Recent progress in 3D printing degradable polylactic acid-based bone repair scaffold for the application of cancellous bone defect
Xulin Hu, Zhidong Lin, and Jian He contributed equally to this study.
Abstract
Large size bone defects have become a growing clinical challenge. Cancellous bone, which has the highest volume ratio, the fastest replacement rate, and interconnected porous structure, plays a major role in bone repairing. Considering the structure and composition of cancellous bone, building a bionic 3D scaffold via customized-3D printing technology is the key to solving the problem. As the earliest degradable medical polymer material approved by Food and Drug Administration, polylactic acid has been proved to have excellent biosafety and can be copolymerized or blended with other synthetic polymers, natural polymers, and inorganic materials to improve its performance to better meet clinical applications. A series of biodegradable bone repair scaffolds based on polylactic acid composites and 3D printing technology are developed to achieve large bone defects. Here, we review the composition and structure of cancellous bone, highlighting the relationship to the requirements of bone repair scaffolds. The different types of polylactic-acid-based materials applied in 3D printing technology are described, emphasizing the connection between materials, preparation methods, and applications.
1 INTRODUCTION
More than 20 million patients worldwide are suffering from the bone defect caused by accidental trauma, bone tumor, and osteomyelitis each year with the increasing number of cases year after year.1, 2 By reason of the aggravation of aging, the large size bone defect has become an urgent medical problem. In general, large-size bone defects exceed the critical size of self-healing of osseous tissue and are prone to cause postimplantation infection and poor revascularization, resulting in serious problems such as bone graft absorption, bone nonunion, and an increased disability rate.3-5 Therefore, establishing blood supply, providing continuous release of bone-inducing substances, and supporting the defect site are the key to the treatment of large-size bone defects.
In recent years, autologous bone graft, allograft, nondegradable artificial bone, degradable artificial bone, and tissue engineering scaffolds have the therapeutic method to large-size bone defects.6-8 As the most commonly used clinical treatment methods, autologous bone, and allografts transplantation are often restricted by the bone shortage, secondary injury, and postoperative rejection immune response.9, 10 For the nondegradable artificial bone scaffolds, long-term fixation, and biological stability are the vital questions to be considered. Tissue-engineered scaffolds are able to furnish the seed cells for the bone repair, but to some extent, apparent deficiency such as the limited select range of scaffold materials, complicated preparation process and the high-cost price confine its application in clinical research.11, 12 Hence, the solutions to large-size bone defects focus on the utilization of functionalized degradable materials and porous scaffolds with excellent mechanical and degradation properties.
Polylactic acid (PLA) is a biodegradable polymer, which was widely used in the fields of bone repairing, adhesion-preventing film, cardiovascular stent, ureteral stent, and surgical suture.13-17 However, pure PLA still has some drawbacks in physical and chemical properties to repair large-size bone defects. For example, the fragment and flaw occur during the degradation of PLA, giving rise to the collapse of the implant area.18, 19 Meanwhile acidic degradation products also lead to the postoperative inflammatory reactions.20-22 Besides, PLA is difficult to be deformed because of its high elastic modulus and glass transition temperature.23, 24 It is necessary and achievable to obtain new degradable replacement materials using blending or copolymerization for bone repair. The modification of PLA was divided into the method of blend with natural polymer materials and inorganic bioactive materials or the strategy of copolymerization.25-27
With the development of materials science and clinical technology, the ideal degradable scaffold for repairing large-size bone defects should meet the following characteristics: appropriate mechanical properties; bionic macro-porous structure that facilitates the interpenetration and adhesion of osteoblast cells; degradation rate, and dimensional stability matched with bone healing; good biocompatibility and certain bone induction.28, 29 In addition, the surface properties of the scaffolds and the addition of active bone-inducing materials also play a crucial role in bone healing. Therefore, selecting the appropriate additive manufacturing technology to construct the three-dimensional bone-like structure scaffold according to the properties of materials the key to solve the problem. For progressives, the bionic scaffolds promote the bone healing from multiple stages and links of osteogenesis.
3D printing was initially designed to rapidly construct objects by layer-by-layer printing based on digital model files. 3D printing is already being used to fabricate all kinds of customized biomedical devices and tissues with controllable precision.30-32 Its extended application in bone regenerative medicine is to construct a three-dimensional spatial structure bionic bone/cartilage scaffold (3D bioprinting) based on 3D modeling (computer-aided design [CAD]). Integrating multifunctional biomaterials, cells, and growth factors during printing endows the scaffold with excellent bioactivity as a substitute for native bone tissue. Furthermore, with regard to 3D printing bone repair scaffolds of degradable synthetic polymer, accurate control of filling rate, scaffold structure, size, and shape is attainable. For degradable PLA polymer-based materials, the common methods are fused deposition modeling (FDM) and low-temperature deposition (LDM).33-35 The material is heated above the melting point or configured into a solvent/solution system to form a fluid for printing.
This study reviewed the development of degradable bone repair scaffolds with a main focus on the research and potential application in PLA-based bone repair scaffolds using 3D printing methods. The purpose of this study is to look for alternative bone repair products that meet more clinical requirements and provide some ideals for research. First, we summarized the key properties and preparation requirements of PLA 3D printed bone repair scaffolds when applied to cancellous bone repair in clinical practice. Second, the application progress of different PLA-based materials to construct bone repair scaffolds using 3D printing technology was discussed and classified. Subsequently, the opportunity and challenge of 3D printing technology to construct PLA-based bone repair scaffolds are proposed.
2 KEY PROPERTIES REQUIRED FOR 3D PRINTING DEGRADABLE PLA-BASED BONE REPAIR SCAFFOLD
2.1 Composition and structure of cancellous bone
Bone is a “smart” material, which could respond intelligently to stress stimuli, changing its own structure and composition36 (As shown in Figure 1). As a dynamic continuous structure, bone tissue plays an important role throughout life, which has three essential functions: supporting the physical activities of the muscle, protecting the vital organs, and providing a storage for calcium and phosphate.37 The main ingredients of the bone tissue are mineral component (similar to hydroxyapatite [HA]) bone collagen (type I collagen accounts for the main part) and water. Bone tissue is mainly divided into bone marrow cavity, cancellous bone, cortical bone, and periosteum. Among them, cancellous bone possesses the largest volume proportion, specific surface area, the fastest metabolic and replacement rate.38 Accordingly, the repair of cancellous bone is the key to the problem of bone defect repair. The cancellous bone is composed of individual trabeculae of about 200 μm in size with interconnected porous structure. This porous foam-like structure (75%–95% porosity) is benefit to nutrient delivery, cell and blood transport. Meanwhile, this unique structure with high porosity and calcified collagen fibers acting as “binders” combine to give cancellous bone a higher deformability that is different from cortical bone. Consequently, departing with the composition and structure, is the optimal strategy for the design and preparation of degradable bone repair scaffold
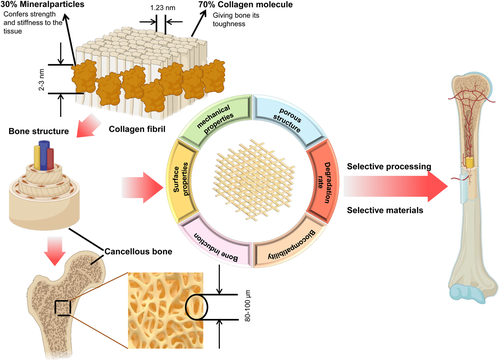
2.2 The crucial requirements for biodegradable bone repair scaffold
With the interactive development of the materials science, engineering technology and clinical, numerous excellent scientists put forward the systematic performance requirements of biodegradable bone repair scaffolds based on the composition and structure of natural bone. First, one of the most influential requirements of a scaffold is its mechanical properties, as this can persistently affect the bone healing process. Excessive compression modulus of the scaffold may cause stress occlusion, lead to secondary fractures after degradation.39 And if the compression modulus of the bone scaffold is lower the natural bone, it will not have enough ability to support the defective area, which will affect the healing effect and make the convalescent patient unable to exercise. The second vital requirement is the surface structure which in favor of cell attachment, helps bone stromal stem cells and osteoblasts to attach, proliferate and differentiate.7 Typically, the surface structure demands a higher roughness as well as a higher specific surface area. Third, to provide a place for cell proliferation, neovascularization, and inward growth of bone tissue to assist in the reconstruction of blood circulation.40 To add more, the bionic microcellular structure with interpenetration should also be featured by the scaffold (the pore size between 200 and 500 μm). The scaffold should be composed with a porosity similar to that of cancellous bone, between 75% and 95%.41 Fourth, possessing a degradation rate compatible with the bone healing and maintaining dimensional stability during the prehealing stages, creating the conditions for new tissue growth. It is necessary to point out that the rate of degradation is not only the rate of mass loss of the scaffold, but also the rate of mechanical Performance penalty of the scaffold. Furthermore, the change rate of the molecular weight of the polymer should also be taken into account. Fifth, superior biocompatibility without causing postoperative rejection immune reactions. Sixth, a certain degree of osteoinductive properties, which induces differentiation of marrow stromal stem cells into osteogenic and osteoblastic cells and stimulates host osteogenic capacity.42 Consequently, we should match the required materials to the performance requirements of the bone repair scaffold and construct a 3D bionic scaffold that can meet them through the appropriate choice of printing technology.
3 PLA-BASED SCAFFOLD MANUFACTURING TECHNOLOGY
In this section, we describe two main technologies-namely, the traditional and 3D additive manufacturing technologies-used to fabricate PLA-based composites scaffolds (As shown in Figures 2 and 3). We also discuss the advantages and disadvantages of each preparation technique.
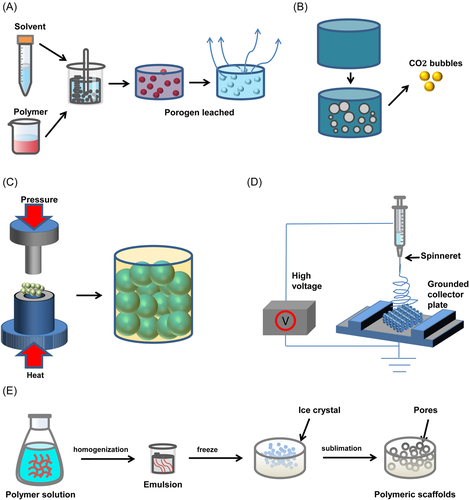
3.1 Traditional technology
3.1.1 Solvent casting-particulate leaching technique
The solvent casting-particle leaching technique is one of the most frequently used techniques in applied research to fabricate tissue engineering scaffolds. There are three steps in any solvent casting-particle leaching technique. First, the particle size and proportion of the porogen were screened. Then the polymer material and the porogen mixed emulsion are added to the mold for evaporative solidification. Finally, residual porogen particles are removed.43-53 The technology relies on molds and porogens, and different shapes of scaffolds can be constructed using different molds. Sodium chloride is usually selected as a porogen and is blended with the polymer to prepare scaffolds in circular/rectangular molds. To avoid excessive temperature effects on scaffold bioactivity, Mao et al. combined room temperature molding technology to construct poly(lactic acid)/ethyl cellulose/HA (PLA/EC/HA) scaffold with good comprehensive properties.44
Further, some scholars choose to combine low-temperature freeze-drying technology to construct scaffolds.45, 46 However, the solvent casting-particle leaching technique has some limitations, and the size and ratio of porogen particles can easily affect the pore structure inside the scaffold. Solvents make it impossible to add bioactive molecules, and residual solvents may even affect cell proliferation. Therefore, the bioactivity of scaffolds prepared by the solvent casting-particle leaching technique is more derived from inorganic particles such as HA or ceramic powder.47-50 The scaffolds constructed by solvent casting-particle leaching technology cannot control the surface structure of the scaffolds, and the pore size is random. In addition, multifunctional modification of the surface is often required to compensate for the lack of bioactivity and biocompatibility of the scaffold. Birru et al. selected human mesenchymal stem cells or natural polymer materials to combine on the scaffold surface to accelerate bone regeneration after scaffold implantation in vivo.51-53
3.1.2 Gas foaming technique
By forming passages of gases or bubbles inside a material, gas foaming is a technology of creating a porous scaffold.54 The technique begins with mixing materials, which includes the substrate, a foaming agent, and a binder. As the mixture is mixed into jelly, it would be put into a mold and let solidify partially. The shape of the scaffold is determined by the mold. The mixture would be put in a solution and let the chemical reaction happen inside the material. The chemical reaction creates gas bubbles escaping from the materials, causing the erosion of the scaffold. The internal porous structures of the scaffold are created by the preset mold and the escaping gases, which include CO2 and N2.
The main advantage of the gas foaming technique is its lower costs and efficiency. Due to its simple foaming process, it can fabricate a mass of scaffolds at one time. However, it also has its drawbacks. Generally, controlling the foaming chemical reaction and the mixing ratio of materials can control the pore size of the scaffold. But the pore can be distributed anywhere on scaffolds and its sizes range from 40 to 800 µm. Another drawback of this process is that the pores inside the scaffold are not interconnected. Many researchers have tried to resolve this limitation by using the foaming agent twice. However, this process may also lead to mechanical deformation and create additional voids in the structure. Furthermore, the main drawback of gas foaming is that it couldn't fabricate the delicately shaped scaffolds. The interconnected geometries are difficult to control through this traditional form.55 Besides, the foaming agent left in the scaffolds still poses a risk to tissue engineering.
3.1.3 Microsphere sintering technique
Polymer microsphere sintering technology is another mainstream method to construct porous tissue engineering scaffolds. Compared with pure polymer scaffolds, microspheres have the advantage of loading inorganic particles, drugs, or bioactive molecules. The basic properties of microsphere-based sintered scaffolds mainly depend on the degree of sintering. Different sintering conditions are required for different polymers. The sintering technologies of PLA-based microsphere scaffolds mainly include high temperature56, 57/laser sintering58-60 (using the glass transition temperature [Tg] of the material) and solvent sintering (using solvents of different compositions). Yan et al. used laser powder bed fusion technology to sinter PLA/nano-HAP microspheres into microsphere scaffolds with good biocompatibility and osteogenicity.58 The scaffold was selected for rapid sintering at 65−70°C for 5 min to avoid excessive influence on the internal pore structure of the scaffold. High temperature/laser sintering (>80°C) technology requires longtime processing at high temperatures, which is not conducive to the loading of bioactive substances. Furthermore, Qutachi et al. developed a PLA composite porous microsphere scaffold constructed at 37°C. Of course, this low-temperature sintering condition still requires the addition of other solvents for assisted molding.61, 62 When using solvent (vapor) sintering techniques to construct PLA-based microsphere scaffolds, the control of sintering time is rigorous. Similar to the solvent casting-particle leaching technique, excess solvent easily deactivates the bioactive substances loaded in the microspheres. The above-mentioned PLA-based microsphere sintering technology can construct a bone repair scaffold with a specific three-dimensional structure. However, the microsphere sintering conditions can have a tremendous impact on the overall performance of the scaffolds.63 In addition, the biggest limitation of microsphere sintered scaffolds is that their porosity is usually low (below 50%), which is not conducive to the recovery of tissue function.
3.1.4 Electrospinning technique
The electrospinning technique is a process that uses electrostatic forces to draw polymer material from a special nozzle.64 Generally, the surface tension of polymer material can also be reduced by using a solution-based electrospinning technique. However, solvents used in solution electrospinning may pose a risk of potential cell toxic effects on tissue when used for tissue engineering.65 Besides, using this technique, the maximum thickness of the 3D printing scaffold would be limited to 4 mm. because the repulsive forces are created between successive layers, which are associated with residual chargers and solvent. Instead of solvents, melt-electrospinning, with a thermal source, this electrospinning can change the state of the polymer from a solid-state to a viscous fluid.66 Using this method, the mats produced larger fiber diameters and wider pore size. Additionally, melt electrospinning is characterized by improved interlayer bonding, due to the absence of residual solvents.
By using the electrospinning technique, scaffolds with nanofiber can be fabricated efficiently. the surface area and aspect ratio of these scaffolds can be controlled by changing the electrospinning parameter straightly. moreover, the diameter of fibers can be controlled and the final form of scaffolds can be modified with a high degree of flexibility.67 However, the electrospinning technique also has its own disadvantages, which include the thickness, porosity, and pore size of the scaffolds. It has also been proved that it is difficult for cells to penetrate inside the fibrous network of the electrospinning scaffold.68
3.1.5 Freeze drying technique
Freeze drying has been a classic preparation technique for tissue engineering scaffolds because of its simple operation and wide range of materials.3 Freeze drying is to prepare the material into a liquid state through a suitable solvent, prefreeze it in the mold to solidify it, and then sublimate it under low pressure to remove the solvent to obtain a porous scaffold.
Serdar Korpayev et al. applied freeze-drying and thermal gelation techniques to develop a biomimetic multilayered scaffold based on chitosan、collagen and genipin.69 This kind of multistage structure scaffolds obtained through structure with gradient pore diameter by using freeze-drying technology under different conditions. At the same time, they verified that the scaffold has a good ability to promote cartilage healing through biological and animal experiments. Dou et al. fabricated PLGA/HA/geltain composite scaffolds via 3D printing combined with freeze-drying method. The highlight of this study is that the characteristics of bone tissue healing at different stages were used to design the scaffolds.70 They first constructed PLHA/HA macro-porous scaffolds with suitable mechanical properties by 3D printing, then injected gelatin solution into the scaffolds and constructed cell-like matrix structure by freeze-drying, so as to obtain gradient degradation composite scaffolds. They verified that the composite scaffold had the highest amount of new bone formation through the SD rat bone defect model. Nevertheless, the pore size control and penetration of freeze-dried porous scaffolds are affected by many factors, such as solution viscosity, freeze-drying rate, and vacuum degree.
3.2 3D additive manufacturing technologies
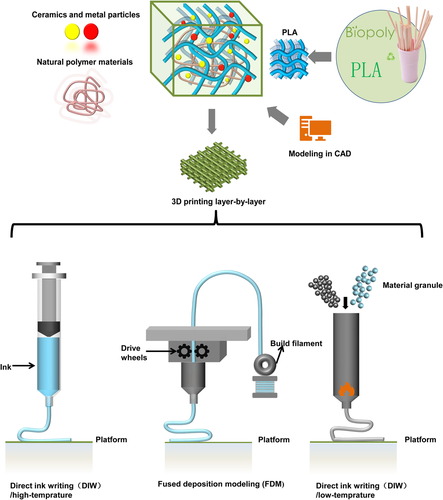
Since the 3D printing technology was developed in the 1990s, after a long period of development, its applications have spread in various fields, such as aerospace, biomedical and other fields.71 At present, the most widely used 3D printing technologies include: FDM, LDM, DIW, SLA, and so on.72 Owing to its ability to achieve free and customized design of structure and size, and a high degree of freedom in the selection of printing materials, it has quickly become a sought-after choice for the preparation of degradable bone repair scaffolds.73
The FDM technology is to utilize the hot melt and adhesive properties of thermoplastic materials to form layer by layer under the control of a computer-aided system.74 First, it preheats the filament and feeds it to the heating nozzle. Then, the heating nozzle moves along the section profile and internal trajectory of the part under the command of the control system, and at the same time extrudes the hot melt material in a semi-fluid state. Next, the viscous molding material and support material are selectively coated on the table and rapidly cured to form the cross-sectional profile. After the current layer is formed, the nozzle rises to a certain height and then coats the next layer, and the layers are stacked to form a three-dimensional product. To sum up, this filament-based printing technology is still limited in material selection and requires pre-extrusion to prepare filaments. Drawing on the principle of twin-screw extruder, high-temperature ink direct writing 3D printing was proposed afterward. This technical proposal greatly expands the freedom of polymer printing. Simultaneously, the direct writing of ink based on solvent system can also be realized by changing the nozzle. The principle of these two printing methods is similar to that of FDM, but only the injection method is changed.75
4 3D PRINTED DEGRADABLE PLA-BASED BONE REPAIR SCAFFOLDS (CLASSIFIED FROM MATERIALS)
4.1 Copolymerization/blending materials with synthetic high polymers
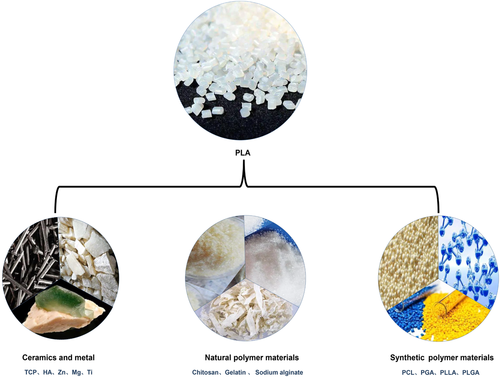
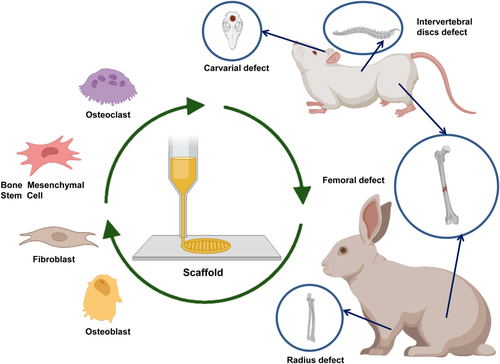
Heretofore, PLA-based synthetic polymer blend materials have been employed for the regeneration of blood vessels, nerves, and bone. This section discusses the application of 3D printed scaffolds based on different types of PLA, PLLA, and PLGA blended with polymer composites materials in terms of their merits for the multifunctional repair of tissue (shown in Figure 4 and Table 1). The types of cells and animal experiments involved in relevant applications are shown in Figure 5.
| Title | Materials | Build method | Basic properties | Application | Animal model | Reference |
|---|---|---|---|---|---|---|
| Enhanced bone tissue regeneration using a 3D printed microstructure incorporated with a hybrid nano hydrogel | Polylactic acid (PLA) + photocurable gelatin hydrogels | Fused deposition modeling (FDM, filaments) | Reinforced the mechanical strength and enhanced the osteogenic differentiation | Stem cell differentiation control and bone tissue regeneration | - | [76] |
| 3D poly (l-lactide)/chitosan micro/nano fibrous scaffolds functionalized with quercetin-polydopamine (PDA) for enhanced osteogenic and anti-inflammatory activities | PLA + chitosan+ quercetin | FDM, filaments | Promoted cell adhesion and proliferation, with excellent cell affinity and osteogenic activity | Bone tissue engineering | - | [77] |
| 3D-poly (lactic acid) scaffolds coated with gelatin and mucic acid for bone tissue engineering | PLA + gelatin+ mucic acid | FDM, filaments | Improved their physicochemical properties, increased the expression of the master bone transcription factor and other osteoblastic differentiation marker | Bone tissue engineering | - | [78] |
| 3D printed biodegradable functional temperature-stimuli shape memory polymer for customized scaffoldings | PLA + chitosan | FDM, filaments | With shape memory property, the scaffolds are possessed with good wettability and cell proliferation | Acute bone deficiencies | - | [79] |
| Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanohydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration | PLA + collagen + minocycline + hydroxyapatite (HA) | FDM, filaments | With uniform macroporous, adequate wettability and an excellent compressive strength, antibacterial activities, osteogenic activity | Bone repair while mitigating the typical infections | - | [80] |
| Evaluation of bone marrow stem cell response to PLA scaffolds manufactured by 3D printing and coated with PDA and type I collagen | PLA + PDA + collagen I | FDM, filaments | The PDA layer improved COL immobilization,the PDA and COL facilitated the robust deposition of extracellular matrix and enhanced the osteoinductivity. | Bone tissue engineering | - | [81] |
| Shape-fitting collagen-PLA composite promotes osteogenic differentiation of porcine adipose stem cells | PLA + minerlized collagen | FDM, filaments | Increased the compressive strength, the addition of PLA does not negatively influence the osteoinductive nature of the mineralized collagen scaffold. | Craniomaxillofacial bone regeneration | - | [82] |
| Meniscal tissue engineering via 3D printed PLA monolith with carbohydrate-based self-healing interpenetrating network hydrogel | PLA + collagen, alginate + oxidized alginate | FDM, filaments | With excellent biocompatibility, showing the feasibility of using carbohydrate based IPN hydrogel embedded in 3D printed scaffold for meniscal tissue development. | Meniscal tissue replacement | - | [83] |
| Fabrication of tissue-engineered tympanic membrane patches using 3D-Printing technology | PLA + chitosan + sodium alginate | Direct ink writing (DIW)/low-temprature | With significantly superior and favorable features in printing quality, the scaffolds are with excellent biocompatibility | Novel patches for repair tympanic membrane perforation | - | [84] |
| A New Bone Substitute Developed from 3D-Prints of Polylactide (PLA) Loaded with Collagen I: An In Vitro Study | PLA + collagen Contained Stromal derived factor | FDM, filaments | Confirms the biocompatibility of PLA and demonstrates an endotoxin contamination clearly below the FDA (Food and Drug Administration) limit, induce neo-vessel formation. | Bone tissue engineering | - | [85] |
| The 3D-Printed Bilayer's Bioactive-Biomaterials Scaffold for Full-Thickness Articular Cartilage Defects Treatment | PLA/polycaprolactone(PCL)/HA/chitosan (CS)/SF | FDM, filaments | The structure increased cell proliferation, with excellent load carrying capacity. | Full-thickness articular cartilage defect | - | [86] |
| BMP-2 and hMSC dual delivery onto 3D printed PLA-Biogel scaffold for critical-size bone defect regeneration in rabbit tibia | PLA + recombinant human bone morphogenetic protein-2 + Mesenchymal stem cells + gelatin and alginate | FDM, filaments | A higher ALP activity, bone related gene expression, bone volume, BV/TV | Bone tissue engineering | Rabbit proximal tibia corticocancellous defect model | [87] |
| PLA short sub-micron fiber reinforcement of 3D bioprinted alginate constructs for cartilage regeneration | PLA + alginate | DIW/low-temprature | Increased the mechanical strength, high biocompability. | Tissue engineering applications | - | [88] |
| 3D printing of PLA/n-HA composite scaffolds with customized mechanical properties and biological functions for bone tissue engineering | PLA/n-HA (1) | FDM, filaments | The biocompatibility and osteogenic induction properties were proved better than that of the pure PLA scaffold | Bone repair | Rabbit femur model | [89] |
| 3D printing of bone scaffolds with hybrid biomaterials | PLA/cHA | FDM, filaments | The mechanical properties are shown to be affected by the reduced interaction between the PLA matrix and the cHA particles | Bone tissue and regenerative engineering | - | [90] |
| 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations | PLA/nHA (2) | FDM, filaments | The mechanical and biological properties of the scaffold are improved by the personalized printing method of low cost, stability, simplicity and fast | Bone regeneration | Rabbit femurs model | [91] |
| Highly loaded HA microsphere/PLA porous scaffolds obtained by FDM | PLA/HA(3) | FDM, filaments)+ spray drying (sd) | Significantly increases the surface roughness of the scaffold, increasing the overall porosity, improving the available surface area and promoting cell adhesion | Treat bone lesions | - | [92] |
| Composite PLA/PEG/nHA/dexamethasone scaffold prepared by 3D printing for bone regeneration | PLA/PEG/nHA/dexamethasone | FDM, filaments | Excellent osteogenic properties and resistance to internal and external inflammatory factors | Bone tissue engineering with anti-inflammatory activities | Rat cranial model | [93] |
| Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering | PLA/Col/MH/cHA | FDM, filaments + alkaline treatment + collagen coating | A combined effect of an enhanced osteogenic activity, mitigating the typical infections associated to bone implants | Bone regeneration with antimicrobial effects | - | [94] |
| Engineering 3D printed bioactive composite scaffolds based on the combination of aliphatic polyester and calcium phosphates for bone tissue regeneration | PLA/PCL/HA | FDM, filaments | Accomplish more favorable properties in terms of biocompatibility, viability, and osteoinduction property. | Bone tissue engineering | - | [95] |
| Porous bone tissue scaffold concept based on shape memory PLA/Fe3O4 | PLA/HA/TCP | FDM, filaments | Supported cell attachment, proliferation, and differentiation, which together with their scalability | Bone regeneration | - | [96] |
| Three-dimensional printed PLA scaffolds promote bone-like matrix deposition in vitro | PLA (1) | FDM, filaments | The open internal structure of macropores and micropores is favorable for oxygen and nutrient transport after implantation. | Bone regeneration | - | [97] |
| 3D-printing of hierarchically designed and osteoconductive bone tissue engineering scaffolds | PLA (2) | FDM, filaments | Porous helical scaffolds with large specific surface area and porous network structure facilitate the growth and differentiation of human fetal osteoblasts | Bone regeneration | - | [98] |
| Simultaneously constructing nanotopographical and chemical cues in 3D-printed PLA scaffolds to promote bone regeneration | PLA (PDA coating) | FDM, filaments + coating | The morphological structure and bioactive surface coating better promote osteoblast adhesion, proliferation, and proliferation. | Bone regeneration | Rat femoral critical size defect model | [99] |
| Biodegradable 3D printed scaffolds of modified poly (trimethylene carbonate) composite materials with poly (l-lactic acid) and HA for bone regeneration | Polytrimethylene carbonate (PTMC)/PLA/HA | DIW/low-temprature | Low toxicity, good biodegradability, good biocompatibility, can enhance the proliferation of osteoblasts | Bone regeneration | Rat femur defect model | [100] |
| Fabrication of hierarchical macroporous biocompatible scaffolds by combining pickering high internal phase emulsion templates with three-dimensional printing | PLLA + PCL(1) | DIW/low-temprature | Drug release, biocompatibility, promotion of osteocyte proliferation and differentiation | Bone regeneration | - | [101] |
| Construction of nanofibrous scaffolds with interconnected perfusable microchannel networks for engineering of vascularized bone tissue | PLLA + PCL(2) | DIW/low-temprature | Enhances blood vessel formation and bone regeneration | Bone regeneration | Rat calvarial model | [102] |
| Rapid prototyping fabrication of soft and oriented polyester scaffolds for axonal guidance | PLLA + PLGA | DIW/low-temprature | Guiding regenerating axons to a linear conformation and promoting the growth of pluripotent stem cell-derived neurons | Nerve repair | Spinal cord injury model in rats | [103] |
| Construction of biomimetic artificial intervertebral disc (IVD) scaffold via 3D printing and electrospinning | PLLA/polyhedral oligomeric silsesquioxane (POSS)-(PLLA)8 | DIW/low-temprature + FDM, filaments + electrospinning | Appropriate mechanical properties, good biocompatibility, facilitated deposition of proteoglycans | IVD degeneration | Rat caudal disc model | [104] |
| Genetically programmed, mesenchymal stromal cell-laden and mechanically strong 3D bioprinted scaffolds for bone repair | Polylactic-co-glycolic acid (PLGA) | DIW/low-temprature | Good biocompatibility, enhanced myoblast adhesion, and proliferation ability | Skeletal muscle | - | [105] |
| PLGA/beta-TCP composite scaffold incorporating cucurbitacin B promotes bone regeneration by inducing angiogenesis | PLGA/TCP/CuB | DIW/low-temprature | Good biomimetic structure, higher mechanical properties, promotion of neovascularization and osteogenesis | Bone Repair (vascularization) | Rat critical size calvarial defect model | [106] |
| A hierarchical scaffold with a highly pore-interconnective 3D printed PLGA/n-HA framework and an extracellular matrix like gelatin network filler for bone regeneration | PLGA/n-HA/Gel | DIW/low-temprature | Hierarchical pore structure, gradient degradation performance, fast degradation, favorable for cell attachment and new bone regeneration | Bone regeneration | Rat femur model | [70] |
| 3D-printed HA15-loaded beta-tricalcium phosphate/poly (lactic-co-glycolic acid) bone tissue scaffold promotes bone regeneration in rabbit radial defects | PLGA/β-TCP/HA15 | DIW/low-temprature | Good biomechanical properties, bone conduction function, local release of osteogenic drugs, promoting osteogenesis, bone regeneration, and angiogenesis | Bone regeneration | Rat radial bone defect model | [107] |
| Preparation and biocompatibility of diphasic magnetic nanocomposite scaffold | PLGA/Col-I-PLGA/n-HA/Fe2O3 | DIW/low-temprature | Proper mechanical properties and cytocompatibility to promote stem cell differentiation | Bone repair (cartilage and subchondral bone damage) | - | [108] |
| A three-dimensional-printed SPION/PLGA scaffold for enhanced palate-bone regeneration and concurrent alteration of the oral microbiota in rats | SPION/PLGA | DIW/low-temprature | Antibacterial, enhanced bone regeneration, altered oral microbiota | Bone regeneration (oral cavity) | Rat jaw model | [109] |
| Modification of PLGA Scaffold by MSC-Derived Extracellular Matrix Combats Macrophage Inflammation to Initiate Bone Regeneration via TGF-beta-Induced Protein | PLGA-ECM | DIW/low-temprature | Increased accumulation of M2 macrophages initiates tissue regeneration and fights inflammation | Chronic inflammation or bone regeneration immune response | Mouse bilateral femoral defect model | [110] |
| Multifunctional magnesium incorporated scaffolds by 3D-Printing for comprehensive postsurgical management of osteosarcoma | PLGA/Mg | DIW/low-temprature | Inhibit tumor recurrence, promote bone regeneration, good biocompatibility | Bone sarcoma | A mouse model for postoperative recurrence of osteosarcoma | [111] |
| Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits | PLGA/TCP/Icariin | DIW/low-temprature | Excellent biodegradability, biocompatibility, and osteogenic ability, enhances new bone and angiogenesis | Steroid-related osteonecrosis | New Zealand rabbit femoral defect | [112] |
| Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect | PLGA/TCP/Mg | DIW/low-temprature | Osteogenesis and angiogenesis, enhancing new bone formation and mass | Steroid-related osteonecrosis | Steroid-related osteonecrosis rabbit model | [113] |
| Cryogenic 3D printing of ss-TCP/PLGA composite scaffolds incorporated with BpV (Pic) for treating early avascular necrosis of eemoral head | bpV(pic)/TCP/PLGA | DIW/low-temprature | Induction of autophagy to prevent apoptosis, promote osteogenesis and angiogenesis | Avascular necrosis of femoral head | Alcoholic femoral head necrosis model of rats | [114] |
| Lithium-containing bioactive glasses enhanced 3D-printed PLGA scaffolds for bone regeneration in diabetes | Li-MBG/PLGA | DIW/low-temprature | Reverse the inhibitory effect of high glucose on the proliferation, migration, and osteogenic differentiation of BMSCs and recruit stem cells | Bone repair (diabetes) | Mouse skull defect | [115] |
- Abbreviations: BMSC, bone marrow mesenchymal stem cell; ECM, extracellular matrix; SPION, superparamagnetic iron oxide nanoparticle.
4.1.1 PLA
PLA, which has the characteristics of low cost and biodegradability, is a renewable substance. Most importantly, PLA is highly safe for the human body and can be absorbed by tissues. Coupled with its excellent physical and mechanical properties, it can construct bone tissue engineering scaffolds for application in the field of biomedicine. Fairag et al. utilized this property to construct 3D-printed PLA scaffolds with excellent penetrability to facilitate the transport of nutrients and metabolites during cell proliferation.97, 98 However, PLA scaffolds suffer from some limitations. Its performance is relatively single, and it is challenging to meet the multifunctional requirements of clinical applications. A variety of synthetic polymers, such as polycaprolactone (PCL)100 and polytrimethylene carbonate (PTMC),99 have been used to construct PLA-based blend composites scaffolds to regenerate different tissue such as cartilage100 and bone.86, 99
To improve the biocompatibility and osteogenic differentiation capacity of the PLA-based composite scaffolds, these authors also compounded bioactive substances, such as HA.99, 100 PLA scaffolds more readily induced bone formation via nano-topography when combined with polydopamine (PDA).86 Thus, the PLA-based composites scaffolds could serve as a biomimetic template for cancellous bone regeneration. PLA scaffolds have a suitable compressive modulus between 110 and 210 MPa. The upper compressive modulus can be significantly increased when compounded with other polymers or inorganic materials, emphasizing these scaffolds' applicability for bone regeneration. Furthermore, cell seeding showed that cells exhibited good adhesion and proliferation inside the scaffold. These studies also examined the bone repairability of the scaffolds in animal models. They constructed a femoral condyle defect model in rats, and the results showed that PLA-based scaffolds could effectively promote bone defect regeneration. However, long-term acidic by-products of PLA degradation may adversely affect the bone repair.
4.1.2 l-polylactic acid(PLLA)
With the continuous development of synthetic chemistry, scientists have synthesized biodegradable PLLA with high strength. During the past few decades, PLLA-based composites to construct multifunctional 3D printed scaffolds have drawn extensive attention. Wang et al. established that PLLA can form composites with other polymers, such as PCL,101, 102 poly(lactic-co-glycolic acid (PLGA),103 and eight-armed polyhedral oligomeric silsesquioxane [POSS-(PLLA)8],104 to fabricate 3D scaffolds for different tissue engineering types in clinical diseases, such as bone repair,102 nerve repair,103 and intervertebral disc (IVD) degeneration.104 These authors developed a series of biomimetic 3D scaffolds with therapeutic and supportive functions using biodegradable PLLA-based composites via 3D printing and electrospinning techniques. In addition to the necessary space support, PLLA-based scaffolds can also achieve controlled drug release and the cultivation of various functional cells.101-103 At the same time, it can also combine with angiogenic factors to promote vascular network formation.102 Further, Zhu et al. imitated the natural IVD structure to construct an IVD scaffold with similar structure and mechanical properties.104 It can simulate the nucleus pulposus (NP) structure in combination with the hydrogel and control the pore structure and mechanical properties of the scaffold by adjusting the 3D model parameters.
These studies demonstrated that PLLA-based scaffolds for different tissue regeneration can match the surrounding tissue microenvironment when implanted in vivo, and can also effectively guide the proliferation and differentiation of functional cells. PLLA-based scaffolds effectively supported the growth and guidance of stem cell-derived axons in a spinal cord severed animal model of injury. Furthermore, PLLA-based scaffolds are validated to match disc height and promote proteoglycan deposition in animal models of IVD degeneration, thereby promoting regeneration of degenerated or damaged IVDs. However, regenerating axons or intervertebral disks may require a slower degradation period since the injury in humans is much greater than in deficient animal models. Therefore, the slow degradation behavior of PLLA-based scaffolds needs to be regulated.
4.1.3 Polylactic acid-glycolicacid (PLGA)
Although PLA and PLLA-based materials have ideal biological properties, their slow degradation rate affects their wide-ranging applications in the medical field. The emergence of PLGA solves this defect with a faster degradation ability and a controllable degradation rate. Many medical products are based on PLGA, such as artificial catheters, drug release carriers, and tissue engineering scaffold materials. However, deficiencies such as poor osteoconductivity and hydrophobicity still limit the application of PLGA in bone repair. In ordinary bone defect repair, it is necessary to satisfy the proliferation and differentiation of osteoblasts and myoblasts, that is, osteoconductivity and osteoinductivity. Abu Awwad et al. constructed PLGA 3D printed scaffolds and found that they had good biological activity and the ability to enhance myoblast adhesion and proliferation.105
The critical factor of any bone regeneration depends on the degree of revascularization after scaffolding. To further validate the potential of PLGA-based composite scaffolds for bone regeneration, Cheng et al. adopted the delivery of cucurbitacin B that stimulate blood vessel and bone formation to enhance angiogenesis in bone tissue engineering.106 However, new bone regeneration needs to mimic the spatial structure of natural bone, endow the scaffold with appropriate gradient spatial structure, degradation properties, and the ability to load drugs to modulate the local inflammatory microenvironment. Dou et al. z prepared PLGA/n-HA/Gel and PLGA/β-TCP/HA15 multifunctional scaffolds.70, 107 These two scaffold systems meet the above conditions and effectively promote bone repair. The mainstream approach to modulating the inflammatory environment is to add clinical drugs, but scientists are also investigating alternatives to modulate the inflammatory environment or microbiota in the microenvironment.109 Huang et al. found that Fe2O3 as a substitute for drugs also regulates inflammation. The developed biphasic magnetic nanocomposite scaffold (PLGA/Col-I-PLGA/n-HA/Fe2O3) can effectively improve cartilage and subchondral bone damage caused by osteoarthritis or trauma.108 In contrast, Deng et al. modified the surface of PLGA scaffolds with extracellular matrix (ECM) (PLGA-ECM) to suppress immune responses and promote tissue regeneration by recruiting macrophages (M2).110
A major challenge in designing tissue-engineered scaffolds under complex disease conditions is the mechanism of interaction between the scaffold and surrounding tissue. For example, diseases such as cancer cells, steroid-induced osteonecrosis, avascular necrosis of the femoral head, and bone defects in diabetic patients have complex pathological conditions. High requirements are placed on scaffold materials when designing corresponding tissue engineering scaffolds. Researchers are more likely to use simple composite structures with multiple functions for repair, avoiding unnecessary side effects caused by complex scaffold systems. Moreover, avoiding the high fabrication associated with loading growth factors will place higher economic pressure on patients, improving the prospects of PLGA-based 3D scaffolds for clinical use. Long et al. used a simple method such as plant extracts icariin,112 bisperoxovanadium [bpV (pic)],114 lithium-containing mesoporous bioactive glass (Li-MBG115 and magnesium (Mg)111, 113 to endow the PLGA scaffold with multifaceted regulation to promote bone regeneration in a complex environment. Among them, the PLGA-based scaffolds blended with Mg can inhibit the recurrence of osteosarcoma and effectively promote the regeneration of new bone. The utilization of icariin and bpV(pic) can promote hematopoietic function, immune function, and bone metabolism, thereby enhancing the repair of bone defects under complex pathological conditions.112, 114 Furthermore, PLGA-based 3D scaffolds containing Li-MBG can relieve cell proliferation, migration, and differentiation in high glucose environments.115 Meanwhile, the authors suggested that more ingenious and multifunctional PLGA-based 3D printed scaffolds should be designed in future studies to adjust the microenvironment to promote bone healing efficiently. Importantly, PLGA-based scaffolds can effectively enhance bone defect site regeneration, whether in rat calvarial/femoral defects or New Zealand rabbit femur/radius animal models. However, the limitations of these studies are that they did not thoroughly dissect the mechanisms of angiogenesis or bone regeneration.
4.2 PLA/natural polymer blend material
Natural polymers, such as chitosan, alginate, gelatin, collagen, and hyaluronic acid are common biomaterials in tissue engineering. They are comparatively softer, materials with weaker mechanical properties than inorganic materials but offer the superiority of the flexibility to adapt their shape and they usually contain bioactive molecules that can stimulate the growth and differentiation of cells in their development. In this section, we would review the composite of PLA and natural polymers applied to 3D printing technology.
4.2.1 Chitosan
Chitosan (CS) is structurally similar to glycosaminoglycans and is degradable by enzymes in humans. With lots of favorable properties, like Biocompatibility, biodegradability, and antimicrobial activity, chitosan is a promising polymer for tissue engineering. Pandey et al.116 fabricated the PLA/CS scaffolds through 3D printing technology. By doping chitosan, scaffolds showed excellent shape recovery property and better biological activity. similarly, Thunsiri et al.117 combined PLA/PCL filament with chitosan/silk firoin to fabricate the bioactive-biomaterials scaffold. By doping chitosan and silk firoin, the cell proliferation property on the scaffolds was improved and indicated that the scaffolds were with better bioactivity. To add more, according to Szymon's research,118 although the addition of chitosan leads to deterioration of the mechanical properties of printing materials, especially Young's modulus and elongation at break. It has also improved the ability to crystallize and provide antimicrobial properties against Staphylococcus aureus and Escherichia coli. In short, with its excellent biodegradability, biocompatibility, and antimicrobial activity, chitosan is considered a favorable additive material used in tissue engineering.
4.2.2 Collagen
Collagen is the main protein component in tissue, for it accounts for 30% of total protein weight in the human body. For decades, collagen has been used as an additive material in tissue engineering and as a delivery of a mass of molecules.119 Collagen has shown its great potential in medical applications. In a research, Ulrike et al.120 create 3D porous scaffolds with PLA and collagen. In their studies, stromal-derived factors are released from collagen cages. The excellent bioactive effect of these materials on endothelial cells was shown. Their research showed that various cells grow, spread, and proliferate on PLA/collagen scaffold, which is loaded with stromal-derived factors, supporting cell growth and formation of neo-vessel. Furthermore, Bruna et al.121 fabricated the PLA scaffolds coated with type I collagen. By evaluating the effect of scaffolds on bone marrow stem cells, They indicated that the osteoinductivity of scaffolds was improved by collagen coating. Moreover, Victor et al.122 constructed the PLA/HA/collagen scaffolds. According to their results, the osteogenic activity of bone marrow mesenchymal stem cells was improved significantly. Although collagen is with excellent bioactive effect, these materials are still weak in mechanical properties. The incorporation of the PLA can also improve this weakness of collagen scaffolds.123 The author also indicated that the PLA improving mechanical property does not negatively decrease the osteoinductive property and bioactive effect. Being denatured, and/or a physical-chemical process, collagen can be denatured to a high molecular weight polypeptide, named gelatin.
4.2.3 Gelatin
Gelatin is a single-strand molecule formed by breaking the natural triple-helix structure of collagen. Due to the biocompatibility, bioactive, and ease of gelatin, gelatin is a commonly used material in tissue engineering, which includes coating and encapsulating different drugs or molecules. For instance, Ashwin et al.78 fabricated the PLA scaffolds coated with mucic acid/gelatin blending. Their results showed the promotion of osteoblast differentiation of mouse mesenchymal stem cells. Besides bone tissue engineering, PLA/gelatin blend can also be used in skin wounds. Chen et al.124 fabricated an in situ degradable PLA/gelatin membrane. According to their results, the 3D printed PLA/gelatin scaffolds with good biocompatibility and layered nanofibers. Invivo experiments showed that the mice in the experiment group had complete skin rebuild. The same as collagen, the main weakness of gelatin gel brings about its poor mechanical properties. Similarly, the addition of PLA can improve the mechanical strength of the blend. As been demonstrated by Pensa et al.,125 by the addition of PLA meshes to the scaffolds, the tensile strength and elastic modulus were markedly increased. In conclusion, all these research indicated that the 3D-printed PLA/gelatin scaffolds show great potential in tissue engineering.
4.2.4 Alginate
Alginate is a special polysaccharide produced from brown seaweeds and some special bacterial species. Alginate can be gelated by using CaCl2 and other divalent cations. with the excellent property of biocompatibility, easy gelation, biosafety, and easy modification, alginate is a common materials in biomedical applications, like would healing, drugs encapsulation, and release of bioactive molecules.126 Han et al.87 prepared a PLA/gelatin/alginate scaffold with FDM technology. By doping bioactive molecule, recombinant human bone morphogenetic protein-2(BMP-2), the scaffolds showed excellent performance in bone formation and bone regeneration. They also constructed the rabbit tibia bone defect model and proved that the gelatin and alginate bio gel could be a great potential delivery system for bioactive molecules. Moreover, Gupta et al.127 constructed a PLA/collagen/alginate scaffold with a special interpenetrating structure. Their study showed the feasibility of using these hydrogels embedded in a 3D printed scaffold for meniscal tissue development. Moreover, PLA scaffolds with alginate can be used for artificial eardrum patches.128 According to the research, different amounts of chitosan add to PLA improved the biocompatibility of the composite eardrum. These artificial eardrums showed great prospects to solve the problem of people with hearing loss.
4.2.5 Hyaluronic acid (HLA)
HLA is a natural polysaccharide and a common glycosaminoglycan in the human body. It is commonly found in the eyes and joints. Due to its biocompatibility, degradability, low immunogenicity, and easy modification, hyaluronic acid is widely used in tissue engineering as a hydrogel. Studies have shown that scaffolds based on hyaluronic acid have an obvious bone-inducing effect in the process of cartilage repair and can significantly promote the repair of defective cartilage, which is a good candidate material in tissue engineering cartilage repair. Farsi et al.129 fabricated the PLA/PVA/HLA scaffolds through the FDM technique. Although the addition of HLA slightly decreases the mechanical properties of the scaffolds, it apparently reinforces the biocompatibility and cell adhesion of the composite, which is confirmed by the cells' behavior. Similarly, Antich et al.130 construct a PLA/HLA composite biogel as a promising bioink for 3D printing. According to their results, the developed biogel presents excellent printability in 3D printing. Moreover, the addition of HA presents suitable mechanical performance for the growth and differentiation of chondrocytes, which improve the expression of the related genes. Furthermore, Niu et al.131 fabricated a PLA/HLA microfiber through electrospinning technique and they found that by adding the HLA, proliferation, and expression of related markers of endothelial cells can be enhanced. Moreover, HLA presents the potential of reducing coagulation degree and hemolytic activity.
4.3 PLA/inorganic blends
In this section, PLA combined with inorganic materials was shown. By doping different inorganic materials, the mechanical strength, degradation property, and biological activity were improved. A variety of studies have begun to investigate PLA-based composite with inorganic materials, like calcium phosphate, bioglass, and metallic oxide.
A lot of studies have investigated inorganic materials that reinforced the mechanical strength of PLA composites in any systematic way. The mechanical property of PLA scaffolds can be modified by the incorporation of inorganic materials. In Zhou Fang's study, they fabricated the PLA scaffolds with HA whiskers, which with high crystallinity and high aspect ratio. The maximum compressive strength of the scaffolds was 0.42 Mpa, 47.2% higher than the corresponding HA/PLA scaffold, and 130% higher than the pure PLA scaffold. Similarly, Wang et al.89 fabricated the composite PLA/nHA scaffolds. Their results showed that the printed scaffolds showed tunable mechanical property accompanied by the proportion of n-HA components. Monshi et al.132 also fabricated PLA/HA scaffold through the FDM technique and they proved that by coating HA to the scaffolds, the mechanical property and bioactive of scaffolds were improved significantly. Esmaeili et al.133 performed a similar study and they confirmed the possibility of 3D printing PLA/HA scaffolds coating ceramics particles. He et al.134 fabricated an SC-PTMC/PLA/OA-HA composite microsphere scaffold via a straightforward technique and their scaffolds exhibited a high compressive modulus relative to the cancellous bone. However, Bankolle et al.135 fabricated a novel hybrid scaffold with PLA and carbohydrate particles (cHA) and their results showed that adjusting the ratio of PLA and cHA can easily control the mechanical property of the composite scaffolds. However, they also explained that because of the dual deposition led to misalignment, leading to the separation between two successive layers, and thus reducing the mechanical strength of the scaffolds. According to the studies above, with the increase of inorganic materials content, the compressive strength of the composite scaffold increased. However, when the content of HA increases to 50%, it turned into brittle-similar material, showing the characteristics of brittle fracture.91
PLA degrades into carbon dioxide, lactic acid, and water,136 and shows intermediate degradation times. Doping inorganic materials can modify the degradation time of PLA in some way. Mao et al.137 fabricated the BG/PLA scaffolds and they found that the degradation rate could be effectively controlled by adjusting the proportion of BG. They explained that doping different content of BG in the composite could be easily adjusted according to the implantation site to achieve the purpose of matching the new bone formation. An increase in porosity was detected in 28-day-aged pure PLA scaffolds and mineral-doped scaffolds, the PLA degradation was balanced by deposition/nucleation of apatite.138 In another study, Boqing Zhang et al.91 explained that during the degradation process, bone-like apatite formation on the surface of the PLA-nHA scaffolds. The increase of nHA content was helpful to alleviate the acidity of PLA degradation products, modify the degradation rate, and improve bioactivity. Similarly, Mehboob et al.139 also found that the fluoride-coated Mg/PLA composite bone plate showed a lower degradation rate with enhanced healing. However, a study conducted by Fahad Alam found that the biodegradation rate of PLA can be improved by doping Fe2O3. In their studies, PLA/Fe2O3 scaffolds showed the highest biodegradation rate probably due to the presence of interconnected structure and increased wettability of scaffolds resulting in more exposure to the degradative solution. All these studies indicated that excessive HA could disturb the structure of the composite scaffolds, thereby accelerating the degradation rate of the materials. What's more, the results also indicated that the addition of HA could neutralize acid degradation products, and thus maintain the pH of the solution.
Originally, the main purpose of researchers doping different inorganic materials is to increase the bone regeneration capacity of the composite. It has been proved by lots of studies that doping nHA to PLA scaffolds can improve osteoinductive activity,92, 93, 122, 140, 141 owing to the high free surface area of HA particles.142 Similarly, Li et al.143 fabricated a black phosphorus-based HA/SiO2 nanofibers scaffold, through microfluidic technology. Their results showed that the black phosphorus-based scaffolds have excellent photothermal properties as well as biomineralization activity for bone regeneration. Similarly, Backes et al.144 compared the Osteogenesis effect between HA and TCP. They fabricated the PLA/HA/TCP scaffolds with different content respectively. They found that a higher proportion of HA and TCP could lead to a better cellular response and cell biocompatibility. The differentiation tests also showed that the PLA/HA scaffolds and PLA/TCP scaffolds exhibited earlier cell differentiation markers as confirmed by alkaline phosphatase and alizarin red assays. In another study,145 the researchers found that the micro-sized HA particles supported cell proliferation and differentiation better than nano-sized ones. They fabricated HA/PLA (20/80 wt.%) scaffolds and both nano-sized and micro-sized HA particles were homogeneously dispersed in the PLA scaffolds. The results showed that the scaffolds added with micro-sized HA particles have better compatibility and cell performance than the nano-HA particles in 10-day cell culture period. Authors also found that the random and aligned fibrous assemblies had a pronounced influence on cell morphology but had no significant impact on cell proliferation and differentiation. Fe3O4 shares the same biological effect. According to Wei Zhao's research,96 doping Fe3O4 to the PLA scaffold can not only improve cell attachment and proliferation ability but also stimulate cell delivery and bone regeneration. What's more, it has also been proved that the incorporation of bioactive glass (BG) significantly enhanced the bioactivity of composite scaffolds.137, 146
5 CONCLUSION AND OUTLOOK
While there is great potential for poly(lactide-acid) based materials to enable the customization and personalization of bone repair scaffolds though 3D printing technology, some limitations and difficulties need to be addressed. In terms of printing technology, limited by the processing performance of PLA, the 3D printing methods applied to PLA-based materials are mainly based on the two different forming method: FDM and DIW. In regard to FDM printing, the materials withstand the thermal process of extrusion and printing, resulting in thermal degradation of the material and affecting its performance in turn. However, the printing based on melt-extrusion method is restricted from the dual parameters of material viscosity and thermal degradation. At the same time, neither of two methods are able to encapsulate drugs and growth factors directly in the printing process due to the high melting point of PLA-based materials. The LDM method allows for direct printing of the active ingredients compared to the two methods mentioned above, however, concerns with the solvent residues increase the amount of work involved in the posttreatment process. Furthermore, in most cases, solvents applied in the preparation of bioinks such as dichloromethane, dioxane and hexafluoroisopropanol are with inadequate biocompatibility and even poisonous in vivo.
It is worth noting that the rate of bone regeneration varies according to the variables of body type, age, and nutritional status. Thus, for implanting materials, it is vital to consider whether the degradation rate of the materials matches the recovery rate of bone regeneration. By incorporating different proportions of inorganic materials, material degradation rate can be prospectively controlled in a targeted manner to cater for the demands of bone regeneration. In general, a large proportion of 3D scaffolds constructed from PLA-based synthetic polymers are effective in moderating the bone regeneration in microenvironment during the initial implantation stage, but without accelerate the bone regeneration cycle by reason of degradation properties. In addition, the central part of the regenerated new bone may necrose under the action of external pressure. The construction of polylactic-based blends with gradient degradation and protein signal modulation is envisaged to accomplish a central and lateral osteogenesis pattern to facilitate the biological recovery of bone integrally.
AUTHOR CONTRIBUTIONS
Xulin Hu: Conceptualization, supervision, investigation, formal analysis, writing—original draft, writing—review and editing; Zhidong Lin: Data curation, writing—original draft, writing—review and editing; Jian He: Data curation, writing—original draft, writing—review and editing; Minchang Zhou: Visualization, data curation. Shuhao Yang: Visualization, data curation. Yao Wang: Writing original draft. Kainan Li: Conceptualization, funding acquisition, resources.
ACKNOWLEDGMENTS
The authors are grateful for the subproject of the national major project generation method and application verification of personalized rehabilitation prescription for patients with balance (No. 2019YFB1311403). Figures were created with BioRender software, biorender.com.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
This review does not require an ethical statement.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




