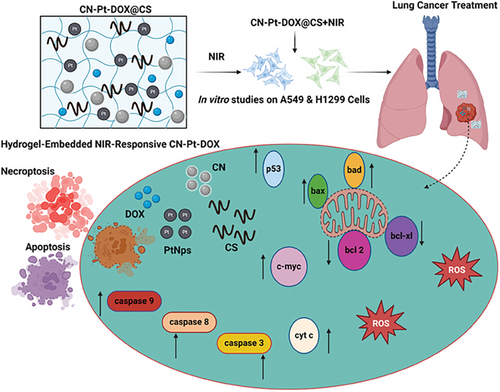
Fabrication of Carbon Nanodots/Platinum Functionalized Nanocomposite Hydrogel Formulation for Near-Infrared Responsive Delivery of Doxorubicin to Promote Necroptosis for Mitigating Lung Cancer Cells: In Vitro Photodynamic Therapy
Abstract
Lung cancer remains one of the deadliest cancers, marked by uncontrolled cell growth in the lungs and often diagnosed at later stages. This study presents a novel hydrogel-embedded nanocomposite responsive to Near-Infrared (NIR) light (CN-Pt-DOX@CS+NIR) to induce necroptosis in lung cancer cells. The composite, created by combining Carbon Nanodots (CNs), Platinum Nanoparticles (PtNPs), and Doxorubicin (DOX) within a Chitosan (CS)-based hydrogel, demonstrated favorable properties for sustained drug release. Upon NIR irradiation, the CNs increase oxidative stress and initiate apoptosis due to the generation of reactive oxygen species (ROS) and caspase activation (3, 8, and 9). The PtNPs enhance ROS production and disrupt mitochondrial membrane potential (MMP), further promoting cell death. DOX, a well-known chemotherapeutic, intercalates DNA and inhibits topoisomerase II, leading to apoptosis. These combined effects result in significant cytotoxicity under NIR stimulation, as shown in vitro on A549 and H1299 cells, confirmed through various assays including AO/EB and DAPI staining, flow cytometry, and RT-PCR. Additionally, the hydrogel matrix provides a controlled release of the therapeutic agents and improves localized delivery. Combining CNs, PtNPs, and DOX with NIR enhanced in vitro cytotoxic effects, apoptosis, ROS generation, and potential for targeted lung cancer therapy.
1 Introduction
Lung cancer remains one of the most challenging and deadly diseases, contributing to a significant public health burden. Despite advancements in diagnostic and therapeutic approaches, lung cancer continues to be the primary cause of cancer-related deaths, accounting for nearly one-fifth of all such fatalities. The aggressive nature and complex biology of lung cancer, coupled with late-stage diagnosis, make it particularly difficult to treat. Often, by the time symptoms become apparent and a diagnosis is made, the disease has already metastasized, complicating treatment and reducing survival rates. Lung cancer primarily manifests in two forms. They are non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), each with distinct biological characteristics and clinical behaviors. NSCLC makes up ≈85% of all lung cancer cases and, although it develops and spreads more slowly than SCLC, it remains highly fatal due to resistance to current therapies.[1, 2] SCLC, while less common, is more aggressive and prone to early metastasis, leading to a poorer prognosis.[3] Both forms of lung cancer have strong links to smoking, but nonsmokers are also at risk due to factors like genetic susceptibility, exposure to environmental carcinogens, and air pollution. In recent years, the landscape of lung cancer treatment has evolved with the advent of targeted therapies and immunotherapies, offering new hope to patients. Targeted therapies, which inhibit specific biochemical pathways involved in tumor growth, have shown promise in extending survival in certain patient subgroups. Immunotherapies, which harness the immune system to identify and attack cancer cells, have also emerged as a promising approach.[4] However, these treatments are not universally effective, and many patients experience limited benefits due to disease heterogeneity and the development of resistance. Given these challenges, there is a critical need for novel therapeutic strategies that more effectively target lung cancer cells, overcome resistance mechanisms, and improve patient outcomes.[5] Current research focuses on developing multifunctional treatment modalities that combine traditional therapies with innovative techniques like nanotechnology-based drug delivery systems, aiming to enhance precision and reduce adverse effects.[6] Researchers continue to explore the complex interactions between tumor cells and their microenvironment to identify new therapeutic targets and biomarkers for personalized treatment strategies.[7]
Nanotechnology-based therapeutics have opened new frontiers in cancer treatment, offering the potential for more precise, targeted, and effective interventions.[8, 9] Hydrogels, in particular, have garnered attention due to their biocompatibility, tunable physical properties, and ability to encapsulate and deliver a wide range of therapeutic agents.[10, 11] Hydrogels can serve as effective delivery platforms, capable of releasing drugs in a controlled manner, responding to environmental stimuli, and localizing therapeutic effects to the tumor site, thereby minimizing systemic toxicity.[12-15]
CS, a natural biopolymer derived from chitin, has shown potential in lung cancer treatment due to its biocompatibility, biodegradability, and ability to enhance drug delivery. The cationic nature of CS allows it to interact with negatively charged cancer cell membranes, facilitating targeted drug release. CS-based nanoparticles can encapsulate chemotherapy agents, improving their stability and controlled release while reducing systemic toxicity. Additionally, the ability of CS to promote immune responses and its use in combination therapies make it a valuable tool in lung cancer management.[16]
A promising approach involves incorporating NIR-responsive nanomaterials within hydrogels.[17, 18] NIR light offers deep tissue penetration with minimal damage to surrounding healthy tissues, making it advantageous in cancer therapy.[19, 20] NIR-responsive nanomaterials can absorb NIR light and convert it into ROS or heat, inducing tumor cell death.[21, 22] Embedding therapeutic agents within these nanomaterials can achieve a synergistic effect, enhancing overall therapeutic efficacy.[23, 24]
CNs, PtNPs, and DOX represent a powerful combination for tackling lung cancer. CNs are emerging as promising nanomaterials due to their small size, biocompatibility, and tunable surface properties. They can be functionalized to specifically target cancer cells and used for drug delivery or photothermal therapy. Their ability to generate ROS under NIR light makes them effective in inducing cancer cell death, offering a potential non-invasive treatment approach.[25-28] PtNPs have gained attention for their potent anticancer properties and ability to enhance chemotherapy efficacy. They promote apoptosis in cancer cells by inducing DNA damage and oxidative stress. Their use in combination with drugs like cisplatin or nanocomposite systems allows for targeted delivery and reduced systemic toxicity, improving treatment outcomes. Their catalytic activity also makes them ideal for enhancing photothermal or photodynamic therapy, providing a multifaceted approach to combating lung cancer.[29, 30] DOX, a widely used chemotherapy drug, intercalates DNA and inhibits topoisomerase II, leading to cancer cell death. While effective against rapidly dividing cancer cells, its use is often limited by severe side effects, including cardiotoxicity. Incorporating DOX into targeted delivery systems, such as nanoparticles, improves its efficacy and reduces toxicity, allowing for more focused lung cancer treatment while minimizing harm to healthy tissues.[31] Together, CNs, PtNPs, and DOX can be encapsulated within a hydrogel matrix, forming a multifunctional platform capable of inducing necroptosis, a form of programmed cell death that stimulates an immune response. This approach targets lung cancer cells through direct cytotoxicity and harnesses the body's immune system.[32] By inducing necroptosis, it releases danger signals (DAMPs) that activate immune cells, enhancing the anti-tumor effect.[33, 34] In lung cancer, immune evasion is a major challenge. This hydrogel system, using NIR-responsive nanomaterials and necroptosis to activate the immune system, offers a novel and advanced therapeutic approach.[35-41]
This study investigates the design, synthesis, and in vitro efficacy of a CN-Pt-DOX system embedded in a hydrogel that responds to NIR light and induces necroptosis in lung cancer cells. By focusing on its innovative mechanisms of action and potential clinical applications, we aim to create a treatment that not only eliminates tumor cells but also activates the immune system to prevent recurrence, offering a novel and advanced therapeutic approach to lung cancer therapy.
2 Results and Discussion
2.1 CN-Pt-DOX@CS Synthesis and Characterization
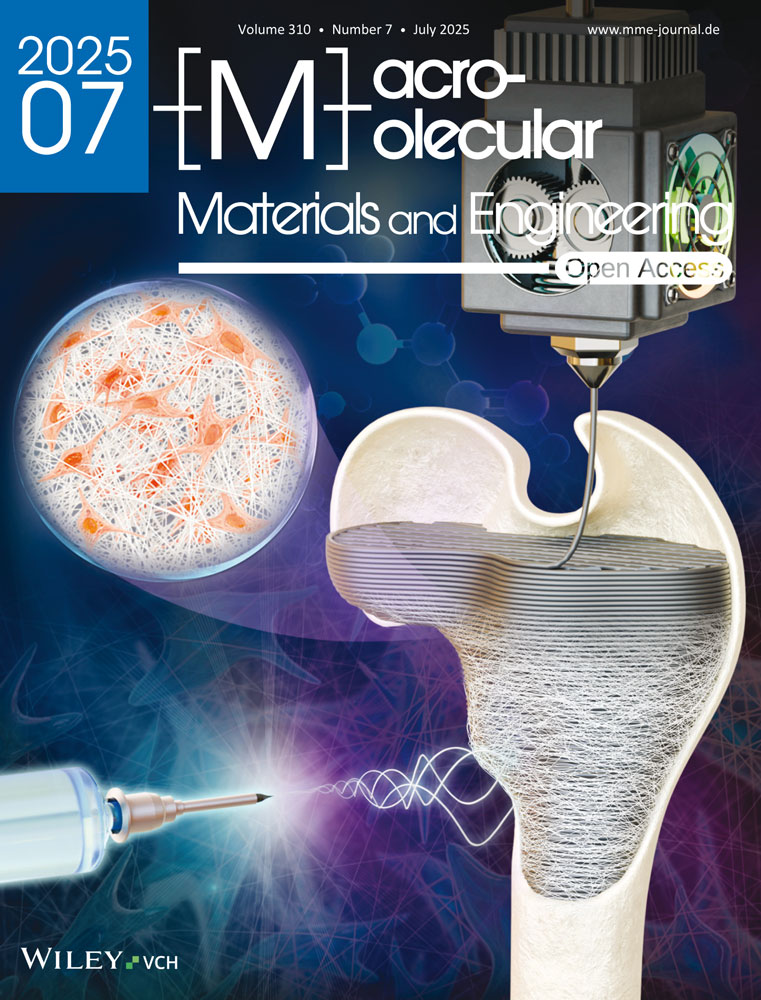
Using the hydrothermal method, citric acid was carbonized at 200 °C to synthesize CNs with a particle size of 2–5 nm, which exhibited strong photoluminescence under UV light. PEG passivation enhanced their biocompatibility and stability in aqueous solutions,[42, 43] making them suitable for biomedical applications.[44, 45] PtNPs were synthesized by reducing H₂PtCl₆ with NaBH₄, using PVP as a stabilizer, resulting in well-dispersed spherical particles. These CNs, PtNPs, and DOX were incorporated into a hydrogel with CS and glutaraldehyde as a crosslinker. A UV visible spectra for CN-Pt-DOX@CS displays characteristic peaks for CNs, PtNPs, and DOX confirming the successful incorporation of nanoparticles and drug in the hydrogel as shown in Figure 1A. The CN-Pt-DOX@CS hydrogel exhibited characteristic peaks for CNs (330–350 nm, n–π* transitions of C═O), PtNPs (≈260 nm, plasmon resonance), and DOX (≈480 nm). Shifts in the DOX absorption band confirmed its conjugation with CNs and PtNPs via EDC/NHS coupling. FTIR confirmed successful nanoparticle embedding as shown in Figure 1B. Peaks for CNs (≈1700 cm⁻¹, C═O stretching; ≈1400–1600 cm⁻¹, C─N/N─H vibrations) and DOX (≈1650 cm⁻¹, amide I; ≈1550 cm⁻¹, amide II) confirmed amide bond formation via EDC/NHS coupling. Shifts in C─N stretching (≈1260 cm⁻¹) and broadening of the ─OH band (≈3300 cm⁻¹) indicated strong hydrogen bonding between CS and nanoparticles. The degree of functionalization (DF) of CN with DOX was determined using UV–vis absorption spectroscopy and FT-IR spectroscopy. UV–vis analysis at 480 nm, based on a calibration curve (R2 = 0.998), revealed a DOX concentration of 4.98 µg mL⁻¹ in the CN-DOX solution, corresponding to a DF of 0.00498 mg DOX/mg CN. FT-IR spectroscopy confirmed conjugation through characteristic peaks, including amide I (1650 cm⁻¹) and amide II (1550 cm⁻¹), with normalized amide I intensity analysis estimating a DOX mass of 0.049 mg, yielding a DF of 0.0049 mg DOX/mg CN. The close agreement between the two methods validates the successful conjugation of DOX to CN with a consistent DF value. SEM revealed uniform nanoparticle distribution within the hydrogel, which exhibited good mechanical strength and elasticity as shown in Figure 1C. Further, the size and morphology of nanocomposite were analyzed using TEM showing that the nanoparticles have spherical morphology[46, 47] as depicted in Figure 1D. SEM and TEM analysis revealed strong integration and adherence of CN-Pt-DOX nanoparticles within the hydrogel matrix, indicating robust particle-matrix interactions. DLS and zeta potential measurements indicated good colloidal stability with average particle sizes as shown in Figure 1E and surface charge as shown in Figure 1F. TEM analysis showed well-dispersed, spherical PtNPs with no significant aggregation, and PVP ensured monodispersity.[48, 49] The compact size and large contact surface of PtNPs boost catalytic activity and ROS generation in NIR-responsive therapy. DOX was conjugated to CNs and PtNPs through the EDC/NHS coupling mechanism, forming stable amide bonds.[50, 51] CN-Pt-DOX nanoparticles were evenly distributed and well-integrated, demonstrating successful embedding and attachment within the hydrogel matrix.[52] SEM images showed the CN-Pt-DOX@CS hydrogel's porous structure, facilitating efficient nanoparticle loading and sustained drug release over time.[53] The uniform dispersion and strong adherence of CN-Pt-DOX within the hydrogel enhance its stability and therapeutic efficacy, making it promising for targeted cancer treatment. FTIR confirmed stable amide bond formation and UV-visible spectral shifts indicated successful interactions of DOX with CNs and PtNPs. Combining CNs' photothermal properties and PtNPs' catalytic activity enhances ROS generation, making the hydrogel a multifunctional platform for NIR-responsive cancer therapy.
2.2 The Hydrogels’ Swelling Ratio
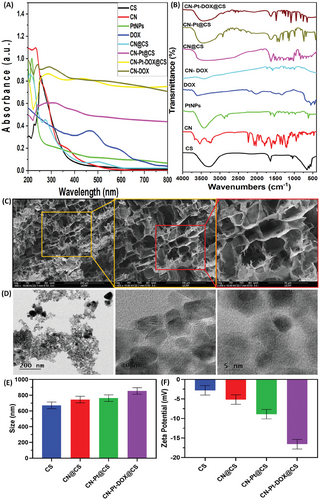
As shown in Figure 2A, the swelling behavior of CS, CN@CS, CN-Pt@CS, and CN-Pt-DOX@CS hydrogels was evaluated using a gravimetric method. All hydrogels exhibited increased water absorption over time, with the extent varying based on their composition. The CS hydrogel group had the highest swelling rate due to its hydrophilic nature, plateauing after reaching equilibrium.[54] In the CN@CS hydrogel group, the incorporation of carbon nanodots slightly reduced swelling capacity due to a denser polymer network limiting water penetration.[55] In the CN-Pt@CS hydrogel group, the addition of PtNPs further decreased swelling due to stronger interactions within the hydrogel, reducing the free space for water absorption.[56] The CN-Pt-DOX@CS hydrogel group had the lowest swelling capacity because the conjugation of DOX increased crosslinking and reduced water uptake, which may aid in controlled drug release.[57]
2.3 Rheological Testing of CN-Pt-DOX@CS
Rheological testing indicated that the hydrogel exhibited shear-thinning properties, with the storage modulus – G' exceeding the loss modulus – G'', indicating the stability of the gel and ensuring it retains its integrity after injection as shown in Figure 2B,C. Physicochemical characterization confirmed the successful synthesis and embedding of CN-Pt-DOX nanoparticles within the hydrogel matrix, crucial for maintaining therapeutic properties. The hydrogel's shear-thinning behavior is beneficial for injectable applications, as it allows easy administration and gel formation in situ. Under shear stress, the hydrogel's viscosity decreases for smooth injection, and it regains viscosity upon stress removal, forming a stable gel.[58-60] The hydrogel's stability and shear-thinning behavior make it ideal for localized drug delivery, where ease of administration and conformability to the application site are crucial. These properties enhance its potential for targeted cancer therapy, offering precise drug delivery with reduced systemic toxicity.
2.4 Drug Release Studies
As shown in Figure 2D, in the absence of NIR irradiation, DOX release from the hydrogel followed a gradual diffusion-controlled pattern, with 25% released over 24 h at 37 °C. The release profile showed an initial burst followed by sustained release. Under NIR exposure (808 nm, 1 h intervals, 1.5 W cm−2), cumulative DOX release significantly increased to 70% over 24 h, with noticeable spikes after each NIR interval, demonstrating effective light-triggered release.[61, 62] UV–vis analysis of release medium samples showed a characteristic DOX peak at ≈480 nm, with increasing intensity over time, indicating cumulative DOX release.[63, 64] Absorbance values were used to quantify DOX concentration and plot release profiles over time. The NIR-irradiated hydrogel showed a steeper slope compared to the non-irradiated control, indicating faster, controlled DOX release under NIR light. This accelerated release is due to localized heating from NIR-responsive CNs within the hydrogel.[65, 66] NIR irradiation generates heat that expands the hydrogel matrix or disrupts interactions holding DOX, leading to rapid drug release. The CN-Pt-DOX system shows a dual-release mechanism: initial passive diffusion is enhanced by NIR heating, creating micro-cavities in the hydrogel that further facilitate DOX release.[67, 68] NIR light serves as a remote trigger for controlled drug release, allowing modulation based on therapeutic needs. This method has significant implications for cancer therapy, enabling precise tumor targeting and minimizing systemic toxicity by concentrating high DOX levels at the tumor site. Enhanced release under NIR irradiation can facilitate higher controlled doses of DOX, maximizing therapeutic efficacy against tumor cells while reducing adverse effects.[69, 70] A comparative analysis of NIR-irradiated and non-irradiated hydrogels shows the superiority of the NIR-responsive system for controlled drug release. The ability to trigger and modulate drug release on demand offers a significant advantage in developing advanced cancer drug delivery systems.
2.5 Photothermal Characteristics
During 808 nm laser exposure, the temperature of the hydrogel system increased quickly within 5 min, with the extent of the rise directly linked to the concentration. The time and temperature data indicated that higher concentrations led to more significant temperature increases. For 300 µg mL−1 concentration, the temperature reached 54.40 °C after 5 min as shown in Figure 2E. The photothermal behavior of the hydrogels was tested under different settings of power. When the power increased, both the temperature of the hydrogel and the peak also increased. At a power of 1.5 W cm−2, the temperature raised from 31.96 to 54.71 °C, while at 2 W cm−2, it raised from 34.13 to 59.85 °C as shown in Figure 2F. The stability of the hydrogels in photothermal conditions was also examined as shown in Figure 2G. The CN-Pt hydrogel with a 300 µg mL−1 concentration of CN-Pt was exposed to NIR at 1.5 W cm−2 for 5 min and then allowed to cool back to its initial temperature naturally. This heating and cooling was done five times to track the changes in temperature over time. Our findings indicated that the hydrogels maintained photothermal stability, with no significant change in photothermal conversion efficiency even after five cycles of heating and cooling. Our findings align with the cited study.[71]
2.6 In Vitro Cytotoxicity of CN-Pt-DOX@CS+NIR
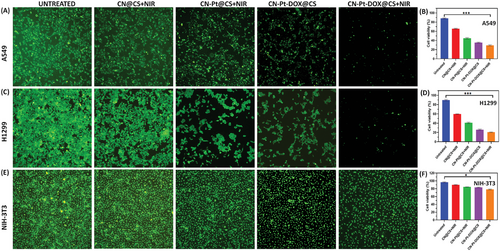
Figure 3A displays the fluorescent cell viability images (using AO staining) for untreated, treated with CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR and Figure 3B displays the quantification of cell viability of A549 cells. Figure 3C displays the fluorescent cell viability images (using AO staining) for untreated, treated with CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR and Figure 3D displays the quantification of cell viability of H1299 cells. In addition to these results, NIH-3T3 (non-cancerous) cells were treated with the samples of CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR, showing high cytocompatibility as shown in Figure 3E. Figure 3F displays the quantification of cell viability of NIH-3T3 cells. The results show that CN-Pt-DOX@CS+NIR effectively kills cancer cells while sparing non-cancerous cells. A549 and H1299 control cells (untreated) displayed high viability with minimal cell death, as indicated by 570 nm absorbance readings. Cells treated with CN@CS+NIR hydrogel nanocomposites showed a slight decrease in viability for A549 and H1299 cells, indicating moderate cytotoxicity.[72] Treatment with CN-Pt@CS+NIR hydrogel nanocomposites significantly reduced cell viability in A549 and H1299 cells, indicating that PtNPs enhance the cytotoxicity of the hydrogel nanocomposites.[73] The CN-Pt-DOX@CS+NIR hydrogel nanocomposites showed the most significant reduction in cell viability for A549 and H1299 cells. This strong cytotoxic effect is due to the synergistic action of PtNPs and DOX, with PtNPs catalyzing ROS production and DOX amplifying the cytotoxicity, resulting in the highest observed cell death among the tested formulations in the presence of NIR.[74, 75] The CN-Pt-DOX@CS+NIR treatment proved to be more effective in killing cancer cells compared to CN-Pt-DOX@CS, due to the added impact of NIR irradiation. P < 0.001 for CN-Pt-DOX@CS+NIR compared to the untreated group for A549 and H1299 cells and P < 0.05 for CN-Pt-DOX@CS+NIR compared to the untreated group for NIH-3T3 cells. The greater sensitivity of H1299 cells to hydrogel nanocomposites, particularly the CN-Pt-DOX@CS+NIR formulation, may be due to inherent biological differences. A549 cell lines (lung adenocarcinoma) and H1299 cell lines (NSCLC lacking functional p53) may respond differently to oxidative stress and chemotherapy.[76] The absence of p53 in H1299 cells may make them more vulnerable to DNA damage from ROS and DOX, leading to lower cell viability.[77] The moderate cytotoxicity of CN@CS+NIR hydrogel nanocomposites indicates CNs alone have limited toxicity due to their inertness and biocompatibility. However, adding PtNPs significantly enhances cytotoxicity, likely through ROS generation and oxidative stress. The CN-Pt-DOX@CS+NIR hydrogel further amplifies this effect, boosting cell death via oxidative damage and DOX-induced apoptosis and necroptosis.[78] NIR activation enhanced the cytotoxicity of DOX, demonstrating its potential for targeted cancer treatment.
2.7 AO/EB Staining
Figure 4A displays the fluorescent live and dead cell images for untreated, treated with CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR and Figure 4B displays the quantification of apoptosis ratio of A549 cells. Figure 4C displays the fluorescent live and dead cell images for untreated, treated with CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR and Figure 4D displays the quantification of apoptosis ratio of H1299 cells. In the untreated control group, A549 and H1299 cells mainly exhibited green fluorescence, indicating high viability and intact cell membranes, with minimal red fluorescence from EB penetration. Cells treated with CN@CS+NIR hydrogel nanocomposites displayed a mix of green and some red fluorescence, suggesting a slight increase in early apoptotic or necrotic processes. The CN-Pt@CS+NIR hydrogel nanocomposites group showed a significant rise in red fluorescence, particularly in H1299 cells, indicating higher cell death due to the cytotoxic effects of PtNPs, which generate ROS and damage cell membranes.[79, 80] The CN-Pt-DOX@CS+NIR group exhibited the most pronounced effect, with most cells showing strong red fluorescence and a significant decrease in green fluorescence.[81] These results show that the combination of CNs, PtNPs, and DOX in the hydrogel nanocomposites induced high cytotoxicity, leading to extensive cell death in both A549 and H1299 cells in the presence of NIR. The strong red fluorescence indicates compromised cell membranes, a marker of late apoptosis or necrosis.[82] The AO/EB staining results highlighted the cytotoxic potential of CN-Pt-DOX@CS+NIR hydrogel nanocomposites. The addition of NIR activation further increased the red fluorescence in the CN-Pt-DOX@CS+NIR group compared to the CN-Pt-DOX@CS group, suggesting enhanced cytotoxicity. P < 0.001 for CN-Pt-DOX@CS+NIR compared to the untreated group for A549 and H1299 cells. The transition from green to red fluorescence in treated groups indicates the progression from viable to non-viable cells. Extensive red fluorescence in the CN-Pt-DOX@CS+NIR group suggests effective disruption of cell membranes,[83] leading to cell death primarily through necrosis or late-stage apoptosis. Differential staining detected live, early, and late apoptotic and necrotic cells. The increased red fluorescence in the CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR groups supports that PtNPs, especially when combined with DOX, significantly enhance cytotoxicity through ROS generation and disruption of cellular homeostasis.[84]
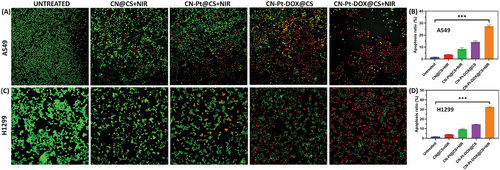
2.8 DAPI Staining
The microscopic images of DAPI staining for untreated, treated with CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR as shown in Figure 5A and the quantification of apoptosis ratio of A549 cells as given in Figure 5B. Figure 5C displays the microscopic images of DAPI staining for untreated, treated with CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR and then Figure 5D displays the quantification of apoptosis ratio of H1299 cells. In the untreated control group, A549 and H1299 cells showed normal nuclei with uniformity and intact DAPI staining, with no signs of chromatin condensation or nuclear fragmentation. Cells treated with CN@CS+NIR hydrogel nanocomposites displayed mild nuclear changes, including slight chromatin condensation, but most nuclei remained intact, indicating limited apoptotic activity. Treatment with CN-Pt@CS+NIR nanocomposites resulted in more pronounced nuclear alterations, including increased chromatin condensation, nuclear shrinkage, and apoptosis, particularly in H1299 cells. The most significant nuclear changes were observed in cells treated with CN-Pt-DOX@CS+NIR nanocomposites, which showed extensive chromatin condensation, nuclear fragmentation, and apoptotic bodies. H1299 cells exhibited a higher degree of these features. These results suggest that the combination of DOX and PtNPs significantly enhances apoptosis induction through both ROS-mediated damage and DOX-induced DNA intercalation in the presence of NIR.[85] The NIR treatment further exacerbated these nuclear alterations, particularly in the CN-Pt-DOX@CS+NIR group compared to the CN-Pt-DOX@CS group, indicating the promotion of cell death through apoptosis or necrosis. P < 0.001 for CN-Pt-DOX@CS+NIR compared to the untreated group for A549 and H1299 cells DAPI staining revealed nuclear alterations induced by the hydrogel nanocomposites.[86] The observed chromatin condensation and nuclear fragmentation[87] in the CN-Pt-DOX@CS+NIR group are indicative features of apoptosis. These nuclear changes are consistent with the mechanism of action of DOX,[88] which intercalates into DNA, causing double-strand breaks, and PtNPs, which induce oxidative DNA damage.[89] The more pronounced apoptotic features in H1299 cells compared to A549 cells may be due to genetic and molecular differences. The p53-null status of H1299 cells likely makes them more susceptible to apoptosis induced by DNA-damaging agents, explaining the greater nuclear alterations observed.[90, 91]
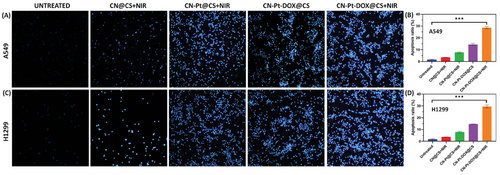
2.9 Cell Apoptosis Quantification Using Flow Cytometry
Cell apoptosis quantification studies were carried out using flow cytometry analysis. Figure 6A displays apoptosis detection using flow cytometry for untreated, treated with CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR and the quantification of apoptosis levels of A549 cells as shown in Figure 6B. Figure 6C displays apoptosis detection using flow cytometry for untreated, treated with CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR and Figure 6D displays the quantification of apoptosis levels of H1299 cells. Flow cytometry with Annexin V-FITC/PI labeling showed that in the untreated control group, most A549 and H1299 cells were viable, with low apoptosis rates. Treatment with CN@CS+NIR hydrogel nanocomposites slightly increased early apoptotic cells but indicated minimal cytotoxicity. CN-Pt@CS+NIR nanocomposites caused significant shifts, increasing both early and late apoptotic populations, and suggesting enhanced apoptosis through ROS generation and DNA damage. The CN-Pt-DOX@CS+NIR group had the most pronounced effects, with a substantial rise in early and late apoptotic cells, indicating effective apoptosis and necrosis induction. These findings suggest that combining CNs, PtNPs, and DOX in hydrogel nanocomposites significantly enhances cytotoxicity and triggers programmed cell death through multiple mechanisms,[92, 93] due to the synergistic effects of DOX, which intercalates into DNA and disrupts replication, and PtNPs, which generate ROS and induce oxidative stress in the presence of NIR.[94] The addition of NIR activation to the CN-Pt-DOX@CS+NIR group resulted in an even greater increase in apoptosis than the CN-Pt-DOX@CS group, with a notable shift toward late-stage apoptosis and necrosis. P < 0.001 for CN-Pt-DOX@CS+NIR compared to the untreated group for A549 and H1299 cells. Higher apoptosis rates in H1299 cells compared to A549 cells may be due to genetic differences. H1299 cells, being p53-null, are more susceptible to apoptosis from DNA-damaging agents like DOX and PtNPs, as they lack p53-mediated DNA repair mechanisms.[95] The results show that CN-Pt-DOX@CS+NIR hydrogel nanocomposites are effective across different genetic backgrounds, suggesting their potential for treating various lung cancer types, including those with p53 mutations. These mutations are often linked to poor prognosis and resistance to conventional therapies.
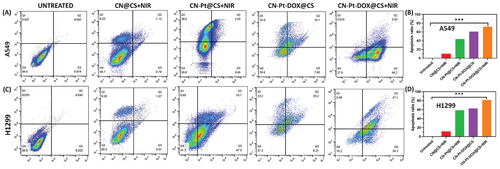
2.10 Semi-Quantitative RT-PCR Analysis
Figure 7A displays a semi-quantitative RT-PCR analysis of pro-apoptotic and anti-apoptotic signaling genes for untreated, treated with CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR in A549 cells. Figure 7B displays a semi-quantitative RT-PCR analysis of pro-apoptotic and anti-apoptotic signaling genes for untreated, treated with CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR in H1299 cells. The schematic representation of the fabrication of carbon nanodots/platinum functionalized nanocomposite hydrogel formulation for near-infrared responsive delivery of doxorubicin to promote necroptosis for mitigating lung cancer cells is illustrated in Scheme 1. In A549 cells, CN@CS+NIR hydrogel nanocomposites moderately upregulated p53 expression compared to controls. CN-Pt@CS+NIR, CN-Pt-DOX@CS and CN-Pt-DOX@CS+NIR treatments significantly increased p53 levels, with the highest expression in the CN-Pt-DOX@CS+NIR group. In contrast, H1299 cells, lacking functional p53, showed no p53 expression. Both cell lines treated with CN-Pt@CS+NIR showed slight upregulation of the pro-apoptotic genes bax and bad, which increased further with treatment, highlighting the apoptotic potential of PtNPs.[96] Cells treated with CN-Pt-DOX@CS+NIR nanocomposites showed the most significant upregulation of bax and bad, indicating a strong pro-apoptotic response from the combined treatment. The transcription of c-myc, a gene involved in cell cycle regulation and apoptosis,[97] was moderately upregulated in A549 and H1299 cells treated with CN@CS+NIR and CN-Pt@CS+NIR nanocomposites. CN-Pt-DOX@CS+NIR treatment significantly increased c-myc expression, indicating enhanced pro-apoptotic pathways. It also significantly upregulated caspase-3, a key effector of apoptosis,[98] was observed in both A549 and H1299 cells treated with CN-Pt-DOX@CS+NIR nanocomposites. The CN-Pt-DOX@CS+NIR group showed the highest expression levels, indicating enhanced activation of the apoptotic cascade via caspase-3. Both A549 and H1299 cells treated with CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR nanocomposites exhibited downregulation of the anti-apoptotic genes bcl-2 and bcl-xl. The most significant reduction was in the CN-Pt-DOX@CS+NIR group, demonstrating effective suppression of apoptosis-inhibiting genes.[99, 100] The addition of NIR further increased the pro-apoptotic gene expression and anti-apoptotic gene suppression in the CN-Pt-DOX@CS+NIR group compared to the CN-Pt-DOX@CS group, suggesting that NIR activation enhances the apoptotic response. P < 0.001 for CN-Pt-DOX@CS+NIR compared to the untreated group for A549 cells’ p53 expression. P < 0.001 for CN-Pt-DOX@CS+NIR compared to the untreated group for A549 and H1299 cells’ caspase-3 expression. P < 0.05 for CN-Pt-DOX@CS+NIR compared to the untreated group for A549 and H1299 cells’ bax and bcl-xl expression. P < 0.01 for CN-Pt-DOX@CS+NIR compared to the untreated group for A549 and H1299 cells’ bad, c-myc, and bcl-2 expression. The RT-PCR results highlight the strong pro-apoptotic effects of CN-Pt-DOX@CS+NIR hydrogel nanocomposites on lung cancer cells. The significant upregulation of pro-apoptotic genes (bax, bad, c-myc, caspase-3) and downregulation of anti-apoptotic genes (bcl-2, bcl-xl) indicate a marked shift toward apoptosis in both A549 and H1299 cells. The CN-Pt-DOX@CS+NIR group showed the most significant pro-apoptotic gene expression and anti-apoptotic gene suppression, suggesting that CNs, PtNPs, and DOX in the presence of NIR synergistically enhance the apoptotic response compared to CNs or PtNPs alone.[101] The graphical abstract representation of the fabrication of carbon nanodots/platinum functionalized nanocomposite hydrogel formulation for near-infrared responsive delivery of doxorubicin for lung cancer treatment is illustrated in Graphical Abstract 1.
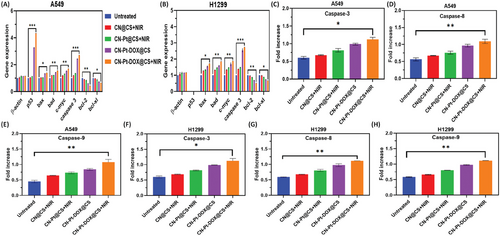
2.11 Analysis of Caspase-3/8/9 Activation
Figure 7C,D,E shows the effect of CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR in A549 cells by the chromogenic assay on Caspase-3, Caspase-8, and Caspase-9 respectively. Figure 7F,G,H shows the effect of CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR in H1299 cells by the chromogenic assay on Caspase-3, Caspase-8 and Caspase-9 respectively. Caspase-3 activity was significantly elevated in A549 cells treated with CN-Pt-DOX@CS+NIR hydrogel nanocomposites, indicating enhanced apoptotic activity. The absorbance at 405 nm showed a substantial increase in cleaved caspase-3 substrate. While CN@CS+NIR and CN-Pt@CS+NIR treatments also increased caspase-3 activity, the effect was less pronounced, highlighting the synergistic effect of combining PtNPs and DOX. H1299 cells exhibited similar trends, with significant caspase-3 activation despite the absence of functional p53, suggesting p53-independent apoptotic pathways. Caspase-8 activity, linked to the extrinsic apoptotic pathway, increased moderately in A549 cells treated with CN@CS+NIR and CN-Pt@CS+NIR, with the most substantial increase in those treated with CN-Pt-DOX@CS+NIR. H1299 cells showed a similar trend, indicating the nanocomposites can trigger apoptosis via the extrinsic pathway regardless of p53 status. The intrinsic pathway marker, caspase-9, also showed significantly increased activity in both A549 and H1299 cells treated with CN-Pt-DOX@CS+NIR, indicating effective activation of the intrinsic pathway even without p53. The caspase activity assay results demonstrate the superior pro-apoptotic efficacy of CN-Pt-DOX@CS+NIR hydrogel nanocomposites, activating both intrinsic and extrinsic apoptotic pathways and leading to robust apoptosis in lung cancer cells.[102] The elevated caspase activities in the CN-Pt-DOX@CS+NIR treatment group compared to individual CN@CS+NIR, CN-Pt@CS+NIR, and CN-Pt-DOX@CS treatments demonstrate the synergistic effect of combining CNs, PtNPs, and DOX in the hydrogel matrix in the presence of NIR. These results suggest that combination therapy enhances drug delivery and amplifies the apoptotic response,[103] making it a more effective treatment option. CN-Pt-DOX@CS+NIR treatment demonstrated significantly enhanced apoptotic effects compared to CN-Pt-DOX@CS, highlighting the synergistic role of NIR activation in amplifying caspase activity and promoting cell death. P < 0.05 for CN-Pt-DOX@CS+NIR compared to the untreated group for A549 and H1299 cell's caspase-3 gene expression. P < 0.01 for CN-Pt-DOX@CS+NIR compared to the untreated group for A549 and H1299 cells’ caspase-8 and 9 gene expressions. The increased caspase-3 activity in both A549 and H1299 cells treated with CN-Pt-DOX@CS+NIR nanocomposites indicates effective apoptosis induction through both p53-dependent (A549) and p53-independent (H1299) pathways, suggesting the nanocomposites could be effective against various lung cancer types,[104] including those with mutations in the p53 gene.[105] The activation of caspase-8 and caspase-9 by CN-Pt-DOX@CS+NIR confirms dual pathway activation, highlighting its versatility and potency in inducing cell death in cancer cells.[106]
2.12 Effect of CN-Pt-DOX@CS+NIR on Cell Migration
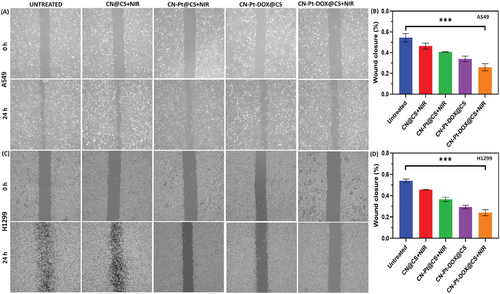
Figure 8A displays microscopic images of scratch assays for untreated, treated with CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR and Figure 8B displays the quantification of wound closure of A549 cells. Figure 8C displays microscopic images of scratch assays for untreated, treated with CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR and Figure 8D displays the quantification of wound closure of H1299 cells. The wound healing assay showed varying effects on cell migration after treatment with CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR hydrogel nanocomposites in A549 and H1299 cells. Untreated control cells showed significant migration, reducing the wound gap after 24 h. CN@CS+NIR treatment moderately inhibited wound closure, slightly reducing cell migration. CN-Pt@CS+NIR significantly slowed wound closure, especially in H1299 cells. CN-Pt-DOX@CS+NIR exhibited the highest inhibition of migration, with a marked delay in wound closure, particularly in H1299 cells, indicating strong suppression of cell migration. These results demonstrate that CN-Pt-DOX@CS+NIR hydrogel nanocomposites significantly inhibit cell migration in A549 and H1299 cells by inducing cytotoxic effects, oxidative stress, and apoptosis.[107, 108] The reduced migration in CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR groups suggests that PtNPs, known to generate ROS, are key in suppressing cell migration.[109] CN-Pt-DOX@CS+NIR group exhibited the least migration compared to CN-Pt-DOX@CS group, suggesting a stronger inhibitory effect on cell motility, likely due to enhanced apoptosis induced by NIR activation. P < 0.001 for CN-Pt-DOX@CS+NIR compared to the untreated group for A549 and H1299 cells. The more pronounced inhibition in H1299 cells indicates that the treatment is effective in p53-deficient cells, highlighting its potential for targeting aggressive, migration-prone lung cancer types. CN-Pt-DOX@CS+NIR nanocomposites present a promising strategy to hinder cancer cell migration and prevent metastasis.[110, 111]
2.13 Effect of CN-Pt-DOX@CS+NIR on ROS Production
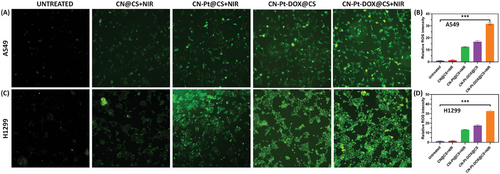
ROS levels were measured in A549 and H1299 cells after treatment with CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR hydrogel nanocomposites using DCFH-DA staining. Figure 9A displays ROS fluorescence images for untreated, treated with CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR and Figure 9B displays the quantification of ROS levels of A549 cells. Figure 9C displays ROS fluorescence images for Untreated, treated with CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR and Figure 9D displays the quantification of ROS levels of H1299 cells. Untreated cells showed minimal ROS levels. CN@CS+NIR treatment slightly increased ROS, while CN-Pt@CS+NIR treatment significantly increased ROS due to the properties of PtNPs. The CN-Pt-DOX@CS+NIR group had the highest ROS levels, resulting from the combined effects of PtNPs and DOX in the presence of NIR.[112, 113] The quantification confirmed that NIR activation significantly increased ROS production in the CN-Pt-DOX@CS+NIR group compared to the CN-Pt-DOX@CS group. P < 0.001 for CN-Pt-DOX@CS+NIR compared to the untreated group for A549 and H1299 cells. H1299 cells exhibited slightly higher ROS levels than A549 cells, indicating ROS generation was independent of p53 status.[114, 115] The results show that CN-Pt-DOX@CS+NIR hydrogel nanocomposites significantly boost ROS production in both A549 and H1299 cells, enhancing apoptosis. PtNPs play a key role in ROS induction, while DOX amplifies oxidative stress. Higher ROS levels in H1299 cells suggest this treatment's effectiveness in p53-deficient cancers. CN-Pt-DOX@CS+NIR nanocomposites leverage ROS to induce apoptosis, offering a promising strategy for lung cancer therapy, especially in resistant types.[116, 117]
2.14 Effect of CN-Pt-DOX@CS+NIR Nanocomposites on MMP
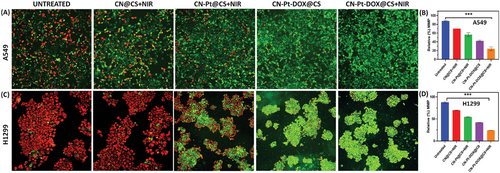
After treatment with hydrogel nanocomposites and NIR, the JC-1 assay showed clear changes in MMP (ΔΨm) in A549 and H1299 cells. Figure 10A displays the fluorescent images for untreated, treated with CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR with JC1 staining, and Figure 10B displays the quantification of the relative percentage of MMP for A549 cells. Figure 10C displays the fluorescent images for untreated, treated with CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR with JC1 staining, and Figure 10D displays the quantification of the relative percentage of MMP for H1299 cells. Untreated cells exhibited strong red-orange fluorescence, indicating healthy mitochondria. CN@CS+NIR treatment caused a decrease in red fluorescence and an increase in green fluorescence, indicating partial depolarization, which intensified after 24 h. CN-Pt@CS+NIR treatment showed more pronounced red-to-green shifts, indicating significant mitochondrial disruption by 24 h. The CN-Pt-DOX@CS+NIR group showed the greatest depolarization, with most cells appearing green by 24 h, indicating extensive ΔΨm loss. Untreated cells maintained healthy ΔΨm, while CN-Pt-DOX@CS+NIR caused the most disruption, followed by CN-Pt-DOX@CS, CN-Pt@CS+NIR, and CN@CS+NIR. CN-Pt-DOX@CS+NIR treatment caused significantly greater mitochondrial depolarization and dysfunction compared to CN-Pt-DOX@CS, emphasizing the enhanced apoptotic potential achieved through NIR activation. P < 0.001 for CN-Pt-DOX@CS+NIR compared to the untreated group for A549 and H1299 cells. These results show that CN-based hydrogel nanocomposites, especially with NIR irradiation, significantly disrupt MMP.[118, 119] While CN@CS caused some mitochondrial damage, the addition of Pt (CN-Pt@CS) amplified this effect, likely due to increased oxidative stress and ROS from Pt.[120] The most severe disruption was observed with CN-Pt-DOX@CS+NIR, consistent with DOX's mitochondrial damage capability.[121] The time-dependent increase in depolarization suggests cumulative mitochondrial damage, which is crucial for cancer therapy as it triggers apoptosis. CN-Pt-DOX with NIR shows the most promise for inducing mitochondrial dysfunction in cancer cells.
3 Conclusion
The NIR-responsive CN-Pt-DOX@CS+NIR nanocomposite embedded in hydrogel shows significant potential as a targeted therapeutic strategy for lung cancer. The combination of CNs, PtNPs, and DOX activated by NIR stimulation, not only enhances cytotoxicity but also induces necroptosis and apoptosis through multiple pathways. The observed effects on cancer cell migration, ROS production, and mitochondrial function further underscore the efficacy of this approach. This innovative formulation offers a promising avenue for improving lung cancer treatment outcomes while minimizing systemic toxicity, highlighting the potential of multifunctional nanocomposites in cancer therapy. Further studies are necessary to optimize its application and evaluate its therapeutic efficacy and systemic toxicity in vivo models.
4 Experimental Section
Materials Required
The following chemicals were procured from Sigma-Aldrich, USA; Polyethylene glycol, deionized water, Citric acid, Chloroplatinic acid (H₂PtCl₆), Sodium borohydride (NaBH₄), Polyvinylpyrrolidone (PVP), Doxorubicin (DOX), N-hydroxysuccinimide (NHS), 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), Phosphate-buffered saline (PBS), Chitosan and Glutaraldehyde.
CN Synthesis
To synthesize CN, 2 g of citric acid was dissolved in 20–50 mL of deionized water and stirred until clear. The solution was transferred into a teflon-coated stainless steel autoclave, and heated to 180–200 °C for 4–8 h, initiating carbonization. After cooling to room temperature, the reaction mixture, which turned dark brown to black, was filtered with a 0.22 µm membrane to remove large particles. The filtrate underwent dialysis against deionized water for 24–48 h to eliminate residual impurities. PEG was then added as a passivating agent to enhance the fluorescence properties, and the solution was stirred at room temperature. The amount of PEG added to the CN dispersion was 2 mg mL−1. The physical properties of the PEG polymer include a molecular weight of 4000 Da, a melting point of 60 °C, and a viscosity of 150 cP at 25 °C. The purified CNs were stored at 4 °C in a clean, airtight container, either as a liquid suspension or freeze-dried powder for long-term storage.[42, 122]
Synthesis of PtNPs
PtNPs were prepared using a reported procedure in the literature.[123] PtNPs were synthesized by dissolving 0.1 g (10 mmol) of H2PtCl6 in 100 mL of deionized water. Simultaneously, 0.1 g (10 mmol) of PVP was dissolved in 100 mL of deionized water, and 0.038 g (10 mmol) of NaBH₄ was dissolved in 100 mL of deionized water in an ice bath to maintain stability. The Pt precursor solution was mixed with the PVP solution and stirred continuously. The NaBH₄ solution was added dropwise, turning the solution from yellow to dark brown/black, indicating PtNP formation. After stirring for 30–60 min to reduce Pt ions and stabilize nanoparticles, the solution was centrifuged at 10,000 rpm for 15–20 min to obtain a black pellet. The pellet was redispersed in deionized water, centrifuged, and washed thrice to remove excess stabilizers and unreacted materials. The purified PtNPs were stored in deionized water or as a dry powder at room temperature or 4 °C.
Conjugation of DOX
CNs (10 mg mL−1) and PtNPs were dispersed in deionized water and sonicated for 10–15 min for a uniform suspension. PBS buffer was added to maintain a pH of 6–7. Then, 2 mmol of EDC and 5 mmol of NHS were sequentially added to activate carboxyl groups on the nanoparticles. This mixture was stirred at ambient temperature for 30–60 min. DOX (5 mg mL−1) solution was added dropwise and stirred at ambient temperature or 30 °C for 4–6 h to form stable amide bonds. Post-reaction, glycine (0.1 m) was added to quench the remaining EDC/NHS and stirred for 30 min. The mixture was centrifuged at 10,000 rpm for 15–20 min to separate the conjugated nanoparticles, which were washed with deionized water or buffer 2–3 times. The purified DOX-conjugated nanoparticles were resuspended in deionized water or buffer and stored at 4 °C or freeze-dried for extended storage.[124]
Preparation of Hydrogel Matrix
CS was dissolved in 1% acetic acid (2–3% w/v) and stirred for 2–4 h at room temperature. A cross-linker solution of glutaraldehyde (0.5–2% w/v) in deionized water was mixed into the hydrogel precursor solution. CN-Pt-DOX nanoparticles were dispersed in PBS and mixed with the hydrogel precursor before solidification to ensure uniform nanoparticle distribution. The mixture was set at room temperature, initiating cross-linking and forming a solid or semi-solid hydrogel. During the setting, the mixture was kept undisturbed. Once set, the hydrogel was removed from molds and stored in deionized water or buffer at 4 °C to maintain its integrity for further studies.[53, 125]
Characterization of the Hydrogel-Embedded CN-Pt-DOX System
Physicochemical characterization of CN-Pt-DOX@CS hydrogel nanocomposites was conducted using various techniques. UV–vis absorption spectroscopy with the Puxi UV-1810 visible spectrophotometer analyzed their optical properties. Functional groups were identified using FT-IR spectroscopy with a Perkin-Elmer RX1 FT-IR spectrometer, with samples mixed with potassium bromide and scanned from 400 to 4000 cm⁻¹.[126] Structural morphology was examined using a scanning electron microscope (JEOL JSM-6490LA), while size and shape were analyzed with transmission electron microscopy (TEM; Hitachi H-7650). Dynamic light scattering (DLS; Brookhaven Instruments Corp., USA) was used to determine hydrodynamic size and zeta potential, providing a comprehensive understanding of the nanocomposites' properties.
Test for the Swelling Rate of Hydrogels
Rheological Testing
Rheological testing assessed the hydrogel's mechanical properties, including viscosity, elasticity, and shear-thinning behavior. A cylindrical hydrogel sample, fully hydrated, was mounted on the Rheometer. The upper plate was lowered until it touched the hydrogel without compressing it. A frequency sweep (0.1 to 100 rad s−1) was used to measure the storage modulus (G') and loss modulus (G''). Strain measurement determined the hydrogel's linear viscoelastic area. Data on gel stiffness and viscoelastic behavior were recorded and analyzed.[127]
Drug Release Studies
To evaluate the release kinetics of DOX from the hydrogel under NIR irradiation, a hydrogel containing CN-Pt-DOX nanoparticles was placed in a dialysis bag and immersed in PBS at 37 °C with continuous stirring. The hydrogel was exposed to NIR light of 808 nm at specific intervals using a laser with a controlled power density of 1–2 W cm−2. At predetermined times, aliquots of the release medium were withdrawn and replaced with fresh PBS. The concentration of released DOX was measured using UV–vis spectroscopy by analyzing its absorbance peak. The cumulative release of DOX over time was plotted to determine the release kinetics and assess the impact of NIR irradiation on the release pattern.[128, 129]
Effects of Hydrogel Concentration, Laser Power, and Stability Under Photothermal Conditions
The effect of the concentration of hydrogel on photothermal effects was studied as follows. Cylindrical hydrogel was prepared and equipped it with a thermometer containing a thermocouple to measure the temperature changes at room temperature. It was then exposed to NIR irradiation (808 nm) with 1.5 W cm−2 power for 5 min, with temperature readings taken every 10 s. The recorded time points were plotted on the x-axis, while the corresponding temperature values were on the y-axis. The photothermal performance of hydrogels containing varying CN-Pt concentrations was compared, with a blank hydrogel serving as the control for comparison.
The effect of laser power on the photothermal performance was studied by exposing the liquid surface to NIR irradiation (808 nm) at varying power levels such as 0.5, 1, 1.5, and 2 W cm−2 for 5 min. A thermometer containing a thermocouple was inserted into the hydrogel and the temperature was noted every 10 s at room temperature with a blank hydrogel serving as the control for comparison.
The stability under photothermal conditions was studied by exposing the hydrogel to NIR irradiation (808 nm) with 1.5 W cm−2 power for 5 min, followed by a cooling period to return to its initial temperature. This cycle was done five times. A thermometer containing a thermocouple was placed inside the hydrogel, and for every 10 s, the temperature was noted at room temperature.
Cell Culture
A549 and H1299 cell lines were obtained from the American Type Culture Collection (ATCC) and cultured in DMEM containing 10% (v/v) heat-inactivated FBS, 1% penicillin/streptomycin, 0.75% glutamine, and 1% sodium pyruvate. Cell cultures were maintained at 37 °C in a humidified atmosphere with 95% air and 5% CO2.
Cell Viability Study
Cell viability of A549, H1299, and NIH-3T3 cell lines treated with 50 µm of CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR hydrogel nanocomposites was assessed using the MTT assay. Cells were inoculated at 10⁴ cells per mL in 96-well plates after being cultured in DMEM. The hydrogel nanocomposites were added to separate wells and incubated at 37 °C for 24 h, with untreated cells as controls. Each well received 10 µL of MTT solution and was cultured for an additional 4 h at 37 °C. After incubation, the insoluble formazan product was dissolved by washing the cells with PBS and incubating with 100 µL of detergent for 2 h. Absorbance at 570 nm was measured using a microplate reader, and the OD values were compared.[130] To compare cell viability, acridine orange (AO) staining was conducted.
Morphological Alterations in the Cells (Staining using Acridine Orange and Ethidium Bromide (AO/EB) and 4′,6-Diamidino-2-phenylindole (DAPI))
A simultaneous AO-EB staining study was conducted to assess cellular toxicity and anti-proliferative capabilities of nanomaterials. Cell lines (2 × 10⁴ cells Ml−1) were cultured in 96-well plates, grouped into untreated control, CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR treatments (50 µm), with 24 wells per condition. After 24 h, cells were trypsinized (20 µL per well), rinsed with PBS, and stained with 4 µL of AO-EB solution (100 µg mL−1 each). The stained cells were transferred to glass slides for observation using a fluorescence microscope. EB binds to DNA in dead cells, causing red fluorescence, while AO penetrates live cells, resulting in green fluorescence. This method differentiates viable cells (green) from non-viable cells (red) based on their fluorescence color.[131]
To examine nuclear morphological changes, cell lines (1 × 10⁵ cells per well) were cultured in DMEM medium in 48-well plates. After 24 h of incubation, the medium was discarded, and 2 mL of freshly prepared medium with 50 µm CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR hydrogel nanocomposites was added. Untreated cells served as controls. Following another 24 h, cells were DAPI-stained for 10 min at 37 °C, washed with PBS to remove excess dye, and observed under a fluorescence microscope to examine nuclear alterations using the DAPI Kit as per the supplier's instructions.[132]
Evaluation of Cell Apoptosis Utilizing Flow Cytometry
Quantification of apoptosis is essential for understanding the efficacy of anticancer composites. Using Annexin V-FITC and PI dual staining, apoptosis was evaluated. Cell lines (2 × 10⁵ cells per well) were cultured in 6-well plates with 2 mL of DMEM medium. After 24 h of incubation, the medium was replaced with 2 mL of fresh medium containing 50 µm CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR hydrogel nanocomposites. Untreated cells served as controls. Following another 24 h, cells were stained with the Annexin V-FITC/PI Staining Assay Kit and analyzed by flow cytometry using Dakewe EXFLOW-206 (Shenzhen, China).
Reverse Transcription Polymerase Chain Reaction (RT-PCR) – (Semi-quantitative)
Gene expression studies were conducted by treating cell lines with 50 µm CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR hydrogel nanocomposites for 48 h, with untreated cells as controls. Total RNA was extracted using TRI reagent (Sigma-Aldrich, USA) and reverse transcribed with Super Script II Reverse Transcriptase (Thermo Fisher Scientific) to synthesize cDNA. The cDNA was used for RT-PCR amplification of apoptotic genes (p53, bax, bad, c-myc, caspase 3) and anti-apoptotic genes (bcl-2, bcl-xl) using Applied Biosystems' reagents. β-actin served as the housekeeping gene reference.
Assay for Caspase Activity
Caspase activity was assessed using chromogenic assay kits for caspase-3, -8, and -9. A549 and H1299 cells (2 × 10⁵ cells per well) were seeded in 6-well plates. After a 24 h incubation, the medium was replaced with 2 mL of fresh medium containing 50 µm CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR hydrogel nanocomposites. Untreated cells served as controls. Cells were lysed with a buffer (50 mm HEPES, 0.1% CHAPS, 100 mm NaCl, 100 mm EDTA, and 1 mm DTT) and centrifuged at 10,000 rpm for 1 min. The supernatant was incubated with the substrate in a water bath at 37 °C for 2 h. A microtiter plate analyzer (Bio-Rad 550) measured the absorbance of the cleaved substrates at 405 nm.
Assay for In Vitro Wound Closure Effect
To assess cell migration, a wound-closure assay was performed. Cell lines were cultured in 6-well plates for 1–4 days and then treated with 50 µm CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR hydrogel nanocomposites for 24 h. Untreated cells served as controls. Cells were plated at a density of 2 million cells per well. After forming a monolayer, a scratch was made using sterile micropipette tips, and the cells were rinsed with PBS to remove debris. Images of the scratched area were taken immediately and 24 h later. The gap distance was measured using ImageJ software from the National Institutes of Health.[133]
Assay for ROS Production
To detect intracellular ROS levels, 2'-7'-Dichlorofluorescin diacetate (DCFH-DA) was used. Untreated and treated cell lines (50 µm CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR) were rinsed twice with PBS, then cultured in serum-free medium containing 10 µm DCFH-DA at 37 °C for 30 min. After incubation, trypsin was used to detach the cells, which were then prepared in PBS for analysis. Intracellular ROS staining was visualized under a fluorescent microscope, and fluorescence intensity was quantified to determine ROS levels.
Assay for MMP
The JC-1 fluorescent probe assessed MMP, where healthy mitochondria emit green fluorescence and compromised ones emit red-orange fluorescence. Untreated and treated cell lines (50 µm CN@CS+NIR, CN-Pt@CS+NIR, CN-Pt-DOX@CS, and CN-Pt-DOX@CS+NIR) were cultured in six-well plates. After 12 and 24 h of exposure, cells were stained with JC-1 (2 µg mL−1) in the culture medium for 30 min. Adherent cells were rinsed with PBS, detached with trypsin-EDTA, collected in PBS, cleaned by centrifugation, re-immersed in PBS, mixed, and examined under a fluorescence microscope with a UV filter (450–490 nm).[134]
Statistical Analysis
All data were presented as mean ± standard deviation (SD). The experimental outcomes were evaluated using one-way analysis of variance (ANOVA) with the help of GraphPad Prism software. A p-value of less than 0.05 was considered statistically significant.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
X.A. and J.B. contributed equally to this work.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.