Advances of Microneedles for Cancer Diagnosis and Therapy
Chunli Yang and Li Zhang have contributed equally to this study.
ABSTRACT
As the global incidence of cancer continues to rise, the need for innovative and precisive alternatives to conventional diagnostic and therapeutic methods has become increasingly urgent. Microneedles (MNs), comprising arrays of micron-scale projections, have emerged as a platform that provides a painless and minimally invasive system for interstitial fluid analysis and transdermal drug delivery. This technology demonstrates potential in cancer diagnostics through continuous biomarker monitoring using integrated biosensors, while enabling controlled release of chemotherapeutics, photothermal/photodynamic, and immunotherapeutic agents. Over the past decades, significant advancements in technology, materials, and medical applications have been achieved in the MN field, which have attracted increasing attention of reseachers. Currently, several MN-based strategies registered on ClinicalTrials.gov are actively investigating their applications in medince, positioning them as a promising new tool for cancer treatment and diagnosis. This review offers a comprehensive summary of the classification, design, fabrication materials, and techniques of MNs, along with their medical applications in cancer diagnosis, therapy, and the management of cancer-associated pain and hair loss. Furthermore, this review summarized ongoing clinical trials in investigating MN-based therapies for cancer patients. And challenges, future perspectives of applying MNs in cancer theranostics are also presented.
1 Introduction
Cancer is the second leading cause of death in the United States and has emerged as a major public health challenge in China [1, 2]. Early detection and treatment of cancer are crucial for improving patient prognosis [2]. Current diagnostic approaches primarily rely on invasive procedures to obtain tumor tissue for pathological evaluation; however, these procedures may carry a potential risk of tumor metastasis [3-5]. An increasing number of emerging technologies and tools are being developed to explore minimally invasive and noninvasive methods for tumor diagnosis [6-9]. These approaches leverage patient blood or tissue fluid to capture tumor cells, proteins, or nucleic acids, showing significant potential for clinical application. Cancer treatment modalities mainly include surgery, chemotherapy, radiotherapy, and immunotherapy [10-13]. In clinical practice, chemotherapy and immunotherapy primarily involve delivering drugs to the patient through intravenous administration or oral intake to target and eliminate tumor cells. While these treatments have indeed resulted in improved therapeutic outcomes, some patients still experience severe adverse effects due to administration routes, such as venous thrombosis [14-16], which can lead to treatment discontinuation or even death. Therefore, selecting an optimal drug administration route can, to a degree, facilitate effective tumor management while minimizing adverse effects, contributing to improved patient survival.
Microneedles (MNs) generally consist of arrays of micrometer-sized needles with a height that ranged from 25 to 1500 μm [17]. They can create micron-scale channels in the skin while avoiding nerves and blood vessels in the dermis, precisely reaching different layers of the skin for various purposes. MNs are painless, offer precise control, and are both flexible and user-friendly, making them advantageous for drug delivery [18-21]. These attributes offer several advantages over conventional invasive injection methods and/or oral delivery strategies. Drug delivery mechanisms based on MNs primarily include transdermal delivery, controlled release, sustained release, and targeted delivery. Transdermal delivery refers to the use of MNs to penetrate the stratum corneum, creating microchannels that allow drugs to directly enter the subcutaneous tissue or systemic circulation. It overcomes the barrier of conventional transdermal drug administration and enhances bioavailability. Controlled release relies on the materials and design of MNs, such as biodegradable polymer MNs or stimuli-responsive MNs. It enables drug release under specific conditions (e.g., pH, temperature, and enzymatic activity) to improve therapeutic efficacy. Sustained release facilitates the stable release of drugs over an extended period, for instance, through the use of sustained-release MNs or MNs encapsulating nanoparticles. It can reduce dosing frequency, maintain consistent plasma drug concentration, and improve patient compliance. Targeted delivery can be achieved by functionalizing MN surfaces with biomolecules such as antibodies and receptor ligands, or by integrating nanocarriers to enable precise drug delivery to specific tissues or cells. It enhances treatment efficiency while minimizing systemic side effects. Optimization of these delivery mechanisms contributes to the advancement of MN technology in clinical drug delivery applications.
Recently, interstitial fluids have been established as reservoirs for various disease biomarkers, including those for tumors. They can also be utilized for early detection, continuous monitoring, and disease management [22-25]. Compared to traditional monitoring methods, MN-based sampling is a minimally invasive and painless approach. It can be integrated with nanomaterials and wearable devices to monitor cancer progression, treatment efficacy, and recurrence while minimizing patient discomfort and the risk of infection [26-30]. Therefore, MNs exhibit significant clinical potential in tumor diagnosis and treatment.
This review comprehensively summarizes the classification, design, and fabrication techniques of MNs, as well as their applications in cancer diagnosis, treatment, and management of cancer-associated pain and hair loss (Figure 1). It also explores ongoing clinical trials investigating MN-based therapies for cancer. Finally, the review addresses the challenges and future perspectives related to the application of MNs in cancer theragnostics.
2 MNs Category, Design, and Fabrication
2.1 Category
Although some drugs are capable of penetrating human skin, their absorption efficiency remains relatively low. MN technology offers a solution by significantly enhancing the skin's permeability to these drugs. According to drug delivery characteristics, MNs can be broadly classified into five types: solid MNs, hollow MNs, coated MNs, dissolving MNs, and porous MNs [18]. As illustrated in Supporting Information Figure S1, these MN designs facilitate the delivery of therapeutics through various manners.
Solid MNs are primarily designed for skin pretreatment, where they create micropores by penetrating the stratum corneum. They facilitate transdermal drug delivery through passive diffusion or drug-loaded patches [31]. Their high mechanical strength ensures robust penetration without bending or breakage, and their manufacturing simplicity allows for scalable production using materials like silicon, metals, ceramics, and polymers [32]. However, solid MNs face challenges such as poor drug dose accuracy and potential risk of bioincompatible toxic residues, which may pose safety concerns [33]. To address these limitations, innovative approaches are being explored, including surface modifications and functional coatings to enhance drug adhesion and release. Smart solid MNs with stimuli-responsive coatings enable controlled drug release triggered by pH, temperature, and enzymatic activity. Additionally, the integration of solid MNs with wearable electronic devices allows for real-time monitoring of skin impedance, hydration levels, and drug diffusion dynamics, optimizing treatment precision. Through continuous advancements in design, functionality, and innovation, solid MNs hold significant potential for enhancing transdermal drug delivery efficiency and expanding their clinical applications [34].
Coated MNs consist of a sharp, solid-core structure with a thin layer of drug-loaded coating that dissolves rapidly upon skin penetration, enabling efficient and minimally invasive transdermal drug delivery [35]. The solid core provides high mechanical strength, ensuring stable insertion without breakage, while the versatile coating technology allows for the precise loading of various therapeutic agents, including small molecules, proteins, vaccines, and nucleic acids [36]. Despite their advantages, coated MNs face challenges such as inconsistent drug loading, potential drug detachment during insertion, and limited drug dosage capacity due to the thin coating layer [36]. To address these issues, advanced coating strategies such as electrostatic deposition, layer-by-layer self-assembly, and bioadhesive coatings have been developed to enhance drug adhesion, stability, and controlled release. Additionally, innovations in nanotechnology and bioengineering have led to functionalized coatings that enhance drug stability, improve skin permeation, and enable combination therapies. With these advancements, coated MNs continue to evolve as a promising platform for needle-free vaccination, localized drug delivery, and personalized medicine [26].
Hollow MNs are designed with a central hollow channel that enables direct injection of liquid drug formulations into skin, following a “poke and flow” mechanism [37, 38]. This unique structure allows for precise and controlled drug administration, bypassing stratum corneum and ensuring rapid absorption into targeted tissue. Hollow MNs can be used for a wide range of applications, including the delivery of biologics, vaccines, and personalized medicine, as they facilitate the administration of large molecules with controlled infusion rates. Additionally, they provide the flexibility to adjust drug dosage dynamically, making them suitable for real-time therapeutic modulation. However, these MNs face challenges such as potential microchannel blockage, which can hinder drug flow and reduce efficacy. To address this issue, advanced designs incorporating off-center bores, multi-channeled architectures, and anti-clogging surface modifications have been explored to improve fluid dynamics and maintain consistent drug delivery. Furthermore, innovations such as pressure-controlled infusion systems, integration with wearable drug delivery devices, and bioresponsive hydrogels aim to enhance the precision and efficiency of hollow MN-based therapies. With ongoing advancements, hollow MNs hold great potential for minimally invasive, on-demand drug administration and real-time precision medicine.
Dissolving MNs are fabricated entirely from biodegradable materials, either incorporating the drug within the matrix or consisting entirely of the active pharmaceutical ingredient [39, 40]. Upon insertion into the skin, these MNs dissolve or degrade at a controlled rate, enabling rapid or sustained drug release depending on the formulation. Biodegradable polymer-based dissolving MNs represent a key advancement in transdermal drug delivery, as they eliminate the need for needle removal. They can reduce the risk of sharp waste and enhance patient compliance [41, 42]. Compared to other types of MNs, dissolving MNs can encapsulate a higher drug payload, making them particularly suitable for vaccines, peptides, and macromolecular therapeutics. Furthermore, advancements in material engineering, such as the incorporation of stimuli-responsive polymers, enable precise control over drug release kinetics based on environmental triggers like pH, temperature, and enzymatic activity. Innovations in fabrication techniques, including 3D printing and layer-by-layer drug loading, further enhance the structural integrity, mechanical strength, and drug distribution efficiency of dissolving MNs. These technological improvements position dissolving MNs as a promising platform for efficient drug delivery, particularly in chronic disease management, immunization, and personalized medicine applications [43].
Porous MNs are primarily fabricated from hydrogels, leveraging their hydrophilic properties to achieve controlled drug delivery [44, 45]. Upon insertion into the skin or tissue, these MNs rapidly absorb interstitial fluid, triggering swelling and facilitating the release of encapsulated therapeutics [46, 47]. Drug loading in porous MNs can be achieved either by incorporating active agents directly into the polymeric network during fabrication or by utilizing an external drug reservoir attached to the MNs. One of their key advantages is the enhanced drug-loading capacity and tunable release kinetics, which are directly influenced by polymer crosslinking density [48]. Unlike traditional MNs, porous MNs enable sustained and responsive drug release without requiring dissolution or degradation, making them ideal for continuous drug administration in chronic disease management. Recent innovations include the integration of smart hydrogels that respond to physiological cues such as pH, temperature, and enzyme activity, enabling on-demand drug release with high precision. Additionally, advancements in microfabrication techniques, such as 3D printing and electrospinning, have further optimized the porosity, mechanical strength, and functional versatility of these MNs, expanding their potential applications in transdermal drug delivery, biosensing, and regenerative medicine [49].
The distinct types and mechanisms of action of MNs result in varying advantages and limitations, enabling them to fulfill diverse roles in cancer diagnosis and therapy. A summary of the types of MNs, along with their respective advantages and limitations, is provided in Supporting Information Table S1.
2.2 Materials, Design, and Fabrication
MNs can be made from various materials, including metals, polymers, glass, and silicon, each offering distinct advantages and limitations [50, 51]. Metal MNs possess sufficient mechanical strength to penetrate the skin without undergoing deformation, while polymer MNs typically require structural reinforcement to achieve comparable strength. In contrast, materials like glass and silicon, although capable of piercing the skin, are inherently brittle and pose a significant risk of breakage during insertion [52, 53]. Fractured MNs embedded in skin tissue can result in complications such as pain, swelling, or granuloma formation. To mitigate these risks, MN development has increasingly prioritized the use of biodegradable and biocompatible materials [54-56]. This strategy aims to prevent adverse outcomes, such as retained needle fragments, by allowing the materials to degrade safely within the body over time. In addition to mechanical properties, material selection is affected by the specific biomedical application, including drug delivery, tissue engineering, and implantable devices. By combining different materials or leveraging advanced fabrication techniques, researchers aim to enhance the safety, efficacy, and versatility of MNs in clinical and therapeutic applications.
The selection of the fabrication for MNs depends on their type, geometry, and material. Researchers in academia and industry have developed numerous methods for MN fabrication at different scales. Several reviews on MN fabrication techniques can be found in the literature [57-61]. In brief, MN fabrication methods include microelectromechanical systems (MEMSs) [62], etching [63], micromolding [64], laser-mediated fabrication techniques (e.g., laser cutting and laser ablation) [65], drawing-based methods (e.g., mechanical force drawing, electro-drawing, and centrifugal drawing) [66-68], atomized spraying method [69], injection molding [70], micro-mechanical machining [71], additive manufacturing (fused deposition modeling (FDM), stereolithography (SLA), digital light processing (DLP), continuous liquid interface production (CLIP), and two-photon polymerization (TPP) [33, 34, 72-74]. Among these, micromolding is the most frequently employed technique to produce MNs.
In addition to the materials and fabrication methods used for MNs, several other critical features should be carefully considered. These include the uniformity of MN performance, structural strength, and geometric factors such as height, width, density, aspect ratio, platform flexibility, needle brittleness, tip thickness, uniform degradation, and signal transmissibility [75-79]. Each of these features can significantly impact MN efficiency, particularly in medical applications. Simultaneously, the drug being loaded should also be considered. For example, cryoMNs may be more suitable for loading living cells, while increasing drug dosage may necessitate the use of dissolving MNs [49, 80, 81].
3 MNs Medical Applications
3.1 MNs for Cancer Diagnosis
Traditional methods of tumor diagnosis primarily include imaging, biopsy, and blood tests [82-84]. While these methods are widely utilized in clinical practice, they often exhibit certain limitations. For instance, although imaging techniques can provide information on tumor location and size, their ability to distinguish between benign and malignant characteristics is limited [85, 86]. Biopsies, despite offering pathological evidence, are highly invasive and may lead a potential risk of tumor metastasis or other complications, making them unsuitable for long-term monitoring [87]. Blood tests often rely on the concentration changes of circulating tumor cells or tumor biomarkers, but these tests tend to have low sensitivity and struggle to provide real-time insights into tumor status [88, 89]. Additionally, these diagnostic approaches are associated with invasive procedures, patient discomfort, and high costs. Therefore, there is an urgent need to develop minimally invasive diagnostic tools that offer greater flexibility and real-time monitoring capabilities to improve cancer detection rates.
The occurrence and progression of tumors are often accompanied by specific molecular and cellular changes, which can be reflected through biomarkers present in interstitial fluid (ISF), such as nucleic acid fragments, proteins, metabolites, and cytokines [90-92]. Effective analysis of these markers can aid in the early detection, monitoring, and evaluation of treatment responses in tumors, laying the foundation for the development of new therapeutic approaches. MNs can extract or directly analyze ISF through the skin, allowing for the detection and monitoring of tumor biomarkers and offering distinct application advantages [24, 29]. For example, by capturing circulating proteins or analyzing specific biomarkers in exosomes, MNs can enhance the specificity of tumor type identification. Additionally, the integration of MNs with nanomaterials and smart sensors facilitates the development of wearable, portable smart diagnostic devices, further enhancing their applications in cancer diagnosis [27].
Yang et al. reported a novel DNA hydrogel-based MN array for rapidly collecting and sensitively detecting specific microRNAs in skin ISF [93] (Supporting Information: Figure S2A). The MN patch, consisted of methacrylate hyaluronic acid (MeHA) equipped with smart DNA circuit hydrogels' system (MeHA/DNA), efficiently collects sufficient ISF and enables sensitive detection of low-abundance microRNAs via DNA displacement reactions. This study presents new ideas for minimally invasive personalized diagnostics and real-time health monitoring, particularly in cancer detection. Chen et al. proposed an early diagnostic method based on ISF analysis for breast cancer [94]. This approach utilizes MNs and a nanosilver/MBL membrane for noninvasive, painless sampling, combined with a nanosilver-based colorimetric immunoassay platform for detection. Compared to traditional blood tests, this method can diagnosis breast cancer 7 days earlier and more accurately reflect the progression of early-stage tumors, offering promising support for early screening and diagnosis. For melanoma diagnosis, Huang et al. developed a wearable MN patch platform combined with trimetallic Au@Ag-Pt nanoparticles to detect tyrosinase (TYR) levels directly on the skin, enabling potential melanoma screening [95]. Wang et al. introduced a two-step strategy involving expandable MNs for ISF sampling and a microfluidic particle dam for the visual quantification of S100A1, offering high sensitivity and specificity for melanoma monitoring and diagnosis [96] (Supporting Information: Figure S2B). Increased interleukin-8 (IL-8) expressions are observed in many cancer types, and elevated IL-8 levels have been identified as a prognostic factor [97, 98]. Song et al. introduced a microporous-based impedance sensor integrated into implantable MNs for the rapid, label-free detection of cytokines and other biomarkers [99] (Supporting Information: Figure S2C). The researchers validated the sensor's specificity and sensitivity through in vitro experiments, demonstrating its ability to measure human interleukin-8 (hIL-8) concentrations in transgenic mice in real time. This technology represents a novel tool for continuous monitoring of cytokine levels, offering significant potential for disease diagnosis and therapeutic monitoring.
3.2 MN-Mediated Drugs Delivery for Cancer Treatment
In current cancer treatment options, aside from surgical intervention, other therapeutic approaches such as immunotherapy, gene therapy, chemotherapy, and photothermal/photodynamic therapy require the delivery of therapeutic agents into the body. MNs offer several significant advantages in cancer treatment, making them a promising alternative to conventional drug delivery methods. As a minimally invasive and painless administration approach, MNs penetrate the stratum corneum without reaching deep nerve endings, significantly reducing pain and discomfort compared to hypodermic injections. Improved patient compliance is another key benefit. MN patches enable self-administration without requiring specialized medical personnel, making long-term and repeated treatments more feasible, especially for cancer management. Enhanced drug stability and bioavailability are achieved through MN-mediated transdermal or localized delivery. It can protect biologics such as proteins, peptides, and nucleic acids from enzymatic degradation in the gastrointestinal tract while facilitating efficient absorption into target tissues. Reduced systemic toxicity is another crucial advantage. MNs enable precise drug delivery to tumor sites. They can minimize systemic drug exposures and associated side effects, which are particularly beneficial for chemotherapy and immunotherapy, where off-target toxicity can be severe. Additionally, MNs can incorporate controlled and sustained release mechanisms, ensuring prolonged therapeutic effects while reducing dosing frequency. These advantages collectively position MNs as an innovative and patient-friendly platform for enhancing the efficacy and safety of cancer treatments. Moreover, MNs can simultaneously load multiple drugs for synergistic therapy, and specifically designed MN patches can enable spatiotemporal drug delivery. In recent studies, various MN-based tumor treatment strategies have been developed, demonstrating excellent therapeutic outcomes. These strategies include immunotherapy, chemotherapy, gene therapy, photothermal and photodynamic therapy, as well as various combination therapies.
The combination of MN technology with immunotherapy, gene therapy, chemotherapy, and photodynamic therapy exhibits significant synergistic effects in cancer treatment. In immunotherapy, MNs enhance antigen delivery to antigen-presenting cells (APCs), such as dendritic cells in the skin, thereby promoting local immune activation and improving immune response to cancer vaccines. Additionally, MNs can be used to deliver immune checkpoint inhibitors locally, facilitating precise immune modulation. In gene therapy, MNs enable efficient delivery of tumor-suppressor genes or RNA interference molecules, overcoming the low transfection efficiency of in vitro methods and potential toxicity of viral vectors while enhancing the specificity of gene editing for tumor cells. In chemotherapy, MNs facilitate localized drug delivery, minimizing systemic toxicity and side effects while enabling sustained drug release through biodegradable MNs or nanoparticle encapsulation. They can increase intratumoral drug concentrations and enhance therapeutic efficacy. In photodynamic therapy, MNs can be used to load and precisely deliver photosensitizers into tumor tissues, improving the efficiency of photodynamic reactions. Additionally, when combined with near-infrared light irradiation, MNs can enhance the localized destruction of tumor cells. By integrating MN technology with these cancer treatment strategies, the targeting and effectiveness of therapies can be improved. Adverse effects can also be reduced, and new avenues for personalized cancer treatment can be explored.
3.2.1 Immunotherapy
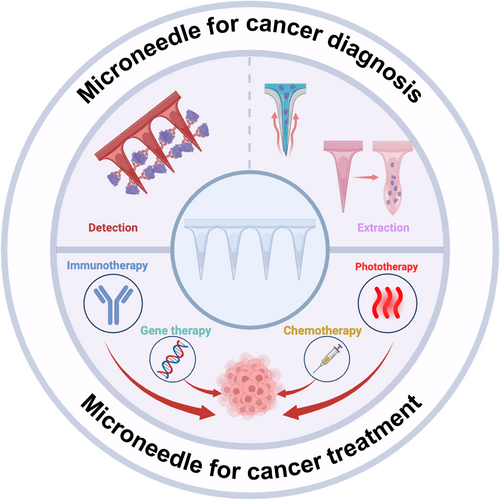
Immunotherapy is an approach that enhances or modulates the body's own immune system to fight diseases and is widely used in cancer treatment. It works by activating or boosting the function of immune cells, enabling them to recognize and attack cancer cells, or by inhibiting suppressive signals to prevent immune system evasion by cancer cells. As a result, it has become a crucial approach in cancer treatment [100, 101]. Currently, various immunotherapies, such as adoptive cell therapy, immune checkpoint inhibitors, cytokines, and cancer vaccines, have achieved promising results in various cancer patients [102-106]. MNs can deliver immunotherapeutic drugs directly to tumor sites or administer intradermal injections to enhance the immune response. Joo et al. combined micro-resolution Digital Light Processing (DLP) 3D printing technology and micro-molding techniques to fabricate self-locking MNs that can fully insert into and lock within skin tissue, aiming to enhance the precision of drug dosage delivery [107]. Dissolving self-locking MNs were fabricated by pouring hyaluronic acid (HA) solution containing (αPD-L1 Ab)/SD-208 (a TGF-β receptor type I kinase inhibitor) into the PDMS molds, followed by centrifugation to fill the micro-cavities of the molds (Figure 2A,B). The transcutaneous application of αPD-L1/SD-208 combination therapeutics-loaded self-locking MN patches onto melanoma tumor sites showed accurate microdosage delivery and significantly enhanced antitumor effectiveness compared with intratumoral injection. It was likely attributed to the precise dosage delivery and sustained local release of drugs from the MN matrix (Figure 2C,D). Chang et al. designed a cryomicroneedle (cryoMN) patch for the intradermal delivery of dendritic cell vaccine [80] (Figure 2E). The patch was fabricated by the micro-molding method, in which optimized cryogenic medium and living dendritic cell (DC) vaccine were added into the mold. The prepared cryoMNs were preserved at low temperatures before usage. In a melanoma model, the cryoMN-delivered DC vaccines induced stronger antigen-specific immune responses and significantly enhanced antitumor capabilities compared to both intravenous and subcutaneous injections.
3.2.2 Gene Therapy
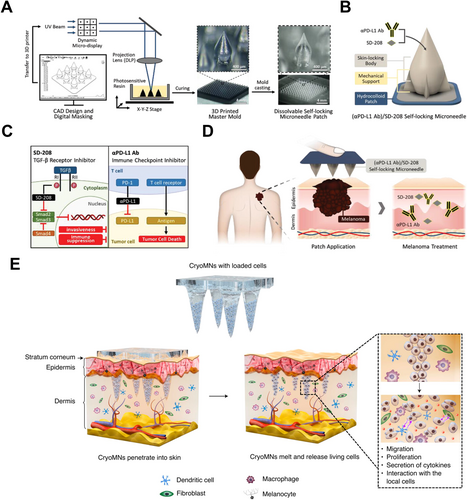
Gene therapy, as an emerging cancer treatment approach, aims to regulate and correct abnormal expression of genes in tumor cells, providing a promising strategy for tumor treatment. The success of this therapy heavily depends on the safe and efficient delivery system. Traditional methods of nucleic acid delivery, such as electroporation, can cause significant damage to cells and are difficult to apply In Vivo, while both viral and nonviral vectors raise concerns regarding safety and immunogenicity [108-111]. So far, MN technology has been recognized as an effective method for gene or drug delivery. By forming microchannels on the skin surface, MNs enable the rapid accumulation of gene-based drugs at skin tumor sites, bypassing the issues of drug degradation, systemic accumulation, and associated side effects at nontarget sites [19, 112, 113]. CRISPR technology is one of the most promising tools in cancer gene therapy, utilizing the Cas9 protein to achieve precise and efficient gene editing [114-117]. Wang et al. developed a dissolving MN system embedded with nanoparticles (IR780-PL/pFBXO44@MNs) capable of delivering both a FBXO44-targeted CRISPR/Cas9 plasmids (pFBXO44) and a hydrophobic photosensitizer [118] (Figure 3A). Under 880 nm irradiation, the IR780-PL/pFBXO44 nanoparticles generate reactive oxygen species for photodynamic therapy. Additionally, the photochemical internalization induced by the therapy accelerates lysosomal escape, leading to the release of the pFBXO44 plasmid into the cytoplasm. It effectively disrupts FBXO44 in cancer cells and inhibits their proliferation and metastasis. Small interfering RNA (siRNA), one of the major nucleic acid drugs in cancer therapy, has been designed to silence key gene expressions in cancer cells [119-122]. Yang et al. designed a rolling MN electrode array (RoMEA) for low-damage and large-area siRNA transfection [123] (Figure 3B). The device features circular blades with sharp MNs at the edges arranged in parallel as electrodes. It allows the MNs to penetrate the stratum corneum while generating a uniform electric field during rolling. The results demonstrated that siRNA delivered via RoMEA effectively suppressed the expression of PD-L1 in tumor cells, preventing immune evasion and significantly inhibiting tumor growth. Furthermore, the coadministration of anti-PD-1 monoclonal antibodies or CpG 2395 (a class C CpG ODN) could block the PD-L1/PD-1 interactions between tumor cells and T cells. It resulted in increased in CD8+ T and CD4+ T cell populations, further enhancing the antitumor response.
3.2.3 Chemotherapy
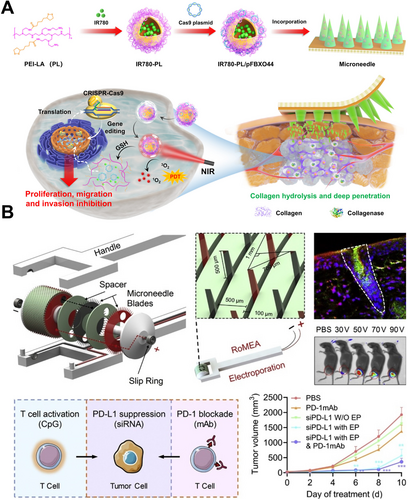
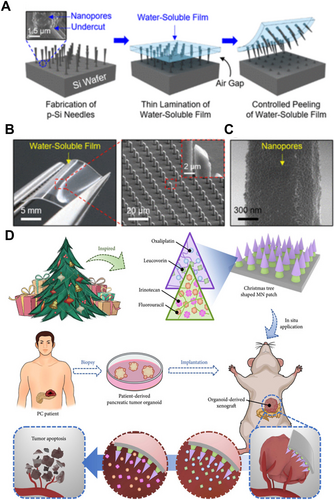
Chemotherapy, as one of the most widely used and well-established cancer treatment modalities, functions by interfering with the division and reproduction of cancer cells, thereby preventing their growth [124]. However, due to its lack of tumor cell specificity, chemotherapy also affects other rapidly dividing normal cells in the body, leading to severe adverse effects [125-127]. Thus, localized delivery of chemotherapy drugs to tumor sites can reduce systemic side effects. MNs for have the advantage of delivering chemotherapy drugs uniformly to tumor sites compared to traditional injection needles. Furthermore, by customizing the materials and structures of MNs, it is possible to achieve intelligent drug delivery and simultaneous delivery of multiple agents. Kim et al. designed a biodegradable small porous silicon (p-Si) MN system (Figure 4A,B), which is fixed onto a flexible water-soluble film and covalently linked to the chemotherapeutic agent doxorubicin (DOX) for melanoma treatment [128]. Upon insertion of the p-Si MNs into tissue, the water-soluble film can dissolve within 1 min when exposed to external physiological saline, while the nanoscale p-Si MNs remain in the tissue, making them inconspicuous (Figure 4C). Experimental results indicated that the p-Si MNs gradually degrade into biocompatible byproducts over several days while continuously releasing the loaded drug at a controlled rate. Huang et al. designed a Christmas tree-shaped bilayer adhesive MN patch combined with the FOLFIRINOX regimen (including fluorouracil, leucovorin, irinotecan, and oxaliplatin) for the treatment of pancreatic cancer [129] (Figure 4D). The researchers encapsulated oxaliplatin and leucovorin in the top layer of the MNs using gelatin methacrylate (GelMA). Irinotecan and fluorouracil were encapsulated in the bottom layer using polyethylene glycol diacrylate (PEGDA). Experimental results demonstrated that the drugs encapsulated in both layers could be sustainably released in a controlled manner, achieving spatiotemporal delivery of multiple drugs from a single MN patch. Additionally, compared to traditional monolayer MN patches, the Christmas tree-shaped MN patch exhibited enhanced adhesion properties, facilitating stable attachment to moist or irregular tumor surfaces.
3.2.4 Photothermal/Photodynamic Therapy
In addition to immunotherapy and chemotherapy, photothermal/photodynamic therapies have also been extensively studied for tumor treatment, primarily for the management of superficial tumors [130-132]. Both photodynamic and photothermal therapy rely on the properties of photosensitizers. Photothermal therapy utilizes photosensitizers to convert energy from the light source into thermal energy, raising the temperature of the surrounding tumor environment and ultimately leading to tumor cell death. Sun et al. fabricated a self-assembly nanomicelle dissolving microneedle (DMN) patch according to the “nano in micro” strategy to co-deliver the first-line chemotherapeutic agent paclitaxel (PTX) and the photosensitizer IR780 (PTX/IR780-NMs @DMNs) for chemo-photothermal synergetic melanoma therapy [133] (Figure 5A). Upon insertion into the tumor site, the DMNs created a regular and multipoint three-dimensional drug depot to maximize tumor accumulation. Accompanied by the DMNs dissolution, the compositions of the needle matrix self-assembled into nanomicelles capable of efficiently penetrating deep tumor tissue. Upon laser irradiation, the nanomicelles not only ablated tumor cells directly by photothermal conversion but also triggered PTX release to induce tumor cell apoptosis. In Vivo results showed that IR780 delivered by PTX/IR780-NMs @DMNs almost completely accumulated at the tumor site compared with intravenous injection. The antitumor results revealed that PTX/IR780-NMs @DMNs could effectively eliminate tumors with an 88% cure rate and without damage to normal tissues. In contrast, under specific wavelengths of light, photosensitizers are activated to release cytotoxic substances that act on tumor tissues, killing cancer cells and stimulating an immune response. This process is referred to as photodynamic therapy. He et al. employ a synthetic biology approach to design a transdermal theranostic MN patch that integrates 5-aminolevulinic acid and catalase within tumor acidity-responsive, copper-doped calcium phosphate nanoparticles [134] (Figure 5B). This co-loading strategy enhances the efficacy of 5-aminolevulinic acid-based photodynamic therapy by maximizing the accumulation of intratumoral protoporphyrin IX. They developed an A375 xenograft tumor model to evaluate the antitumor effects of the MN-CCPCA patch for ion interference-enhanced repeatable 5-ALA-PDT. Under laser irradiation, the MN-CCPCA (+) group showed effective tumor suppression with a tumor inhibition rate of 91.2%, which was significantly higher than those observed in the MN-CCPA and MN-CCPCA groups.
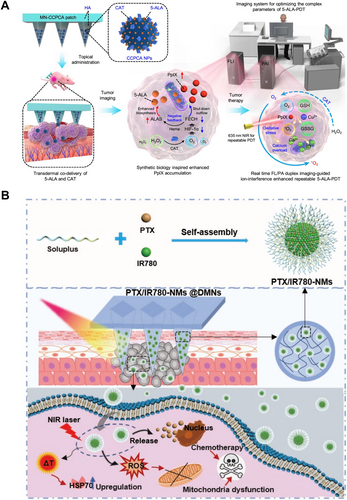
Combination therapy refers to the integration of two or more treatment modalities to achieve synergistic effects, enhance therapeutic efficacy, and reduce side effects. A common approach involves combining photothermal/photodynamic therapy with chemotherapy/immunotherapy, which enables localized tumor heating while simultaneously releasing chemotherapeutic agents, thereby improving drug penetration and therapeutic outcomes [135]. Alternatively, photodynamic therapy can induce tumor cell death while activating the immune system, further enhancing antitumor immune responses [136]. Additionally, specific immunotherapeutic agents can be co-administered, such as the combination of PD-1 inhibitors with R848 [137]. Compared to traditional drug delivery methods, such as intravenous or oral administration, microneedle-mediated combination therapy can significantly increase local drug concentrations in tumors while minimizing systemic adverse effects, thereby achieving superior localized therapeutic outcomes.
3.3 MN-Mediated Cancer-Associated Pain and Chemotherapy-Induced Hair Loss Management
3.3.1 MN-Mediated Cancer-Associated Pain Management
Pain is widely recognized as a significant threat to the quality of life in cancer patients, making pain relief a priority in oncology care [138, 139]. The prevalence of cancer-related pain is estimated to be around 25% in newly diagnosed patients, 33% in those undergoing active treatment, and over 75% in individuals with advanced disease [138]. Pharmacologic therapies, including nonopioids, opioids, and adjuvant analgesics, form the cornerstone of cancer pain management, are often supplemented by anticancer treatments [140-144]. While the oral route is the most common method for administering nonopioids and opioids, it frequently leads to gastrointestinal adverse effects such as nausea, vomiting, gastric ulcers, and constipation. Although intramuscular and intravenous injections of opioid and adjuvant analgesics are effective, they are associated with discomfort and require medical expertise for administration [145-149]. In contrast, transdermal administration offers a promising alternative by mitigating these side effects [150-153]. MN-assisted painless transdermal delivery represents a particularly promising approach for delivering analgesics [154-157]. As drug dosages delivered intradermally via MNs increase, they not only reduce gastrointestinal side effects but also provide comparable pain relief, positioning this method as a potential key tool in the future cancer pain management.
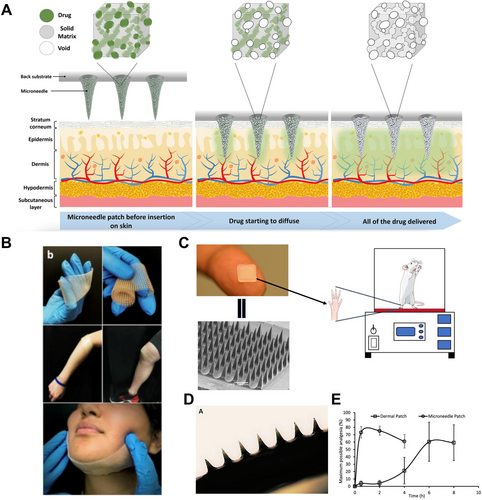
Sadeqi et al. developed polymeric porous MNs utilizing a formulation of the nonsteroidal anti-inflammatory drug ibuprofen embedded in a UV-curable biocompatible hard polymer [158] (Figure 6A,B). The drug was distributed throughout the pores of these solid MNs, and Fourier-transform infrared spectroscopy (FTIR) analysis confirmed that the chemical structure of ibuprofen remained intact during fabrication. The drug release profile indicated that each patch released approximately 1 mg of ibuprofen into a Dulbecco's phosphate-buffered saline (DPBS) solution within 2 h via a dissolution-diffusion mechanism. In a separate investigation, Maurya et al. explored the localized antinociceptive effects of dissolving MNs loaded with fentanyl, using hyaluronic acid (HA) as the matrix material [159] (Figure 6C,D). The analgesic efficacy of the fentanyl MNs was compared to that of traditional fentanyl patches through the hot plate analgesia test in an animal model. The results demonstrated that fentanyl MNs achieved a significantly faster onset of action, providing analgesic effects within half an hour, in contrast to the 6-h onset associated with regular fentanyl patches (Figure 6E). Despite a lower drug load in MNs, the study indicated effective antinociceptive responses, as evidenced by prolonged paw withdrawal latency in rats. These findings suggest that analgesics-loaded MNs may represent a rapid and effective strategy for pain management in cancer patients.
3.3.2 MN-Mediated Chemotherapy-Induced Hair Loss Management
Hair loss caused by chemotherapy drugs usually brings a significant psychological burden to patients [160, 161]. Common chemotherapy drugs, such as the antimicrotubule drug paclitaxel, the anthracycline drug doxorubicin, the alkylating agent cyclophosphamide, and the topoisomerase inhibitor etoposide, can all cause varying degrees of hair loss [162-165]. The extreme anxiety related to this cosmetic disfigurement reportedly drives 8% of patients to reject chemotherapy [166]. Chemotherapy-induced hair loss most prominently affects the highly proliferative matrix keratinocytes of anagen hair follicles, located in the hair bulb [167, 168]. In some cases, hair-follicle stem cells are also damaged, which can lead to permanent hair loss [169, 170]. The micro-damage produced by MNs can stimulate Wnt signaling protein expression and promote the release of growth factors and neoangiogenesis, contributing to hair growth promotion [171, 172]. It not only breaks the traditional limitations of the stratum corneum but also improves the transdermal efficiency of the drug coated on the skin surface. In addition, skin microinjuries induce hair regeneration through macrophage recruitment and subsequent secretion of growth factors or TNF-α [173, 174].
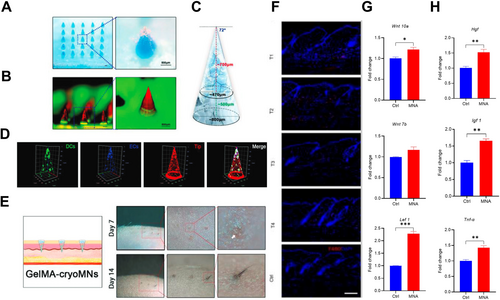
For patients with hair follicle impairment, hair follicle implantation is a viable option. Zheng et al. developed methacrylated gelatin cryoMNs (GelMA-cryoMNs) loaded with hair follicle organoids (Figure 7A–D), enabling efficient transdermal delivery while preserving the viability of the embedded organoids [175]. Both In Vitro and In Vivo studies demonstrated that the organoids delivered via cryoMNs maintained cell viability, self-assembly, and differentiation potential. In mouse models, GelMA-cryoMNs loaded with hair follicle organoids were successfully delivered to the superficial dermis, leading to the hair follicle development and subsequent formation of biomimetic hair tufts through skin surface (Figure 7E). Li et al. used DLP 3D printing technology to fabricate customized MNs to precisely control the hair regeneration [172]. In the mouse model, MNs treatment could induce hair regrowth in a defined area corresponding to the customized shape of the MN. Cellular and molecular analysis indicated that MNs treatment could recruit macrophages In Situ and then initiate the proliferation of hair follicle stem cells, thereby improving hair regeneration (Figure 7F). Meanwhile, the activation of the Wnt/β-catenin signaling pathway was observed in hair follicles. The expressions of Hgf, Igf 1 and Tnf-α were also upregulated in the treated skin that may further support MNA-induced hair regeneration (Figure 7G,H). Although there are currently no reported applications of MNs for the treatment of chemotherapy-induced hair loss, theoretically, MN-mediated physical stimulation or the delivery of hair growth-promoting drugs, such as minoxidil, could potentially accelerate hair regrowth.
3.4 Clinical Trials of MN-Mediated Antitumor Drug Delivery
To date, the majority of studies in MN-mediated cancer treatment have remained at the preclinical stage, either animal experiments or In Vitro experiments [176-181]. We retrieved clinical trials of MNs in cancer treatment from https://clinicaltrials.gov/ and extracted the key information in Table 1 (date of search Mar. 11, 2025). In these clinical trials, the primary focus of MN applications has been the treatment of skin cancer and precancerous skin lesions. The use of dissolving MNs for chemotherapy drug delivery, as well as the pretreatment with solid MNs in combination with photodynamic therapy, have emerged as key areas of clinical research for addressing skin cancer and precancerous lesions.
| Number | Phase | Diseases | MN types | Drugs | Dose (μg) | Aim |
|---|---|---|---|---|---|---|
| NCT06418945 | Phase 3 | Esophageal cancer | NM | Tinidazole | — | Prevention recurrence |
| NCT06722300 | NM | Esophageal cancer | DMNs | Tinidazole | — | Prevention recurrence |
| NCT05377905 | Phase 1b/2 | Cutaneous squamous cell cancer | DMNs | Doxorubicin | 50 | Treatment |
| NCT04928222 | Phase 1 Phase 2 |
Basal cell carcinoma | DMNs | Doxorubicin | 100 | Treatment |
| NCT03646188 | Phase 1 | Basal cell carcinoma | DMNs | Doxorubicin | 25–200 | Treatment |
| NCT02192021 | Phase 1 | Cutaneous T-cell lymphoma | DMNs | Doxorubicin | 25–200 | Treatment |
| NCT06608238 | Phase 2 | Nodular basal cell carcinoma | NM | Doxorubicin | 100–200 | Treatment |
| NCT05329532 | Phase 1 Phase 2 |
Triple- negative breast cancer, squamous cell carcinoma of the head and neck, ovarian carcinoma, renal cell carcinoma | Hollow MNs | Modi-1/Modi-1v vaccine | NM | Treatment |
| NCT02632110 | Phase 2 | Actinic keratosis | Solid MNs | Aminolevulinic acid | NM | Treatment |
| NCT02622594 | Phase 4 | Actinic keratoses | Solid MNs | Aminolevulinic acid | NM | Treatment |
| NCT02594644 | NM | Actinic keratoses | Solid MNs | Aminolevulinic acid | NM | Treatment |
| NCT01812837 | NM | Actinic keratoses | Solid MNs | Aminolevulinic acid | NM | Treatment |
- Abbreviations: MNs, microneedles; NM, not mentioned.
The completed clinical trials reported in the literature mainly explored the efficacy and safety of MNs combined with photodynamic therapy in the treatment of actinic keratosis. Actinic keratosis is a chronic progressive precancerous lesion that can progress to squamous cell carcinoma of the skin [182, 183]. The role of MNs as a combination therapy is also highlighted in the European clinical guidelines for the treatment of actinic keratosis [184]. Pretreatment with solid MNs before photodynamic therapy has been shown to significantly reduce the incubation time of photosensitizers [185]. In existing clinical trials, solid MNs are predominantly used over DMNs for delivering photosensitizers in MNs-mediated photodynamic therapy (PDT), despite the latter's potential for higher bioavailability. The current reliance on solid MNs for photosensitizer delivery in PDT—despite the theoretical advantages of DMNs—stems from two key limitations: (1) the inherent instability of photosensitizers (e.g., aminolevulinic acid), which complicates their long-term storage; and (2) the insufficient drug-loading capacity of DMNs to meet therapeutic thresholds. Consequently, under current MN fabrication technologies, the selection of MN types must be tailored to clinical needs to optimize therapeutic outcomes.
Based on the unique advantages of minimal pain and ability to avoid systemic adverse effects, MNs can increase treatment compliance of some cancer patients. Furthermore, as relatively accessible devices, MNs can be easily applied to clinical applications on a large scale. However, due to the challenges in drug-loading capacity, MNs are still unable to completely replace the systematic use of drugs for anticancer treatment. Compared with surgical removal of skin tumors, MN-based therapies can reduce the risk of infection and scarring associated with surgical procedures, but they may struggle to reach the necessary treatment depth for some deep-seated skin cancer. In the future, MNs customized for different clinical conditions may become an important component of cancer treatment. Moreover, MNs can not only serve as a drug delivery system but also act as mediators for light and heat with the aid of novel materials and processes, potentially further enhancing the efficacy of photodynamic and photothermal therapy.
4 Challenges and Outlooks
MNs, as innovative and painless tools for ISF analysis and drug delivery, are gaining increasing attention and are creating new strategies for tumor diagnosis and treatment. Compared to traditional administration methods, MNs provide significant advantages in delivering drugs efficiently to the dermis, enhancing therapeutic efficacy, minimizing patient discomfort, enabling self-administration, and reducing the risk of infection, thrombosis, and other complications commonly associated with syringes.
Despite many advantages, MNs still face several challenges. Due to their diminutive size, MNs may capture only limited quantities of ISF, potentially compromising detection sensitivity and accuracy. Consequently, a critical challenge in the detection of trace tumor biomarkers lies in enhancing both sampling efficiency and detection sensitivity. The clinical application of MNs in tumor diagnosis is still in its early stages, with limited large-scale clinical trial data to confirm their efficacy and safety. The development of standardized protocols and evaluation systems plays a pivotal role in promoting the widespread clinical application of MN technologies. Additionally, the penetration of MN-delivered drugs into target tissues is critical to their efficacy. Given the small size of MNs, delivering larger doses can be challenging, and a significant portion of the dose may dissipate on the skin surface. Another challenge lies in ensuring dose consistency in MN delivery systems for sustained drug release, including control over the amount of drug released, release frequency, and drug stability. Although this area has received limited research attention, addressing these challenges is essential for the future clinical translation of drug-loaded MNs. Furthermore, the penetration depth of MNs is limited, which may hinder the effective delivery of drugs or diagnostic molecules to deeper tumor tissues, especially in larger tumors. Issues related to material selection and stability also need to be resolved, as the biodegradability and mechanical strength of MNs may not remain stable under different treatment conditions, thereby affecting their efficacy. Lastly, the manufacturing cost and large-scale production of MNs remain significant barriers to their widespread clinical application. Tackling these issues will require interdisciplinary collaboration across fields such as pharmacology, oncology, and biological engineering.
Looking ahead, MN technology is expected to develop according to emerging needs in tumor diagnosis and treatment. For example, through the integration of various technologies, MN chips could become platforms capable of real-time processing and analysis of optical, electrical, and chemical signals [186]. They can hold high-density, precisely fixed biomolecules and integrated circuits and devices, offering advantages such as high throughput, miniaturization, and fast processing speeds that make them suitable for applications in tumor diagnosis and dynamic monitoring of drug metabolism. The development of smart MNs will enable precise drug release and tumor microenvironment modulation in response to external stimuli, thereby enhancing therapeutic efficacy while minimizing side effects. The application of 3D printing will further improve the precision and structural complexity of MNs, facilitating personalized designs tailored to individual patients and large-scale production, thus increasing their clinical feasibility in cancer therapy. Additionally, integration of MNs with wearable devices will enable real-time cancer monitoring and precision treatment. For example, combining MNs with biosensors can detect tumor biomarkers, dynamically assess disease progression, and intelligently adjust drug release based on individual patient needs. Furthermore, AI and machine learning algorithms will play a crucial role in optimizing microneedle-based therapies by analyzing vast amounts of patient data, predicting disease progression, and fine-tuning drug delivery in a real-time adaptive manner. These technologies will contribute to a new era of precision oncology, where treatment strategies are continuously refined to maximize efficacy and minimize adverse effects. As these cutting-edge innovations continue to evolve, MNs are poised to become vital tools in cancer diagnosis and treatment, paving the way for more precise, efficient, and highly personalized therapeutic strategies. In this context, increased involvement of clinical doctors is essential for advancing this field, particularly in initiating clinical trials focused on tumor diagnosis and treatment utilizing the MN platform. Key applications include localized drug delivery for superficial tumors, tumor vaccine administration, management of cancer-associated pain and hair loss, and critical aspects such as early tumor detection and recurrence monitoring.
In summary, the prospects for cancer diagnosis and treatment through MN systems represent a significant potential in the field of cancer theragnostics. Advances in materials science, nanotechnology, and biosensor technologies are expected to further enhance the detection sensitivity and therapeutic efficacy of MNs. Additionally, the accumulation of clinical trial data and the development of standardized protocols will provide a robust foundation for their broader applications in clinical settings. With continued research and technological innovation, MNs hold the potential to become pivotal tools in tumor diagnosis and treatment, providing cancer patients with more precise and convenient theragnostic solutions.
Author Contributions
Chunli Yang: investigation (equal), writing – original draft (lead), writing – review and editing (lead), funding acquisition. Li Zhang: investigation (equal), writing – original draft (equal), writing – review and editing (equal). Siyi Wang: investigation, writing – original draft. Angxi Zhou: investigation, writing – review. Run Tian: investigation, writing – review. Maya Xiang: investigation, writing – review. Ya Ren: investigation. Yang Yu: investigation. Rong Li: investigation. Maling Gou: conceptualization, funding acquisition, project administration, supervision, writing – review and editing (lead). All authors have read and approved this manuscript.
Acknowledgments
We thank Boya Li for the English polish. This study was supported by grants from the National Natural Science Foundation of China (32271468, 82073363), the Natural Science Foundation of Sichuan Province (2023NSFSC1629), the Sichuan University Postdoctoral Interdisciplinary Innovation Fund (JCXK2204) and the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYYC23005).
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
Author Li Zhang is an employee in Huahang Microcreate Technology Co., but has no potential relevant financial or non-financial interests to disclose. The other authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




