Carbon nanomaterials-based electrically conductive scaffolds for tissue engineering applications
Abstract
In tissue engineering, the pivotal role of scaffolds is underscored, serving as key elements to emulate the native extracellular matrix. These scaffolds must provide structural integrity and support and supply electrical, mechanical, and chemical cues for cell and tissue growth. Notably, electrical conductivity plays a crucial role when dealing with tissues like bone, spinal, neural, and cardiac tissues. However, the typical materials used as tissue engineering scaffolds are predominantly polymers, which generally characteristically feature poor electrical conductivity. Therefore, it is often necessary to incorporate conductive materials into the polymeric matrix to yield electrically conductive scaffolds and further enable electrical stimulation. Among different conductive materials, carbon nanomaterials have attracted significant attention in developing conductive tissue engineering scaffolds, demonstrating excellent biocompatibility and bioactivity in both in vitro and in vivo settings. This article aims to comprehensively review the current landscape of carbon-based conductive scaffolds, with a specific focus on their role in advancing tissue engineering for the regeneration and maturation of functional tissues, emphasizing the application of electrical stimulation. This review highlights the versatility of carbon-based conductive scaffolds and addresses existing challenges and prospects, shedding light on the trajectory of innovative conductive scaffold development in tissue engineering.
1 INTRODUCTION
Tissue engineering, a multidisciplinary science bridging together biology, materials science, chemistry, and engineering, aims to repair, replace, or regenerate tissues or organs by applying fundamental knowledge from each of the fields into practical and effective materials or devices and clinical strategies. The fundamental objective of tissue engineering is to close the significant gap arising from the escalating number of patients awaiting organ transplantation due to end-stage failure and the constrained availability of donated organs for such procedures.1-3 In addition, tissue engineering, as a subfield of regenerative medicine, increasingly targets prevalent conditions. It aims to provide therapeutic solutions for severe chronic diseases concerning major organs like the heart, kidney, and liver, extending the impact of tissue-engineering technologies even to those not yet on transplantation waiting lists.1
Among different factors, one of the most crucial players in the success of tissue engineering is scaffolds, intricately designed matrices that provide a structural framework for cellular activities during tissue regeneration. The fundamental function of a scaffold includes providing a foundation for cell attachment, signaling, and their subsequent development into a three-dimensional (3D) tissue construct. For successful tissue regeneration, a scaffold should feature certain functionalities. Among them, the most important and must-have is biocompatibility, which ensures a harmonious integration with host tissue and a preferable immune response.4, 5 Apart from that, mechanical properties, including elasticity and tensile strength, are equally crucial, dictating the scaffold's ability to withstand physiological forces while supporting cellular processes.6, 7 The imperative nature of these properties has led to the exploration of a diverse range of materials, including naturally occurring polymers,8, 9 synthetic polymers–both biodegradable and nonbiodegradable ones,10, 11 metals,12 and ceramics.13 The pursuit of enhanced regenerative outcomes often asks for additional scaffold properties. Electrical conductivity is one of such properties. Electrically conductive scaffolds offer a distinct advantage over their nonconductive counterparts by actively influencing cellular behavior through the application of electrical cues. These cues play a fundamental role in processes such as cell adhesion, migration, and tissue development.14, 15
Among different materials explored for electrically conductive scaffolds, carbon nanomaterials have garnered significant interest in the relevant scientific community. These materials, existing in various carbon allotropes such as carbon nanotubes (CNTs), graphene, and fullerenes, exhibit exceptional properties derived from the unique arrangement of carbon atoms. The versatility of carbon nanomaterials extends across a spectrum of sizes and forms, allowing for precise tailoring of their physicochemical characteristics. Carbon and carbon-based materials feature unique mechanical, electrical, thermal, tribological, and biological properties, which yielded their usage in a wide range of applications, from biotechnology16 to space technology17 and from energy applications18 to quantum devices.19 They have also demonstrated remarkable versatility in the biomedical field, contributing to advancements in drug delivery, imaging, diagnostics, and biosensing.20-24 In the realm of tissue engineering, carbon nanomaterials have been at the forefront since the early stage of this field. The carbon family offers a versatile portfolio of biomaterials, encompassing mechanical properties that span the entire spectrum relevant to interactions with the human body.25 When compared to alternative synthetic biomaterials designed for tissue replacement, carbon-based materials stand out by minimizing mechanical disparities and showcasing compelling biocompatibility. The nanoscale dimensions of carbon nanomaterials further deliver an expanded surface area, mimicking several extracellular matrices of native tissue. Additionally, carbon nanomaterials render tunable electrical conductivity, making them prime candidates for scaffolds designed to leverage the power of electrical cues in guiding regenerative processes.
Due to the immense promise offered by carbon nanomaterials, researchers have been using them in different tissue engineering applications, as schematically illustrated in Figure 1A. The popularity of carbon-based materials in tissue engineering can be realized by the astounding number of publications generated each year. We plotted the year-wise number of publications on carbon nanomaterials in Figure 1B by conducting a search in the Web of Science. Among different carbon allotropes, graphene, and CNTs stand out significantly higher than the other allotropes due to their impressive material properties. However, it should be mentioned that not all the publications utilized the electrical conductivity aspect of carbon nanomaterials in tissue engineering. In this article, we specifically take an interest in the electrical conductivity of carbon nanomaterials and their impact on tissue regeneration under electrical stimulation. Therefore, we review the literature published in the last 10 years (since 2013) on carbon nanomaterials-based electrically conductive tissue engineering scaffolds with electrical stimulation to provide an in-depth exploration of current research, delineate potential challenges, and illuminate future directions. By scrutinizing the multifaceted interactions between carbon nanomaterials, cellular environments, and external electrical stimulation, this review seeks to contribute to the evolving narrative of tissue engineering, offering insights into the transformative potential of these innovative materials.
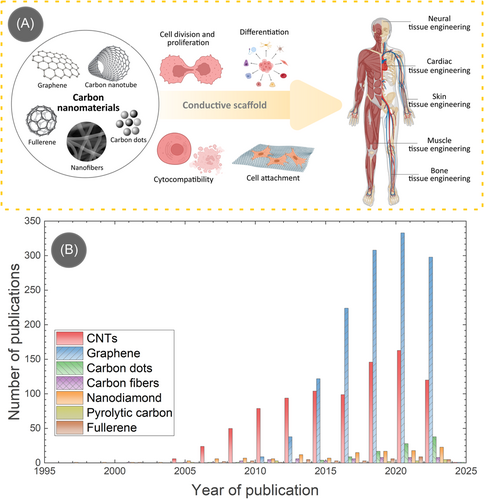
2 IMPORTANCE OF SCAFFOLD ELECTRICAL CONDUCTIVITY IN TISSUE ENGINEERING
Electrical conductivity is a critical factor for tissue engineering scaffolds as it enables the integration of electrical cues into engineered tissues. This feature is essential for mimicking the natural physiological environment of various tissues in the human body. There is a prevailing notion that electroconductive materials, such as metals or conductive polymers, capable of carrying electronic currents can interact with bioelectronic signals, particularly in cardiac, muscle, nerve, and bone tissue engineering.15 However, the mechanisms facilitating this interaction remain unclear and are often inadequately discussed. Many authors equate electronic conductance in artificial systems with ionic conductance in bioelectronic systems, assuming their equivalence and compatibility.15 Nevertheless, scaffold conductivity enhances cell proliferation and differentiation, influences tissue formation, and supports the regeneration of complex tissues like bone and muscle. The controlled release of therapeutic agents through conductive scaffolds and their role in monitoring cellular activities further extend their significance in tissue engineering applications. Furthermore, electrical conductivity facilitates the application of external electrical stimulation, which has proved to fasten tissue maturation and growth.26-30 The following section delves into the influence of electrical stimulation, elicited with scaffold conductivity, in different tissue engineering applications.
The concept of utilization of external electrical stimulation is based on the fact that the human body has bioelectrical properties that are vital in maintaining homeostatic functions such as wound healing, nervous system signaling, and muscle contraction.31 Furthermore, cellular events such as cell division, development, and migration are known to generate endogenous electric fields.32 For example, weak direct current electric fields present in some biological systems influence cell division, differentiation, migration, and the extension of motile processes.33
2.1 Bone healing
The effect of electrical stimulation on tissue was first observed on compressed bones in the 1960s.34 It was found that bone deformation or compression generated negative electrical potentials in both living and dead bones. Removing the inorganic fraction from the area abolished the stress-induced electrical potentials in the living and dead bone. It was concluded that this negative electrical potential activity directly influenced osseous cells and the aggregation patterning of macromolecules in the extracellular matrix.34 More than a decade of research has gone into electrically stimulating bone tissue to promote bone growth in nonunion fractures35-37 and spinal fusions.38 One example is using pulsed electromagnetic fields to prevent bone loss in immobilized patients.39 This is partly due to the fact that electromagnetic and electric fields regulate the synthesis of proteoglycan and collagen, two components of the extracellular matrix.40 Multiple studies have investigated and shown the enhanced healing effects of electrical stimulation on fractured bones. However, many of the implemented electrical stimulation methods found in the literature are not standardized, which leads to ambiguous interpretations of the best method for bone healing.41 A series of investigations have looked at the interconnection between bone's electrical properties, mechanical characteristics, and microstructure that contribute to the creation of bone scaffolds.42-45 As a result of these investigations, interdependencies were established with the trabecular bone's electrical permittivity and loss factor, but not with the bone's electrical conductivity.43 Instead, conductivity was associated with the bone's density and water content based on the measured phase shifts of the bulk properties of osseous tissue rather than the tissue's surface effects.43 To clarify, electrodes and bone boundaries differ from the bulk properties of osseous tissue. Surface effects are extremely important to consider in phase boundaries such as electrode and bone boundaries since these phases induce capacitive behavior, which can be measured with impedance spectroscopy.46 Fundamental properties of materials, such as pore structure, specific surface area, surface chemistry, and electrical conductivity, are a function of material composition. Just the basic properties of scaffold surface area and conductivity overwhelmingly influence scaffold capacitance.47 For example, specific capacitance gradually drops with increasing CNT content as a result of specific surface area loss.47 However, high electrical conductivity was achieved with a CNT content above 15% in weight, making this an optimal composite composition. Electrically induced capacitance from electrode and bone boundaries cannot be canceled with conductive gels, even though adding gels can guarantee contact between the bone and electrode head.46 Considering surface effects, electrical impedance spectroscopy has been used to correlate measured conductivities to the architecture and density of osseous tissue. Thus, this information can then be used to construct a map of tissue resistivity to improve the conductivity of engineered bone scaffolds.14, 48
Investigations on understanding the mechanism supporting electrical stimulation's bone healing effects began in earnest in the last decade. Several studies suggested electrical stimulation's in vitro effect on improving bone stem cell migration, proliferation, alignment, differentiation, and attachments in scaffold materials.49-52 Furthermore, electrical stimulation has been shown to increase mineralization and extracellular matrix deposition, as well as enhance the expression of multiple genes involved in bone regeneration.53, 54 For example, externally applied electrical stimulation increased the metabolic activity of mesenchymal cells after 3 days of culture, resulting in significant upregulation of osteogenic gene expression patterns including Runx2, a key transcription factor for osteoblast differentiation; osteopontin, an extracellular structure protein; and CoL2A1, a gene involved in type II collagen synthesis.54, 55 In a set of in vivo experiments, rat large-bone defect models were exposed to electrically conductive scaffolds with mesenchymal stem cells.56 The results showed that electrical stimulation further enhanced bone healing in addition to the already established healing effects from the scaffold comprising of the stem cells. After 8 weeks, additional scar tissue combined with less newly formed bone and cartilage was found in the controls plus sham cohorts compared to the electrically stimulated cohorts. Although complete bone healing was not observed in any of the electrically stimulated cohorts in the eighth week, new bone growth was observed at the center of the bone defect.56 Thus, electrical stimulation has notable applications in bone regeneration with reduced scarring. However, further investigations on exogenous electrical stimulation parameters and protocols are required to understand their effects on bioelectric signals that regulate bone regeneration.
2.2 Nerve tissue regeneration
There are multiple theories surrounding the effects of electrical stimulation on nerve regeneration. One theory postulates that electrical stimulation could act directly on the nerve cells, leading to several intracellular changes, which include the redistribution of cytoplasmic materials within the cell, the activation of growth-controlled transport activity across the plasma membrane of the cell, and the electrophoretic accumulation of surface molecule that influence neurite growth or the cell's adhesion to substrates.57, 58 A second theory hypothesizes that endogenous electric fields are produced by steady inward currents at the cell's growth cone tips, which occurs only during active growth.59 A third theory proposed that electrical stimulation affects protein synthesis, which stimulates neurite outgrowth in vitro.60 This theory was supported by observing an increased gene expression of neural growth factors via electrical stimulation under the alternating potentials.61 In a fourth theory, fibronectin, a component of the extracellular matrix, was shown to have increased absorption into electrically stimulated cells.62 Increased fibronectin leads to improved neurite growth on electrically stimulated polypyrrole films. Neurons, which are electroactive cells, utilize the properties of electrical systems by generating electromotive force, maintaining membrane potentials, increasing or decreasing membrane potentials, utilizing electrical resistance in series or parallel, turning current on or off, controlling current flow, correcting current flow, and storing electrical charge.63
An electrical voltage that exists across the plasma membrane of a neuron cell characteristically has an internal potential that is more negative than the external potential of the cell.64 Thus, the internal membrane potential is −60 to 100 mV while the external potential is 0 mV. This is referred to as the resting potential and is maintained during the inactive states of neuron cells.64 When neuron cells become activated by electrical stimulation, an ion influx occurs across the plasma membrane, resulting in action potentials that influence intracellular signal transduction pathways.65 Action potentials transmit information along the nervous system via axons that interconnect the cell's soma to the dendrites of the next nerve cell. Initial investigations have observed that extracellular electric fields with direct current between 0.1 and 10 V/cm reversibly influenced the neurite growth and orientation of Xenopus neurons.66 Further research has shown that applied electric fields in the form of electromagnetic fields, direct current, and alternating current influence orientation, neurite outgrowth, neuron differentiation, and calcium ion influx.65, 67-69
2.3 Muscular and cardiac tissue repair
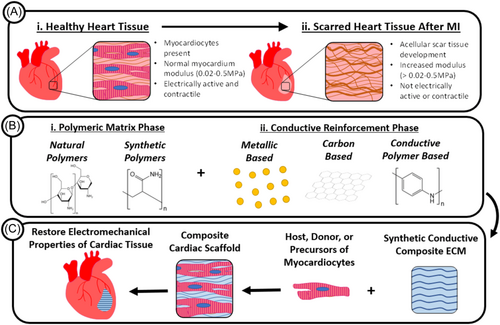
Cardiac muscle synchronicity relies on intricate coordination of action potentials in combination with calcium signaling that is facilitated by membrane potential depolarization, pacemaker cells, and a network of intracellular communication.70 To consider the synchronicity for heart tissue engineering, the myocardium, or involuntary striated muscular tissue that occupies the inner mass of the heart wall, must be present as a framework of parallel fibers.71 These fibers comprise contractile muscle cells called cardiomyocytes, which confer the heart's characteristic twist motion during the contraction cycle. Strands of connective tissue hold together cardiomyocytes.72 Other cells, such as endothelial cells, smooth muscle cells, macrophages, and cardiac fibroblast, also support heart tissue remodeling during development or after pathological conditions. These cells are surrounded by a cardiac-specific extracellular matrix, which makes up 5% of the myocardial dry weight.73 In addition, the cardiac-specific extracellular matrix provides support to the myofibers and is responsible for mechano-transduction, a mechanical stimulus that converts to electrochemical activity that results in morphological changes as well as the deposition of structural and functional proteins.
Electrically propagating cells, including pacemaker, atrial, atrioventricular, and Purkinje cells, initiate electrical impulses to the ventricular contractile cell to activate muscle contraction of the heart.71 As a result, each sarcomere, the functional and structural unit of the cardiac muscle within a cardiac muscle fiber, will shorten. Electrical activity is initiated by specialized cells in the sinoatrial node of the right atrium, which generates a stimulus between 60 and 100 times per minute at regular intervals.74 At an action potential of −65 mV, diastolic depolarization begins, while the nodal action potential triggers at −45 mV. This electrical signal propagates along the atria to the atrioventricular node, causing the atria to contract.74 The signal continues to the bundle of His and then reaches the Purkinje fibers that extend and propagate the action potential to each cell within the myocardium.70
Given the intricate role of electrical signaling of the native myocardium, scaffolds composed of electroconductive biomaterials are an emerging success for cardiac tissue engineering.75 Although scaffolds for regenerative medicine are not able to successfully support and drive cell behavior to achieve mature and functional tissue, the use of biomaterials in the scaffold composition is able to mimic the physiological electroconductivity of the heart as well as have adequate degradation kinetics for cardiac tissue engineering (Figure 2).76, 77 These conductive biomaterials include extrinsically conductive materials such as graphene and CNTs, which have chemical and physical properties that can dictate the cytotoxicity and engineered cardiac tissue's outcome in in vitro and in vivo studies.74
3 CARBON NANOMATERIALS
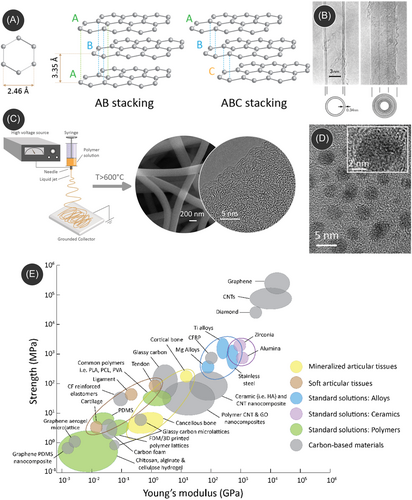
In its ground state, carbon features an electronic structure of 1s22s22p2, revealing four electron vacancies in its outer electron shell. This particular electronic configuration enables carbon atoms to engage in the formation of robust covalent bonds with other carbon atoms across various hybridization states (sp, sp2, sp3), thereby facilitating the emergence of diverse carbon allotropes in the solid state.78, 79 Notably, diamond and graphite are the sole naturally occurring variants among different carbon allotropes. Other notable carbon allotropes include graphene, CNTs, fullerene, carbon quantum dots (CQDs), amorphous carbon, diamond-like carbon, and glassy carbon. The allotropic electronic configurations determine the electrical and mechanical properties of carbon materials. For example, despite both being composed solely of carbon atoms, diamond and graphite showcase distinct properties. Diamond, for instance, reigns as the hardest known material and serves as an electrical insulator.80 In contrast, graphite features a soft texture while exhibiting remarkable electrical conductivity. The contrasting attributes of these two allotropes can be attributed to differences in their atomic arrangements. The variation of their allotropic nature yields a wide range of their mechanical performances. Figure 3E presents an Ashby diagram of the mechanical properties of carbon materials in relation to tissue engineering applications while comparing them with other relevant materials such as ceramics, alloys, and polymers.
3.1 Graphene and graphene oxide
Graphene is a single layer of graphite, which is basically stacked graphene layers in parallel configurations, forming a 3D and crystalline structure (Figure 3A). Graphene's two-dimensional structure is composed of a monolayer of carbon atoms covalently bound to three adjacent molecules to form a honeycomb-like model.84 The graphene family consists of a number of derivatives that contrast in structure and properties, such as graphene oxide, reduced graphene oxide, graphene quantum dots, graphene nanosheets, and few-layer graphene.85 Graphene's unique physical and electrical properties make it an ideal candidate for incorporation into scaffold composites to enhance specific properties. The unique properties of graphene are exceptionally high Young's modulus (~1.0 TPa),86 a large surface area of 2630 m2/g,87 intrinsic mobility of 200,000 cm2 V−1 s−1,88 thermal conductivity of ~5000 Wm−1 K−1,89 optical transmittance of ~97.7%,87 as well as exceptional electrical conductivity.90 Within the graphene family, another popular member is graphene oxide, which can be produced by treating graphene materials with oxidative agents. The oxidative treatment often introduces contrasting properties within graphene oxide when compared to graphene.91, 92 For example, graphene oxide exhibits hydrophilic surface characteristics, whereas pristine graphene is hydrophobic. Furthermore, the electrical conductivity of graphene oxide is suppressed significantly by the oxidative functional groups.93 However, the conductivity can be regained through an additional reduction process, resulting in reduced graphene oxide.94
3.2 CNTs
Since Iijima's groundbreaking work in 1991, CNTs have been the subject of extensive investigation across various research fields.81 CNTs are composed of sp2-hybridized carbon atoms and are created by rolling up graphene sheets into a seamless cylindrical structure. CNTs can exist in the form of single-walled (SWCNT) or multi-walled (MWCNT) configurations. As the nomenclature suggests, SWCNTs are formed by rolling up a single-layer graphene sheet. They typically feature a diameter of 1 nm.95 When multiple graphene layers form the CNTs, they are termed as MWCNTs. The interlayer spacing between adjacent layers is ≈3.4 Å, as depicted in Figure 3B. The diameter of MWCNTs typically ranges from 10 to 50 nm, and the length ranges from 1 to 20 µm. CNTs feature important physical and mechanical properties, including high tensile strength (≥50 GPa), extremely high Young's modulus (≥1 TPa), high electrical conductivity of ≥107 S/m, and a maximum current transmittance of ≥100 MA/cm2.96 Even though CNTs feature high electrical conductivity, they can be metallic or semiconducting depending on their chirality or orientation of the graphene planes. Popular methods for CNT preparation include chemical vapor deposition (CVD), arc discharge, laser ablation, and plasma torch.
3.3 CQDs
CQDs (also known as carbon dots) are quasi-zero-dimensional (0D) carbon nanoparticles with an average size below 20 nm, as illustrated in Figure 3D.83, 97 These nanoparticles primarily consist of sp2-hybridized carbon atoms and feature multilayer graphene sheets, as depicted in the inset of Figure 3D. These sheets are interconnected with chemical groups located at their edges or within the interlayer graphene planes. CQDs showcase outstanding fluorescence properties stemming from the quantum confinement effect inherent in these nanoparticles. This distinctive characteristic contributes to their notable optical behavior, making them valuable in various applications.
3.4 Carbon nanofibers (CNFs)
CNFs are distinguished by their fibrous morphology with a diameter ranging from a few nanometers to a few micrometers. The most popular method for fabricating CNFs is electrospinning, where a droplet of a polymer solution is deformed into a conical shape (known as the Taylor cone) under a strong electric field, followed by ejection of a fiber jet, which is collected on a ground collector.98, 99 A schematic of a typical electrospinning process is presented in Figure 3C. The electrospun polymer fibers are further carbonized in an inert atmosphere, where the polymer goes through a thermochemical degradation reaction, yielding the formation of CNFs (see the scanning electron microscope [SEM] image in Figure 3C).82, 100, 101 The electronic structure of CNFs resembles that of glassy carbon, characterized by a mixture of sp2 and sp3 carbon (see the SEM image in Figure 3C), and depends on the carbonization temperature. Low-temperature carbonization results in more of an amorphous matrix, with sp3-heavy carbons, whereas high-temperature carbonization leads to more graphitic carbon, characterized by sp2 carbons.100 Due to their versatile carbon configurations, the electrical conductivity of CNFs can range from semiconducting to conductive, often falling within the range of 100–1000 S/cm, depending on specific conditions and applications. Furthermore, CNFs exhibit exceptionally high mechanical properties, with a stiffness ranging from 50 to 600 GPa and a strength ranging from 0.05 to 12 GPa.101 Such interesting properties make them versatile for applications in composites, energy storage, and sensing devices.
3.5 Processing of carbon nanomaterials for tissue engineering scaffolds
Typical processing methods of carbon nanomaterials include CVD, physical vapor deposition, microwave synthesis, hydrothermal methods, and plasma arc synthesis methods.102, 103 However, these methods majorly generate carbon nanomaterials in powder form. Several studies have reported that carbon nanomaterials often induce cytotoxicity while freely interacting with cells in powder form, depending on the size, shape, concentration, surface functionalization, and rate of aggregation of these nanomaterials. It has been demonstrated that these nanomaterials can permeate cell membranes, resulting in significant damage to the cell membranes.104 Moreover, their subsequent translocation into cells and cell nuclei has the potential to trigger inflammation and, in more severe cases, genotoxicity.105 In comparison, when these carbon nanomaterials are used in composite materials, their free movement is restricted, obstructing the free interaction with the cells, thereby resulting in minimal or no cytotoxic effects.
Carbon nanomaterials are typically insoluble in organic solvents. Therefore, they are used as dispersions while preparing composite materials. Carbon nanomaterials dispersed in a solvent are meticulously blended with a polymeric, ceramic, or composite matrix, undergoing thorough agitation through processes like mechanical stirring, sonication, and vigorous shaking.106 The composite matrix is further processed through various methods for fabricating the final scaffold structure. Two of the most popular methods are electrohydrodynamic printing and 3D printing. Electrohydrodynamic printing involves both electrospinning107, 108 and melt-electrowriting.109 The common methodology includes drawing a fiber jet from a polymeric droplet under a strong electric field. Electrospinning generates the droplet through a viscoelastic solution, whereas melt-electrowriting relies on generating the droplet by melting the polymeric material. Electrospinning further allows fabricating CNF-based freestanding scaffolds without needing a composite scaffold.110 The 3D printing approach mainly relies on extrusion-based additive manufacturing (such as fused deposition modeling printing and gel printing) for fabricating carbon-composite scaffolds.25 Other methods for fabricating the carbon-based composite scaffold include freeze-casting and phase separation. An alternative approach to fabricated carbon nanomaterial-based scaffold involves employing suitable surface coating or functionalization.111 The coating of a 3D template with carbon nanomaterials can further allow for etching the 3D template, resulting in a 3D scaffold of carbon nanomaterials. For example, Selhuber-Unkel and her team utilized zinc oxide (ZnO) tetrapod structures as templates, onto which carbon or CNT layers were deposited through CVD or dip-coating methods. Subsequently, the ZnO was etched to generate aerographite or CNT tube (CNTT) structures.112, 113
4 CARBON NANOMATERIALS-BASED CONDUCTIVE SCAFFOLD FOR TISSUE ENGINEERING
4.1 Bone tissue engineering
CNTs and graphene have been extensively used as promising electroconductive biomaterials for bone tissue engineering. Notably, various review papers that discuss and review the use of CNTs and graphene-based materials in bone tissue engineering are already available.106, 114-118 Due to their good electrical conductivity, CNTs, and graphene-based scaffolds have also demonstrated the ability to stimulate osteogenesis by fostering improved intercellular communication in the presence of endogenous electric field (EnFE). Composite scaffolds employing CNTs and graphene-based materials can influence cell fate commitment, leading to enhanced osteoblastic differentiation and the upregulation of osteogenic signal expression in stem cells. Such behavior was observed for different polymeric and nonpolymeric composites.119-122 For example, Duan et al. evaluated the incorporation of CNTs and graphene in a poly(l-lactide) (PLA) nanofibrous scaffold for enhanced osteogenic differentiation of bone mesenchymal stem cells (BMSCs).123 They found that both CNTs and graphene significantly promoted enhancement in cell biological behaviors compared to the pristine PLA materials. They demonstrated that the BMSCs proliferated faster, expressed higher levels of alkaline phosphatase (ALP, a bone marker), and produced bone formation-related proteins richer with the presence of CNTs and graphene. The authors argued that both carbon nanomaterials had a strong ability to absorb proteins due to the interaction of π electron cloud of carbon and the hydrophobic part of the protein. Furthermore, the π–π bond of carbon nanomaterials could interact with bioactive components dexamethasone, β-glycerophosphate disodium, and ascorbic acid, leading to strong adsorption, which further promoted osteogenic differentiation and mineralization. However, many of these studies do not utilize the electrical conductivity properties for electrical stimulation-derived cell behaviors.
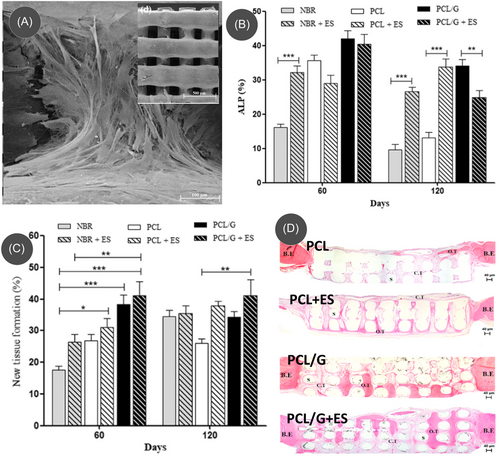
Recent studies have started utilizing the conductive properties of carbon nanomaterials, as the inclusion of carbon nanomaterials within an insulating matrix (either polymer or ceramic) introduces electrical conductivity to the composites, enabling them to investigate the effect of electrical stimulation in bone tissue engineering.124-126 For example, Wang et al. fabricated an electroconductive 3D scaffold of poly(ε-caprolactone) (PCL)/graphene (Figure 4A) by melt extrusion 3D printing of a composite pellet and performed in vivo studies for bone formation using the 3D scaffolds under electrical stimulation.124 An electrical stimulation of 10 µA for 5 min was applied to the bone defect of the subject male Wistar rat twice a week until 120 days. During the in vivo experiments, graphene and electrical stimulation exhibited a synergistic activity. The PCL/graphene scaffold induced the highest ALP expression under the electrical stimulation by Day 60, compared to natural bone regeneration (NBR) conditions, even to PCL/graphene without electrical stimulation (Figure 4B). However, the ALP expression decreased by Day 120 due to the completion of bone tissue formation and initiation of bone remodeling. The results suggested that the synergistic activity of graphene and electrical stimulation accelerated bone repair and modulated the remodeling phase at both cellular and molecular levels. This was further confirmed by the quantification of newly formed tissue, as the PCL/graphene scaffold with electrical stimulation showed the highest amount of newly formed tissue (Figure 4C). Similarly, it was also possible to observe the more organized bone formation and greater portions of new bone in the graphene/PCL scaffold at both 60 and 120 days (Figure 4D).
Other than direct incorporation, coating polymeric or ceramic scaffolds with carbon nanomaterials is another approach for fabricating electroactive scaffolds.127-130 For an example, Li et al. coated 3D printed poly(propylene fumarate) (PPF) structures with a negatively charged ssDNA@CNT nano-complex for generating an electroconductive 3D surface for preosteoblast MC3T3-E1 cell culture.127 Furthermore, the scaffolds with cells were stimulated for 2 h per day under a voltage of 100 mV/mm and frequency of 20 Hz. Their study showed that ES resulted in enhanced proliferation for all the scaffolds, with an increase observed in the ssDNA@CNT treatment when compared with the nonconductive 3D-PPFA control. However, the scaffold with CNTs had significantly more cell proliferation after electrical stimulation. The authors speculated that the synergistic activity of electrical conductivity of CNTs and surface properties originating from the ssDNA under ES yielded improved cell viability and proliferation. Furthermore, under ES, the hybrid scaffold resulted in an enhanced expression of osteogenic markers alkaline phosphatase (ALP), osteocalcin (OCN), bone morphogenic protein 2 (BMP2), and RUNX family transcription factor 2 (Runx2). These results proved that when in the presence of a conductive substrate, electrical pulses can promote osteogenesis and mineral formation. The authors further discussed that ES-induced enhanced proliferation of MC3T3-E1 could be due to the activation of voltage-gated calcium channels, leading to an increase in transforming growth factor-1 (TGF-1) messenger RNA (mRNA) levels,131 which is an important regulator of osteogenesis. TGF-1 mRNA directly interacts with the calcium-binding protein calmodulin, enhancing the responses from other proteins linked to tissue mineralization.132 Such activation process led by the ES and electroconductive scaffold could be the reason behind the enhanced osteoblast growth, differentiation, and mineralization.
4.2 Cardiac tissue engineering
Engineered cardiac tissues intended for the treatment of damaged heart muscles are commonly generated by seeding neonatal cardiomyocytes onto a synthetic scaffold. The myocardium cells possess distinctive structural, mechanical, and electrical properties, and the goal of constructing these engineered cardiac tissues is to closely mimic these inherent characteristics. The cardiomyocytes, in their habitant conditions, feature a size of 20–200 µm and are organized anisotropically into quasi-lamellar sheets. The myocardial extracellular matrix (ECM) comprises both structural elements, such as collagen, and nonstructural proteins, like glycoproteins, proteoglycans, and glycosaminoglycans, present in the extracellular fluid at physiological pH (∼7.4).133 Intercellular connections among cardiomyocytes are established through intercalated discs, encompassing fascia adherens, desmosomes, and gap junctions. The fascia adherens, desmosomes, and extracellular matrix collectively contribute to the approximate 425 kPa compressive (elastic) modulus observed in native heart tissue.134 Electrical communication between cardiomyocytes is facilitated by gap junctions. Longitudinally, the electrical conductivity is approximately ∼0.16 S/cm, while transversely, it is around ∼5 × 10−3 S/cm.135 These intricate structural and electrical features are pivotal in maintaining the physiological functions of the myocardium, and efforts in engineered cardiac tissues aim to replicate these characteristics for effective therapeutic interventions.
Typical materials used in cardiac tissue engineering include hydrogels and polymeric materials. Even though these materials offer easy processability, they often lack mechanical stiffness native to the cardiac cells and electrical conductivity. The utilization of carbon nanomaterials to enhance these scaffolds represents one of the most promising avenues in advancing the design of cardiac tissue constructs. The integration of carbon nanomaterials into polymeric matrices not only enhances the physical properties of the scaffolds but also contributes to the improvement of cell behavior and the physiological properties of the cardiac tissue construct.136 For example, the incorporation of 1.5% CNTs into poly(glycerol sebacate):gelatin (PG) electrospun nanofibers yielded an increase in tensile strength from 250 to 1475.3 kPa and toughness from 275.7 to 1948.7 kJ/m3.137 In another example, the integration of CNTs within a chitosan/PVA nanofibril matrix enhanced Young's modulus from 275 to 733 MPa, and electrical conductivity from 10−8 to 10−3 S/m.138 These improvements in cardiac scaffold properties also resulted in improved cellular proliferation and better attachment of cardiomyocytes.137-142 Additionally, graphene oxide-enhanced scaffolds exhibited superior cell adhesion and spreading than CNT-based scaffolds.139 The enhancements observed in graphene oxide-enhanced scaffolds could be attributed to cytoplasmic prolongations, facilitating connections between cells across layers. This phenomenon likely contributed to enhanced cell–cell communication and improved permeability of oxygen and nutrients within the scaffold.139 Furthermore, these scaffolds, owning to their properties close to the native cardiac tissue constructs, were also capable of inducing stem cell differentiation into cardiac cells.138 The carbon nanomaterial containing scaffolds further yielded spontaneous beating functions of contractile cardiomyocytes.143, 144 For example, cardiomyocytes cultured on graphene-containing electrospun aligned polycaprolactone (G-PCL) fiber scaffolds exhibited spontaneous beating within 48 h of culturing (Figure 5A).144 Moreover, the G-PCL scaffold elevated expression levels of myosin heavy chain (MHC) and cardiac-specific cardiac troponin (cTnT) in comparison to PCL scaffolds after 6 days of culture (Figure 5A). Notably, the expression of connexin43 (Cx43) was significantly upregulated on aligned graphene-containing scaffolds when compared to PCL scaffolds, and this difference became more pronounced with an extended culture period of 14 days.
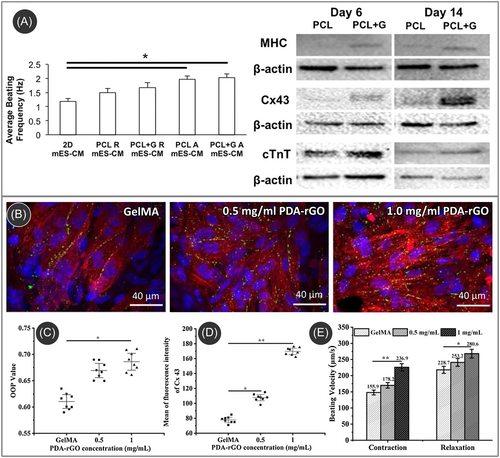
Integrating carbon nanomaterials into polymeric scaffolds allows for electrical stimulations of the cultured cardiomyocytes.138, 143, 145 Li et al. reported that external electrical stimulation on cardiomyocytes cultured in a PDA-rGO-incorporated hydrogel scaffold resulted in faster maturation of the cultured cells.143 Under electrical stimulation, the rGO-based scaffold yielded improved sarcomeric orientation and significantly increased Cx43 protein expression compared to the pristine hydrogel scaffold (Figure 5B–D). Furthermore, higher contraction and relaxation velocities of the cardiomyocytes were observed with an increased amount of rGO within the scaffold, as shown in (Figure 5E). The contraction velocities for 0, 0.5, and 1 mg/mL rGO concentration were 147 ± 8.9, 170 ± 8.2, and 227 ± 9.9 µm/s, respectively, whereas the relaxation velocities were 218 ± 10.7, 242 ± 11.0, and 269 ± 11.6 µm/s, respectively. The external stimulation induced the process of hyperpolarization at the anode end and depolarization at the cathode end of the stimulation, which promoted the expression of cardiac-related specific markers, resulting in accelerated maturation. Furthermore, electrical stimulation promoted cardiomyogenic differentiation of human-induced pluripotent stem cells on carbon nanomaterial-based conductive scaffolds through positively influencing the extracellular Ca2+ influx.138 In the context of cardiomyogenic differentiation in brown adipose-derived stem cells on SWCNT-collagen substrates, it was noted that the facilitative effect of CNTs in cardiac differentiation was modulated through the β1 integrin-dependent TGF-β1 signaling pathway.146
4.3 Muscle tissue engineering
Carbon nanomaterials have been successfully integrated into polymeric matrices for applications in skeletal muscle tissue engineering (SMTE). Several studies have explored the effectiveness of carbon nanomaterials as substrates for cell culture and have delved into their interactions with muscle cells. For example, films created from poly(ethylene glycol)-linked multi-walled CNTs (PEG-CNTs) using a drop-drying method have been investigated for their potential to spontaneously induce myogenic differentiation in human mesenchymal stem cells (hMSCs).147 These films exhibited nanoscale surface roughness and increased hydrophilicity compared to a glass substrate, potentially contributing to the upregulated expression of MyoD, desmin, and myosin heavy chain 2 (MHC) on PEG-CNT films compared to glass. Notably, the PEG-CNT film also upregulated the expression of troponin C (TnC) and ryanodine receptor 1 (Ryr) in comparison to glass, whereas myogenic induction alone did not. Additionally, the PEG-CNT film downregulated osteogenic and chondrogenic markers, suggesting its specific influence on promoting myogenic differentiation in hMSCs. In contrast to hydrogels, electrospun fibers offer the added advantage of directionality and mimicking the native myofiber structure, providing a 3D substrate for myoblast alignment and fusion. Carbon nanomaterials have also been incorporated within electrospun polymeric fibers for producing electroactive fibril scaffolds for SMTE. Chaudhuri et al. developed GO-PCL composites, producing a mesh with approximately 85% porosity tailored for the growth of C2C12 cells.148 On the GO-PCL scaffolds, the expression of myogenic proteins such as Desmin and MyoD, along with cell signaling, exhibited improvements, indicating a superior myogenic differentiation potential attributed to the enhanced conductivity of the GO-containing mesh. The same research team demonstrated that human skeletal muscle cells derived from mesenchymal stem cells from umbilical cord blood can form myotubes on GO-PCL scaffolds.149 Interestingly, GO-PCL appears to influence the insulin-like growth factor-1 (IGF-1) pathway, a key player in myotube formation and maturation.148 These findings underscore the potential of GO-PCL composites in promoting myogenic differentiation and maturation of muscle cells, offering promising implications for tissue engineering applications.
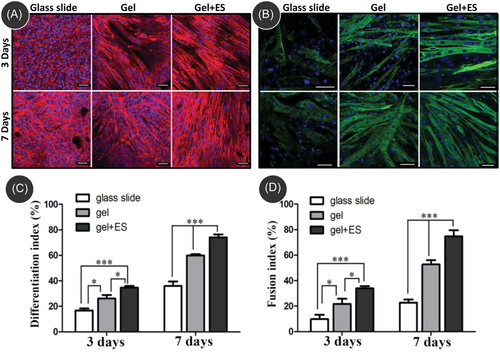
Even though successful SMTE works were demonstrated using carbon nanomaterials-incorporated electroactive scaffolds, limited research has been undertaken with the application of external electrical stimulations. However, the existing literature demonstrated that electrical stimulation on skeletal muscle cells cultured on a carbon-nanomaterial-incorporated electroactive scaffold enables faster maturation of the cultured muscle cells by promoting enhanced fusion of myoblasts to form myotubes.150-154 For example, Wang et al. fabricated a polydopamine (PDA)/reduced graphite oxide (rGO) ultralight and conductive aerogel and studied the cell culture behavior of C2C12 cells under an electrical stimulation of 5 V, 10 ms, 1 Hz for 4 h every day.154 The PDA/rGO scaffold exhibited significantly higher expressions for differentiation index and fusion index when compared to the control on glass slides (Figure 6). Furthermore, aerogel under electrical stimulation exhibited the highest quantified indexes of myosin heavy chain (MHC) expression, indicating an advanced differentiation state. Moreover, quantitative real-time polymerase chain reaction (qRT-PCR) analysis demonstrated a notable increase in the expression levels of genes, including α-actinin, MHC, and myogenin within PDA/rGO aerogels. Notably, the combined effect of electrical stimulation and PDA/rGO aerogels during a 7-day differentiation period further enhanced the upregulation of these gene expressions, emphasizing the synergistic impact of electrical stimulation and PDA/rGO aerogels on promoting myogenic differentiation. Furthermore, the synergistic effect also resulted in a synchronous contraction of myotubes within the PDA/rGO aerogel, implying the electroactive property of the aerogel, indicating its capability to facilitate the transmission of electrical signals throughout the muscle tissue.
4.4 Neural tissue engineering
Carbon nanomaterials have been popularly investigated for neural tissue engineering due to their superior electrical conductivity, mechanical stability, high mobility of charge carriers, and ability to mimic the native physiological conditions of neural cells.155-158 These properties also enabled the study of electrical stimulation on the neural cells cultured in carbon-based scaffolds.159-163 In a detailed and extensive study, Dong et al. investigated the use of graphene-incorporated electrospun PCL/collagen electrically conductive nanofiber scaffold (GCFS) for sciatic nerve regeneration and studied the effect of electrical stimulation both in vitro and in vivo experiments.164 The electrical conductivity of the scaffold increased with the amount of graphene incorporated; however, a higher amount of graphene imposed nanotoxicity on the cultured cells, limiting the concentration to 2 wt.%. The fibril scaffold featured good directionality of the fibers, closely resembling the architecture of nerve fibers and preferable for promoting axonal outgrowth, alignment, and elongation. The GCFS scaffold accelerated the neural differentiation of the cultured mesenchymal stem cells (MSCs), evaluated through enhanced expression of the early neural marker, Tuj1. The mature neuronal marker, NF-M, and the astrocytes-specific marker, GFAP, also exhibited higher expression levels on the CGFSs than on the scaffolds without graphene. The application of electrical stimulation (2 Hz, 10 min daily) further accelerated the neural differentiation of MSCs, particularly on the GCFS scaffolds, compared to the scaffolds without graphene (Figure 7A). Notably, comparable or better differentiation results were achieved at a lower intensity of the electrical pulse for the GCFS scaffold (10 or 20 mV/cm) in a short time period (3 days) than the nongraphene scaffold (50 mV/cm) (Figure 7B). Furthermore, in vivo studies yielded results indicating that exogenous electrical stimulation significantly enhances sciatic nerve regeneration and functional recovery when utilizing the GCFS conduit to bridge the severed sciatic nerve. Daily electrical stimulation exhibited high therapeutic efficacy in the recovery of motor and sensory function, nerve conduction function, targeted gastrocnemius muscle morphology, as well as regeneration and remyelination of the injured nerve. These outcomes surpassed the effects of a 1-h brief electrical stimulation and were comparable to the gold standard autograft. A similar improvement was also observed while culturing HT22 hippocampal neuron cells on CNT-reinforced chitosan scaffold and applying electrical stimulation,166 where CNT/chitosan scaffolds induced adhesion of HT22 cell line and promoted neurite extension like pyramidal cells (radial neurite extension), which is necessary for the scaffolds intended to regenerate brain cells, due to better mechanical stability, morphological alignment, and electrical conductivity. In another study, Nekounam et al. investigated the effect of different surface functionalization of electrospun CNFs obtained from nitric acid, ethylenediamine, and oxygen plasma treatment on the neural differentiation of MSCs under electrical stimulation (10 min each day by a 1.5 mA current with a frequency of 500 Hz).160 The surface functionalization led to better differentiation results than pristine CNFs. The CNFs functionalized with amine and oxidative groups exhibited accelerated neural differentiation, which could be attributed to better scaffold-cell interaction. Even though the aminated CNFs yielded the highest proliferative effects on MSCs in the absence of electrical stimulation, plasma-treated CNFs with oxidative functional groups exhibited the highest neural gene expression under electrical stimulation, which could be due to enhanced hydrophilicity and unaltered surface morphology.
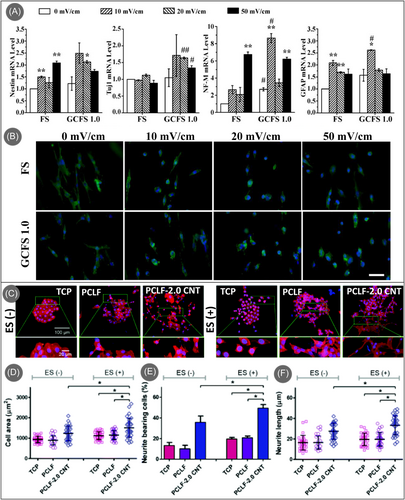
Under electrical stimulation, carbon-based conductive scaffolds result in accelerated neural differentiation and can impact the neural cells’ morphology. Zhou et al. reported that PC-12 cells cultured on CNTs-incorporated polycaprolactone fumarate (PCLF) scaffolds showed significantly more cell spreading and neurite extension under electrical stimulation (Figure 7C), whereas control TCP and pristine PCLF scaffolds did not result in any difference.165 The increased cell area, percentage of neurite-bearing cells, and neurite length of the cultured cells in the CNT/PCLF scaffold, as presented in Figure 7D–F, demonstrated that electrical stimulation along with the presence of CNTs promoted neurite outgrowth and neuronal differentiation, suggesting better neuronal maturity. Furthermore, this synergistic activity led to the enhanced intercellular junction when compared to the absence of electrical stimulation, which is critical for intercellular communication and nerve regeneration.
4.5 Skin tissue engineering
Electrical stimulation has been reported as a tool to accelerate the wound-healing process by promoting the migration and proliferation of fibroblasts and epithelial cells.167 Carbon nanomaterial-based structures have been previously reported for skin tissue engineering.168 However, they have been minimally investigated as conductive scaffolds for wound healing, probably due to the already extensive range of strategies reported in this research area.169 For example, Liang et al. reported the use of CNTs on an injectable composite hydrogel for wound dressing. The addition of CNTs improved the electrical conductivity of the hydrogels and showed a faster in vivo wound closure.170 However, the authors did not study the effect of external electrical stimulation on the rate of wound healing, which would be an interesting future direction yet to be evaluated. Similarly, Li et al. investigated the potential of a graphene foam scaffold to improve skin wound healing. These scaffolds, loaded with mesenchymal cells, provided key cues for the healing outcome for both the transplanted and the host cells, as shown in a rat model.171 Nevertheless, the authors did not study how the foam's electrical stimulation could impact cell viability or cell differentiation, as it has been shown for neural stem cells.172
Nonetheless, Di Luca et al. reported the use of graphene oxide hydrogels as a drug delivery system for smart skin bandages upon external electric stimulation.173 They showed how the release profiles of curcumin were modulated by the introduction of graphene oxide nanoparticles in the presence of external voltages (12–48 V) as a consequence of the swelling degree and the electrostatic repulsive forces between the curcumin and the hybrid hydrogels. This study is an example of the capabilities of carbon nanomaterial-based conductive scaffolds for skin regeneration. We believe that more comprehensive works in future research will unveil the potential of these conductive structures.
5 CURRENT CHALLENGES AND FUTURE PERSPECTIVE
In this article, we reviewed carbon nanomaterial-based conductive scaffolds for tissue engineering applications, where specific emphasis was given to the cases with the application of external electrical stimulation. In all tissue engineering applications, the conductive carbon-based scaffolds and electrical conductivity exhibited a synergistic activity in accelerating cellular differentiation from the parent cell lines and faster maturation, proving carbon materials an important biomaterial for regenerative medicine. Even though carbon nanomaterials have been successfully used as tissue engineering scaffolds in conjunction with electrical stimulation, the carbon nanomaterials were majorly limited to CNTs, graphene, graphene derivatives, and CNFs. Other carbon allotropes, such as carbon dots, fullerenes, and glassy carbon, have not been used extensively as conductive tissue engineering scaffolds.
Fullerenes are not good electricity conductors but may become conductive or superconductive when synthesized with alkali metals.174 Their biomedical applications, mainly linked to cancer treatment, anti-HIV activity, drug delivery, and biosensing, have been previously reviewed.175 Regarding applications in tissue engineering, fullerenes provide good support for colonization with human osteoblast-like cells of the lines MG-63, Saos-2, and U-2 OS, primary osteoblasts, and also human MSCs, which is promising for articular tissue engineering.176 Considering neural tissue engineering, water-soluble fullerenes bearing alanine residues have been investigated as companions for neural stem cells (NSCs), demonstrating the proliferation of NSCs promotion, differentiation induction for NSCs into neurons, and migration of NSCs inhibition. All this indicates remarkable potential as biomaterials for regeneration in nerve tissue engineering for diseases related to the nervous system.177 Processability of highly aligned 1D fullerene whisker scaffolds by interfacial alignment enables concurrent control over cellular orientation and differentiation to skeletal muscle cells and cardiomyogenic differentiation.178, 179 Nevertheless, the electrical properties of fullerenes, fullerene 1D whiskers, and 2D fullerene mats, their tunability, their eventual impact on enhancing cellular responses in vitro with fullerene-based tissue engineering scaffolds, and finally, their impact under electrical stimulation still need to be investigated in detail.
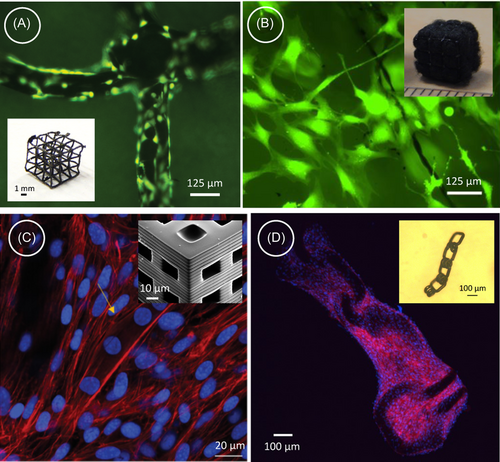
In recent years, 3D printing has emerged as an innovative method for fabricating design-controlled patient-specific scaffolds for tissue engineering. Carbon nanomaterials have also been incorporated within 3D printed scaffolds for producing conductive 3D scaffolds.25, 180, 181 However, in almost all cases, the carbon nanomaterials must be embedded within a polymeric matrix to enable the 3D printing process. Even though carbon-based polymeric composites exhibit promising results in tissue engineering, the carbon counterparts' electrical conductivity and mechanical cues are often compromised by the polymeric matrices. 3D printed pyrolytic carbon can be a viable solution to this. 3D printing of polymeric precursors and their subsequent pyrolytic conversion to carbon can lead to the fabrication of 3D complex scaffolds of pyrolytic carbon with resolutions ranging from a few hundred nanometers to a millimetric scale.182 The electrical and mechanical properties of these 3D-printed carbon structures can be further tuned by precise control over the process parameters. Recent studies have also investigated these 3D carbon structures for cellular interactions, evaluating their biocompatibility and ability to induce 3D cell culture for neural,183 bone,184, 185 and musculoskeletal applications.186 During cell culture, architected 3D pyrolytic carbon demonstrates superior cell adhesion. The intrinsic surface porosity of pyrolytic carbon offers abundant anchor sites, thereby promoting enhanced cell proliferation compared to the results obtained with the precursor counterpart.187 The hierarchical structure of multiscale 3D pyrolytic carbon further creates favorable microenvironments for cell growth, facilitating 3D cell colonization, as illustrated in Figure 8A,B for cultured osteoblast-like murine MC3T3-E1 cells cultured on microarchitected 3D pyrolytic carbon scaffold. This is particularly advantageous for applications in complex articular reconstructions and osteochondral regeneration. Furthermore, the 3D microarchitected carbon scaffold also induces myogenic differentiation owing to their high mechanical stiffness, as developed myotubes are indicated in Figure 8C.186 Apart from stationary rigid scaffolds, 3D printing technology further allows the fabrication of shape-morphing compliant structures of pyrolytic carbon. An example of such a compliant structure is presented in the inset of Figure 8D, which is also demonstrated for hosting myoblasts and inducing directional alignment of actin fibers.186 However, the electrical properties of these 3D carbon scaffolds and their influence on cell culture are yet to be evaluated. There is a vast space for research on 3D-printed carbon scaffolds and their implications in tissue engineering, particularly under electrical stimulation.
Carbon dots' nearly 0D nature, characterized by sizes of less than 10 nm, limits their application as standalone structural materials for repairing large defects in articular injuries and in the broader field of tissue engineering. However, their captivating properties position them as excellent companions to other structural materials, allowing for advancements in highly innovative diagnostic procedures and therapies. However, incorporating carbon dots into tissue engineering scaffolds is promising, as recent research highlights. For instance, evidence demonstrates that carbon dots can serve the dual purpose of tracking and enhancing the osteogenic differentiation of mesenchymal stem cells, showcasing their potential relevance in advancing bone tissue engineering.188
Even though carbon nanomaterials have been successfully employed in every aspect of tissue engineering, the potential of conductive scaffolds and the implications of electrical stimulations have not been explored yet in dental tissue engineering. Dental tissue engineering is a special area, as it strongly overlaps muscle tissue engineering and bone tissue engineering.189, 190 Carbon nanomaterials have also exhibited great promise in this area, mainly to improve relevant properties for dental implants, such as mechanical properties, corrosion resistance, osteogenic properties, and antibacterial properties.191, 192 However, the distinctive electrical properties of carbon nanomaterials offer a versatile opportunity for integration with microelectronics. This integration allows for stimulating, controlling, and manipulating cellular behaviors. Additionally, it facilitates the diagnosis and monitoring of oral and periodontal diseases.
Another interesting research direction is linked to taking advantage of the electrical properties of carbon scaffolds for the development of self-sensing implants and for the extracorporeal evaluation of scaffolds' integrity and healing or regenerative processes. Indeed, electrical conductivity modifications can be monitored from outside the body in noninvasive manners employing adequate antennae193 or near-field communication.194 During the healing of large-scale bone defects, small-sized carbon scaffolds, introduced in a minimally invasive surgical way and designed for self-assembly in connection with four-dimensional printing,195 would arguably become interconnected, and the whole regenerative construct would decrease its electrical resistance under load. These evolutions could be monitored and correlated to innovative figures of merit describing healing and regeneration. Conversely, an electrical conductivity decrease could detect eventual cracks initiated during the carbon scaffold lifecycle, leading to mid or long-term failure. Incorporating biodegradable antennas into the scaffolding structure could also provide useful information regarding postsurgical infections.196 If the scaffolds are employed for tissue engineering after tumor surgery, recurrence could also be monitored from outside the body.197 Analyzing the compatibility of carbon scaffolds with these extracorporeal assessment strategies is hence proposed.
Anticipating continued advancements in the field, we expect increased efforts dedicated to carbon-based conductive scaffolds in tissue engineering and regenerative medicine. With the ongoing innovation in scaffold fabrication, particularly through advanced technologies like 3D printing, there is a growing potential to create scaffolds with controlled porosity, tailored properties, and high bioresorbability, as evidenced by preliminary explorations. The research space can be wider by including more carbon allotropes for additional functionality and electrical conductivity. This feasibility could significantly expedite the applications of carbon materials as tissue engineering scaffolds and further open opportunities for investigating the applications of other external stimuli.
Notwithstanding all the above, a special reference should be made to the relevance of fulfilling medical regulations and performing exhaustive in vitro tests before progressing towards animal models and preclinical evaluations for demonstrating the safety and effectivity of developed medical devices based on the use of electrically conductive carbon nanomaterials. Both in the United States according to the FDA regulations and in the European Union under MDR 2017/745, most implantable solutions analyzed in this study would be classified as high-risk medical devices (Class III), which are subject to controls and premarket approval by competent authorities or notified bodies. Although the biocompatibility of carbon-based materials and nanomaterials is often praised and carbon nanomaterials have been even considered ideal for tissue engineering applications,96, 198, 199 some studies have also put forward specific concerns regarding the long-term performance or integration of carbon implants after several months or years inside the body.200-202 Accordingly, to progress towards technological transfer to society, the guidelines of ISO standard 10993 for systematically evaluating the biocompatibility of developed solutions employing electrically conductive carbon nanomaterials should be considered a requisite. In agreement with this standard, the employment of ISO 14971 for promoting risk management strategies in developing medical devices is also advised. Ideally, for minimizing animal trials following the 3Rs principles (Replace, Reduce, Refine), methodic use of simulations or digital twins, characterization results, and in vitro/ex vivo studies should be fostered to approach in vivo studies with the highest possible responsibility and care. Research projects should be conducted specifically focused on systematically gathering and generating biocompatibility data linked to the variety of electrically conductive carbon nanomaterials available and to their imaginable configurations as medical devices. The use of open-science strategies and FAIR data principles for sharing the obtained results would also empower the straightforward usability of these materials and the development of biodevices based on them.
AUTHOR CONTRIBUTIONS
Genevieve Abd: Data curation (equal); formal analysis (equal); investigation (equal); writing—original draft (equal); writing—review and editing (equal). Raquel S. Díaz: Data curation (supporting); writing—original draft (supporting); writing—review and editing (supporting). Anju Gupta: Formal analysis (supporting); writing—review and editing (supporting). Tagbo H. R. Niepa: Formal analysis (supporting); writing—review and editing (supporting). Kunal Mondal: Conceptualization (equal); data curation (supporting); formal analysis (supporting); investigation (supporting); methodology (supporting); project administration (supporting); supervision (supporting); writing—original draft (equal); writing—review and editing (equal). Seeram Ramakrishna: Formal analysis (supporting); investigation (supporting); writing—original draft (supporting); writing—review and editing (supporting). Ashutosh Sharma: Formal analysis (supporting); project administration (supporting); writing—review and editing (supporting). Andrés D. Lantada: Data curation (supporting); formal analysis (supporting); investigation (supporting); validation (supporting); writing—original draft (equal); writing—review and editing (equal). Monsur Islam: Conceptualization (lead); data curation (lead); formal analysis (lead); investigation (lead); methodology (lead); project administration (lead); writing—original draft (lead); writing—review and editing (lead). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors have nothing to report.
CONFLICT OF INTEREST STATEMENT
Author Raquel S. Díaz is an employee in Zoetech S.L., but has no potential relevant financial or non-financial interests to disclose. The other authors have no conflicts of interest to declare.
ETHICS STATEMENT
The authors have nothing to report.
Open Research
DATA AVAILABILITY STATEMENT
The authors have nothing to report.




