Currently, cancer immunotherapy approaches mainly include (1) using cytokines such as IL2 and interferon to activate and promote the immune cell killing ability of the patient; (2) monoclonal antibody therapy such as ibritumomab, nabumomab, pembrolizumab that targeting immune checkpoints; (3) using adoptive cell transfer therapy such as NK cells, DCs, cytokine-induced killer (CIK) cells, genetically engineered modified T cells; (4) immunizing patients with tumor vaccines made from the patient's own tumor cells, tumor proteins, peptides, and so on to activate the autoimmunity and enhance the antitumor effect; (5) immunotherapy targeting inhibitory cells such as Treg, TAM, and MDSC. And these immunotherapeutic treatments have achieved a certain degree of effectiveness. For instance, CIK cellular immunotherapy provides a pivotal role in clinical trials for the treatment of cancer.296 The CIK cell therapy combination with DCs has been relatively successful in lung cancer clinical trials.297, 298 Combination therapy with CIK cells and PD1 blocking antibodies showed potential for profound durable responses in NSCLC. And those NSCLC subpopulations with PDL1 positivity and high CD8+ TIL counts had better ORR with the combination of PD1 inhibitors and CIK cells.299 However, it is worth emphasizing that the response rate to tumor immunotherapy is still not satisfactory, therefore there is an urgent to find the cause, and identify more biomarkers that can indicate the response rate to immunotherapy and thus improve personalized treatment for tumor patients.
From this review, we understood that epigenetic modifications in cancer are involved in the modulation of immune cell activity and function. Epigenetic regulation influences the interactions between cancer cells and immune cells as well as the state of TIME. Epigenetic regulation can be used as an intervention to induce robust antitumor immunity. Combining epigenetic modulators with cancer immunotherapy solutions is a rational strategy to further enhance the efficacy of immunotherapy and has shown promising applications in clinical studies.
Most studies on tumor metabolism have focused on the overall assessment of in vitro tissue culture samples, yet with the emergence of single-cell sequencing technologies, the impact of tumor metabolism at the single-cell level cannot be ignored. In addition, whether metabolic changes are responsive or instructive throughout functional changes in immune cells is now still required more detailed researches. And whether the metabolic dysregulation of immune cells in TIME is due to immune cell-intrinsic programs or to competition with cancer cells for limited nutrients still requires further study.
The current view is that cancer immunotherapy efficacy largely depends on the viability and function of tumor antigen-specific CD8+ T cells in the immunosuppressive TIME. So, strategies to improve immune efficacy are mainly focused with CD8+ T cells, but the role of other Immune cells such as NK cells with tumor-killing function as well as Treg and MDSC with tumor-suppressive function on cancer immune efficacy is also worth attention. In addition, the ineffectiveness of tumor immunotherapy may be attributed in part to the complex effects of tumor immune escape and the microenvironment. Although several literatures have identified potential biomarkers for assessing the efficacy of immunotherapy and have also reported some models for assessing immunotherapy, there is still a demand for a large amount of clinical data to validate them. In addition, the combination of multiple biomarkers to assess immunotherapy efficacy is also well worth investigating. With further understanding of tumors and immune cells, it is to be expected that the immunotherapy field will achieve more breakthroughs in the future, providing a more powerful weapon to overcome the great challenge of cancer.
AUTHOR CONTRIBUTIONS
D. X. and Y. T. designed the study. S. Q. drafted the manuscript. S. Q., B. X., Q. W., R. Y., J. S., C. H., and S. L. collected the data and conducted the picture processing. D. X., Y. T., and S. L. revised the manuscript. All authors have read and approved the final version of manuscript.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (82073136, 82272982, 82302889), the Natural Science Foundation of Hunan Province (2021JJ30909), and Aid Program for Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article.




