Advancing polypharmacological drug design: A dual-targeted approach for treating psychiatric disorders
In a recent publication in the journal Cell, Chen et al. devised the compound IHCH-7179, which targets both 5-HT2AR and 5-HT1AR as an agonist,1 which represents a paradigm for enhancing treatment through polypharmacology, where multiple targets are simultaneously modulated. The utilization of flexible scaffold-based chemoinformatics approach (FSCA) in this study also paves the way for future advancements in drug design strategies.
Mental illnesses pose significant challenges to healthcare systems worldwide, characterized by complex symptoms and heterogeneous etiologies.2, 3 Traditional drug development approaches focusing on single targets have shown limited efficacy in treating these diseases. Recognizing the necessity for innovative methods, researchers have turned to polypharmacology. However, designing a versatile bifunctional compound remains a formidable challenge.
The dual-targeting approach in this study was inspired by understanding the structural binding pockets of serotonin receptor subtypes. Their research utilized IHCH-7179, developed using FSCA, to target receptors associated with dementia-related psychiatric disorders (DRP): 5-HT1AR and 5-HT2AR. IHCH-7179 serves a dual-purpose effect, much like hitting two birds with one stone. It functions as a key to open deadlock, where different targets resemble distinct locks. Guided by these targets, IHCH-7179 adapts its shape according to the configuration of the lock to induce the drug effect.
In their investigation, the authors described that the binding modes of lysergic acid diethylamide (LSD) and antipsychotic drugs prompted the discovery of flexible scaffolds. LSD's primary pharmacophore occupies orthogonal binding pockets in different receptors, while antipsychotic drugs bind downward, bringing their secondary pharmacophore (SPS) to deep binding pockets (DBPs). These findings support the idea to design structurally similar drugs, where the binding pattern to receptors can predict their pharmacological activity.
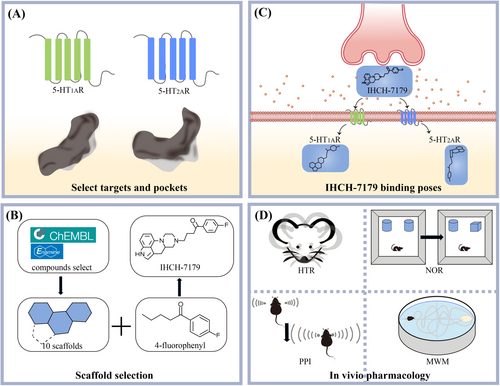
To achieve this goal, Chen et al. planned to combine flexible tricyclic/tetracyclic scaffolds with positively charged amino groups. Using computational screening of a large number of compounds on ChEMBL and Enamine databases, they selected 10 virtual compounds that met specific criteria from over 200 molecules. Analysis of published structures of 5-HT1AR and 5-HT2AR revealed that their shallow binding pockets (SBPs) are larger than DBPs, capable of accommodating the 4-fluorophenyl group. This group, common in antipsychotic drugs, is an ideal SPS for occupying G protein-coupled receptor (GPCR) DBP.4 By selecting ideal linkers, they found that various antipsychotic drugs share the same 4-fluorophenyl group and butanoyl linker as antagonists of 5-HT2AR. Therefore, they chose 10 virtual compounds incorporating a butanoyl linker and a 4-fluorophenyl group. Subsequent docking rounds selected seven compounds that fit SBP. Among these compounds, six are tricyclic ring systems and one is a tetracyclic ring system. One tricyclic and one tetracyclic compound were synthesized. Having analyzed the synthesized compound coupled with docking predictions, they found that two compounds acted as agonists at 5-HT1AR and as either an antagonist or a weak partial agonist at 5-HT2AR. IHCH-7179, with a balanced effect of agonism on 5-HT1AR and antagonism on 5-HT2AR, was selected for further in vitro and in vivo evaluation (Figure 1A,B).
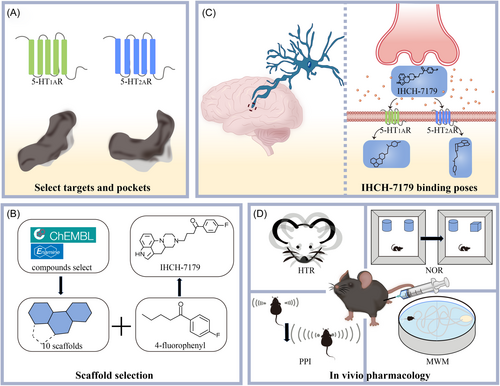
Subsequently, Chen et al. confirmed the different binding poses of the compound on 5-HT1AR and 5-HT2AR by structural-functional study with cryoelectron microscopy and x-ray crystallography. In 5-HT1AR, IHCH-7179 adopts a flat conformation, forming crucial salt bridges with the receptor, resulting in agonist activity. However, in 5-HT2AR, IHCH-7179 binds in a downward orientation, interacting with the receptor's DBP through the 4-fluorophenyl group, exhibiting antagonist activity (Figure 1C). Furthermore, studies on IHCH-7179 analogs revealed that subtle changes of chemical structures could significantly affect ligand binding modes and functional activity on GPCRs. Specifically, key motifs such as agonist filters and conformation shapers were emphasized in determining compound binding modes.
To validate the findings, Chen et al. then conducted in vivo experiments using APP/PS1 mouse models (that were widely used in Alzheimer's disease research),5 comprehensively assessing IHCH-7179s potential in treating psychiatric disorders and cognitive impairments. Pharmacokinetic studies of IHCH-7179 in mice exhibited reasonabley improvede half-life and significant brain penetration. Using the 5-HT1AR selective antagonist WAY-100635, they confirmed IHCH-7179s improvement of cognitive function through 5-HT1AR agonism. Administration of IHCH-7179 resulted in significant reductions in mouse psychiatric symptoms and enhancements in cognitive abilities in behavioral assessments, such as head-twitch responses (HTR), prepulse inhibition (PPI), Morris water maze (MWM) experiments, and novel object recognition tests. Their analyses confirmed that IHCH-7179 alleviates mouse psychiatric symptoms by inhibiting 5-HT2AR and improves cognition in schizophrenic and demented mice by activating 5-HT1AR (Figure 1D). These results demonstrate the potential therapeutic value of IHCH-7179 in managing psychiatric and cognitive impairments, particularly, for diseases like DRP.
While the design of dual-target drugs theoretically holds significant therapeutic potential, it faces a series of challenges in clinical application. Evaluating dual-target drugs in clinical trials is more complex than single-target drugs because the optimal activity levels for the two targets may differ. Incorrect dosing can affect therapeutic outcomes or cause adverse reactions. To ensure effective regulation of both targets, dual-target drugs require precise dose optimization. Trials must be designed to accurately assess the impact on each target and monitor multiple biomarkers. Additionally, stricter patient selection criteria are needed to ensure that the observed therapeutic effects are due to the drug's action on both targets. When dual-target drugs enter the market, their development costs are higher than those of traditional drugs due to the increased complexity of their research and manufacturing processes. Consequently, the cost-effectiveness of these drugs will influence the acceptance of new treatment options by doctors and patients. Educating healthcare providers and patients about the unique benefits and potential risks of these drugs is crucial for successful market entry.
Overall, this study represents significant advancement of polypharmacological drug design for mental illnesses. The utilization of the FSCA method integrates disciplines such as chemoinformatics, structural biology, cellular functional studies, and behavioral pharmacology, addressing the development of dual-target compounds for psychiatric symptoms and cognitive deficits. Additionally, the identification of critical molecular motifs provides a framework for the rational design of polypharmacological ligands with different pharmacological characteristics. Further research aimed at optimizing compound properties, elucidating detailed structure–activity relationships, and validating therapeutic efficacy in clinical settings is crucial for fully harnessing the potential of polypharmacology in psychiatric treatment.
AUTHOR CONTRIBUTIONS
Yawen Pan wrote the manuscript and drew the figure. Yinghao Zhi initiated, supervised, and revised the manuscript writing. Jianliang Shen read and revised this paper. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
Figure 1 was created with Biorender.com. This study is supported by the fifth batch of the National Traditional Chinese Medicine Clinical Outstanding Talents Training project (National Administration of Traditional Chinese Medicine Talent Education letter No. 2022). The Zhejiang Province Traditional Chinese Medicine Inheritance and Innovation Team for Diagnosis and Treatment of Cerebrovascular Diseases (Zhejiang Provincial Health Commission Document [2023] No. 31) supported this study.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors have nothing to report.
Open Research
DATA AVAILABILITY STATEMENT
The authors have nothing to report.