Optimizing Electrospun PVA Fibers with MXene Integration for Biomedical Applications
Abstract
MXene-based materials have gained attention in the biomedical field due to their promising biocompatibility, improved mechanical strength, and conductivity. In this study, the focus is on optimizing MXene-incorporated electrospun fibers and subsequent characterizations to assess their potential for biomedical applications. Polyvinyl alcohol (PVA) is used as the appropriate matrix material and process parameters are finetuned to ensure effective incorporation of MXene. XRD and spectroscopic analysis confirm the successful synthesis and integration of MXenes into the nanofibers. Morphological analysis shows that MXene led to the formation of sub-micrometer fibers with smooth surfaces and reduced the fiber diameter (587 ± 191 nm) compared to pure PVA (696 ±160 nm). Investigations on the electrical characteristics demonstrate a fourfold increase in conductivity of nanofibers (σ = 1.90 ± 0.45 × 10−8 S cm−1) after MXene addition (compared to σ = 0.46 ± 0.05 × 10−8 S cm−1 of PVA-only fibers). Furthermore, the MXene-PVA system demonstrates a nearly twofold increase in mechanical stiffness, with E = 136.87 ± 19.63 MPa than 71.42 ± 16.56 MPa for PVA. Moreover, the initial in vitro experiments indicate improved L929 cell viability. These findings position MXene-PVA composites as a highly versatile platform for advanced biomedical devices, such as electroactive tissue scaffolds and wearable sensors.
1 Introduction
In recent years, there has been considerable interest in the development of advanced materials for biomedical applications aimed to address emerging challenges in bioengineering and related medical fields. MXenes, a family of 2D transition metal carbides and nitrides have emerged as strong contenders because of their remarkable electrical conductivity, hydrophilicity, mechanical strength, and biocompatibility.[1] These distinctive characteristics make MXenes suitable for various applications, such as tissue engineering, drug delivery, biosensors, and wearable technology.
The incorporation of MXenes into polymer matrices has shown great potential in enhancing the thermal, mechanical, and electrical properties of composite fibers. Polyvinyl alcohol (PVA), as a biodegradable and cost-effective polymer offers significant environmental and economic benefits.[2] Its integration with MXene enables the development of nanofiber composites with controlled degradation rates and reduced production costs. For example, Ti₃C₂T₂ MXene, one of the most studied members of this family, is particularly well-suited for fiber-shaped supercapacitors (FSCs), which are integral to portable and wearable electronics.[3] When combined with conductive binders like poly(3,4-ethylenedioxythiophene) (PEDOT), MXene-based hybrid fibers demonstrate remarkable energy storage capabilities, further expanding their applicability in wearable devices.[4] Additionally, MXene fibers have shown promise in terahertz shielding,[5] surpassing traditional carbon-based materials in effectiveness due to their exceptional conductivity and processability.
In the biomedical realm, the combination of MXenes with hydrogels and biodegradable polymers has led to the development of materials with superior chemical activity, flexibility, and biocompatibility. Graphene-PVA composites are widely recognized for their exceptional mechanical properties and electrical conductivity, often surpassing those of MXene-PVA systems.[6] However, the hydrophobic nature of graphene poses challenges in its dispersion and biocompatibility within aqueous systems ─ a critical factor for biomedical applications. In contrast, MXenes, with their hydrophilic surface resulting from functional groups such as ─OH, ═O, and ─F, offer improved dispersion and better interaction with biological environments. Similarly, Carbon Nanotube (CNT)-PVA composites exhibit excellent electrical conductivity and tensile strength, but their potential cytotoxicity and biocompatibility concerns remain major hurdles for biomedical use.[7]
Recent studies have highlighted the versatility of MXenes in various biomedical applications. For instance, MXenes have been explored for their potential in drug delivery, tissue engineering, antimicrobial activity, and biosensing, owing to their large surface area and high electrical conductivity.[8] Additionally, the integration of MXene with polymers has led to the development of advanced nanocomposites with applications in drug delivery, imaging, diagnostics, and environmental remediation.[9] Furthermore, MXene-reinforced bioactive polymer hydrogels have shown promise in biomedical applications due to their enhanced mechanical properties and biocompatibility.[10] These advancements include support for bone regeneration, antibacterial effects, and tissue repair capabilities of MXene composites.[11, 12] When mixed with PVA (a hydrophilic polymer possessing film-forming ability), its composites show a synergistic enhancement in its electrical, mechanical, and biological properties.[13] For instance, the PVA/MXene/nano-hydroxyapatite (n-HA) composites showed improved mechanical strength, bioactivity, and osteogenic potential and thus appeared to be promising matrices for guided bone regeneration applications.[14] These developments confirm the promise that MXene-based materials hold in the biomedical world, and one can expect additional research into properties and applications. Among the versatile techniques for fabricating nanofibers, the electrospinning technique has advantages in creating high-surface-area materials with well-defined architecture. Electrospun PVA fibers loaded with MXene showed enhanced conductivity and mechanical properties while finding applications ranging from wound healing to tissue engineering.
In this paper, we focus on the optimization of the fabrication of MXene-PVA nanofibers by electrospinning with a view to developing materials possessing enhanced functional properties. We thus focus on tuning the electrospinning parameters to achieve fibers with uniform morphology and improved performance characteristics such as electrical conductivity and mechanical stiffness. The biocompatibility and bioactivity of the resulting fibers, concerning cell adhesion, proliferation, and differentiation, are very important in tissue engineering applications. The insights drawn from this work add to the literature reported on MXene-polymer composites while opening ways toward next-generation biomedical materials with tailored functionality.
2 Experimental Section
2.1 Materials
For MXene synthesis, Ti3AlC2 MAX phase precursor was purchased from Carbon-Ukraine (Particle Size ≤ 40 µm). Hydrofluoric acid (HF, 48–51%) was obtained from ACROS Organics. Hydrochloric acid (HCL, ≥ 37%, ACS Reagent) and Lithium Chloride (LiCl, 99%) were purchased from Sigma–Aldrich. A Durapore 0.22 µm pore size, 47 mm PVDF hydrophobic membrane was used for vacuum filtration and film preparation. All reagents were used without further processing. For MXene/PVA fiber preparation, Polyvinyl alcohol (PVA) (Mw 130 000 Da, 99+% hydrolyzed) was purchased from Sigma–Aldrich (St. Louis, MO, USA).
2.2 Synthesis of MXene and Delamination
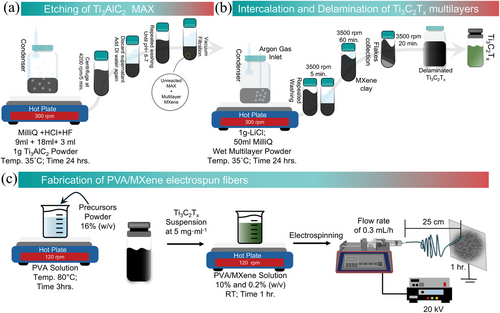
Following the mixed acid method,[15] 1 g of Ti3AlC2 MAX was gradually added to the etchant solution consisting of Milli-Q water, HCl, and HF (9, 18, and 3 mL, respectively) in a Nalgene bottle. The mixture was continuously stirred for 24 h using a Teflon-coated magnetic stirrer at 400 rpm at 35 °C in an oil bath. After 24 h, the solution was washed repeatedly with Milli-Q water via centrifugation at 4200 rpm for 5 min until the pH of the supernatant reached ≈7 (Figure 1a). Subsequently, the sediment containing multilayer MXene (ML-Ti3C2Tx) was collected via vacuum-assisted filtration. To obtain single flakes, multilayered MXene was obtained by LiCl intercalation, and the process of delamination was followed as demonstrated in Figure 1b. Briefly, 1 g of LiCl was mixed in 50 mL of Milli-Q water and the previously obtained etched MXene sediment was added and stirred at 400 rpm for 24 h at 35 °C under continuous argon (Ar) purging. The solution was then washed 2–3 times with Milli-Q water at 3500 rpm for 5 min, and the clear supernatant was discarded. Once a dark supernatant was obtained, the centrifugation time was increased to 1 h. Following that, the sediment was further dispersed with a known amount of Milli-Q water and mixed well with a glass rod, and shaking was performed for 30 min. The delaminated flakes were collected at 3500 rpm for 20 min. To further concentrate the delaminated single-layer MXene (SL-MXene), the dark black supernatant was centrifuged at 9000 rpm for 30 min and the final delaminated MXene suspension was treated with Ar gas and stored at 4 °C until further use. The remaining sediment, consisting of unetched MAX and ML-MXene, was discarded.
Hydrofluoric acid (HF) is an extremely hazardous chemical that requires adherence to safety protocols during use. HF is colorless, highly corrosive (irrespective of concentration), and poses significant risks due to its ability to penetrate deep into tissues and bones, potentially causing severe damage that may remain asymptomatic for up to 24 h. Handling HF necessitates the mandatory use of personal protective equipment (PPE), including safety glasses, acid-resistant gloves, aprons, and a face shield to minimize risks of inhalation. All procedures involving HF must be conducted within a well-ventilated fume hood to prevent inhalation of vapors. Special caution is required during the initial stage of synthesis, particularly when mixing MAX phase powders with the etchant solution, as well as during subsequent washing until the pH approaches ≈6–7.[16, 17] All waste materials collected should be neutralized properly in compliance with local environmental and safety regulations. Emergency protocols include the application of calcium gluconate gel for skin exposure and prompt medical consultation must be in place to further mitigate the risks. Alternative methods, such as molten salt etching, microwave-assisted etching, and electrochemical etching, have recently been reported.[18] However, challenges such as high energy consumption, synthesis complexity, and lower yields limit their wider applicability.
2.3 Production of PVA/MXene Nanofiber Mats
PVA aqueous solution was prepared at 16% (w/v), heated to 80 °C, and stirred (120 rpm) for 3 h. After cooling to room temperature, Ti3C2Tx MXene at a concentration of 5 mg mL−1 was mixed into the PVA aqueous solution and stirred at 120 rpm for 1 h to obtain a PVA/MXene composite with final concentrations of 10% and 0.2% (w/v), respectively (Figure 1c). The concentration for MXene was determined by gradually diluting the stock solution and analyzing the UV–vis absorbance spectra (Figure S1, Supporting Information). Electrospun nanofibers were fabricated using a vertically arranged electrospinning (NANON01; MECC, Fukuoka, Japan). Five milliliters of the PVA/MXene mixture was loaded into a 5 mL syringe equipped with a 27G metallic needle. The syringe was connected to an additive syringe pump (KD Scientific, Holliston, MA, USA) to allow controlled solution feeding. The electrospinning process was carried out at room temperature with a voltage of 20 kV, a flow rate of 0.3 mL h−1, and 25 cm between the needle tip and the collector. The fibers were collected for 1 h. Of note, preliminary attempts with higher concentrations of MXene result in significant process challenges, such as needle clogging and spray-like jetting.
2.4 Morphology and Structural Characterization
A field-emission scanning electron microscope (FE-SEM, Quanta FEG 200, FEI; Eindhoven, The Netherlands) with an accelerating voltage of 10 kV was used to evaluate the morphological features of the electrospun fibers. Rounded electrospun samples, each with a diameter of 6 mm, were placed into the sputtering machine, operated at 20 kV for 30 s, to deposit a thin Pd–Au coating ≈15–20 nm thick, improving surface conductivity. Open-source image analysis software (ImageJ 1.8; Fiji) was used to measure the average fiber diameters on selected SEM images. To analyze the surface elemental composition of the fibers in terms of weight and atomic percentages, energy dispersive X-ray spectroscopy (EDS) was conducted. Fourier-transform infrared spectroscopy (ATR-FTIR Spectrum; PerkinElmer; Waltham, MA, USA) was performed from 650 to 4000 cm−1. Wide angle X-ray scattering measurements (WAXS) were collected at room temperature with a PANalytical Empyrean diffractometer operating in reflection geometry (θ−2θ scans, with 2θ the scattering angle) using a Ni-filtered Cu-Kα radiation (λ = 1.5418 Å).
2.5 Electrical Conductivity
Electrical properties were assessed by depositing 1 µm thick films made of pure PVA and PVA/MXene fibers on multilayer substrates consisting of a 500 µm thick highly doped silicon (Si++), a 200 nm thick SiO2 dielectric barrier, and 150 nm thick pre-patterned interdigitated gold electrodes. Before film deposition, the SiO2 surface was cleaned in ultrasonic baths of acetone and isopropanol and then dried by nitrogen gas. For all samples, four active channels were achieved with two different pairs having lengths (L) of 20 and 40 µm, respectively. The channel width (W) was scaled so that the ratio between W and L was invariably 550. This layout has been previously employed to reliably assess the conductivity and mobility performances of a wide range of organic and inorganic compounds.[19, 20] All the electrical tests were carried out in the air, with the samples being contacted by a Signatone probe station, equipped with micro-manipulators bearing metallic tips and connected to a Keithley 2612A dual channel source meter. Using this experimental setup, the background current noise level was ≈0.1 nA.
2.6 Mechanical Properties
Mechanical tensile tests were carried out using the Instron dynamometer 5566 (Instron, Bucks, UK) equipped with a 10 N loading cell. Dog bone-shaped specimens were obtained through the Ceast cutting machine equipped with an ASTM D1708 cutting die. The non-contact laser sensor (Micro-Epsilon optoNCDT 1420-10, MICRO-EPSILON UK & Ireland Ltd., Ortenburg, Germany) was used for measuring specimens’ thickness. Each specimen was clamped on the dynamometric equipment through titanium chucks, and a preload of 0.01 N was applied before running the tensile test at an elongation speed of 1.0 mm min−1 up to rupture. Force and elongation data were acquired at a speed of 10 pts s−1. Stress and strain were obtained by dividing the load and the elongation by the specimen's cross-section area and the initial length, respectively. Mechanical properties (i.e., Young's modulus, maximum stress, and maximum strain) were detected on stress versus strain diagrams. Analysis of variance was performed at a probability level of p = 0.05 using the OriginPro 2018 software (OriginLab Corporation, Northampton, USA).
2.7 In Vitro assay
To evaluate the biological influence of MXene on the PVA fibers, L929 cells (fibroblasts derived from mouse, Sigma–Aldrich, St. Louis, MO, USA) were used to perform Cell Proliferation Kit II (XTT assay, Roche Diagnostics Deutschland GmbH, Mannheim, Germany, purchased by Sigma–Aldrich). This assay indicates cell viability based on the metabolic activity of living cells. First, L929 cells were cultured in Dulbecco's Modified Eagle´s Medium (DMEM, Sigma–Aldrich, Milan, Italy) containing 2 mm of L-glutamine (Sigma–Aldrich, Milan, Italy), 10% fetal bovine serum (FBS, Sigma–Aldrich, St. Louis, MO, USA), and 1% antibiotic solution (streptomycin 100 µg mL−1 and penicillin 100 U mL−1, Sigma–Aldrich, Milan, Italy). The cells were incubated at 37 °C with 5% CO2. Before the in vitro assay, samples were cut, placed into a 96-well cell culture plate, and sterilized with a solution of 70% ethanol. The samples were then washed with phosphate-buffered saline (PBS, Sigma–Aldrich, Milan, Italy) and dried under a hood. For biocompatibility tests, a density of 1×104 cells per well was seeded onto the PVA and PVA/MXene fibers. The XTT assay was conducted according to the manufacturer's protocol after 1, 3, and 7 days in cell culture. Briefly, the cell culture media was removed and replaced with fresh culture media containing XTT reagent and incubated for 4 h under standard conditions. After that period, the supernatant from each well was collected and placed into a 96-well plate reader to measure the absorbance at 450 nm using a spectrophotometer (Wallac Victor 1420, PerkinElmer, Boston, MA, USA). The experiments were performed in triplicate (n = 3) and are presented as mean ± standard derivation. Results were analyzed using a Student t-test to determine significant differences, with p-values < 0.05 considered statistically significant.
3 Results and Discussion
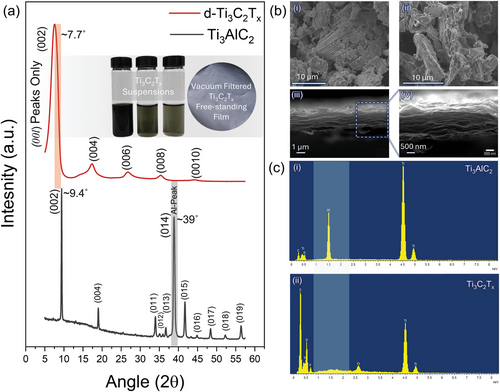
Figure 2a shows the XRD profile of Ti3AlC2 MAX and the corresponding Ti3C2 MXene. The peak at 2θ = 39°, indicating “Al”, and the (002) peak provide crucial information as the XRD patterns change from MAX to delaminated MXene and reassembled films. In Ti3AlC2 MAX, all crystallographic peaks were evident, as expected from the p63/mmc structure. After treatment with the etchant solution, the typical Al peak (at 2θ = 39°) of the MAX phase disappears, and the (00l) peaks broaden and downshift. This confirms the successful removal of aluminum and its conversion into the MXene phase. Additionally, a pronounced shift of the (002) peak to lower angles (from ≈9.4° to ≈7.7°) and noticeable broadening suggests an increase in the c-lattice parameter (c-LP) (from 18.8 to 22.94 Å). This arises from the replacement of the strong metallic/covalent bonds between the M-A layers with weaker van der Waals interactions and hydrogen bonds, making them capable of being intercalated. Furthermore, the exposed Ti-atoms on the surfaces are stabilized by suitable surface functional groups (such as F, O, OH, and Cl), denoted as Tx. Thus, the increase in c-LP signifies the replacement of Al-atoms, the appearance of Tx groups, and the inclusion of water molecules and Li+ ions (delaminating agents) within the sheets.[21, 22] The diluted suspension of MXene exhibits a characteristic greenish color at lower concentrations, while the dried film obtained through vacuum filtration shows its typical appearance (Figure 2a inset). The typical greenish color suggests Ti3C2Tx MXene production with better quality and stable colloidal dispersibility. For delaminated MXene reassembled into a film, only (00l) peaks can be observed, indicating proper delamination and alignment of the flakes in the plane.[23] Ti3AlC2 MAX particles of considerably significant size show a well-defined graphite-like compact layered structure of individual MXene sheets (Figure 2b–i). After HF+HCl acid treatment, the accordion-like morphology is less evident, characteristic of the mixed-acid etching route (Figure 2b–ii).[24] Figure 2b–iii-iv shows SEM images of delaminated SL flakes reassembled in films, demonstrating a layered structure with MXene sheets stacked over each other. Further investigation through UV–vis. Spectroscopy indicates the presence of a typical plasmonic peak at ≈780 nm (Figure S1, Supporting Information). As suggested by the EDXS elemental profile, indicates Ti3AlC2 with evident Al-content (Figure 2c–i) which is significantly removed when MXene was delamination to single flakes (Figure 2c–ii). However, in ml-MXene, some “Al” may still be present as AlFx salt or unreacted MAX (Figure S2, Supporting Information) which is removed during the multiple washing cycles after intercalation.
A solution of PVA and MXene was employed to fabricate electrospun nanofibers. The average diameter of electrospun fibers is a crucial parameter that significantly influences various applications relying on size-dependent properties, such as tissue engineering and sensor technologies.[25, 26] Scanning electron microscopy (SEM) was used to examine the impact of MXene addition on fiber diameter distribution. SEM micrographs of the composite nanofibers are presented in Figure 3a,b. The nanofibers appear smooth without beads, with diameters of 696 (±160) nm for PVA and 587 (±191) nm for PVA/MXene (Figure 3c). The reduced fiber diameter could be attributed to the strong polar interactions of MXene into the solution that enhanced jet stability during electrospinning. In detail, MXene flakes tend to interact with water molecules in solution promoting their intercalation into the polymer solution with an overall stretching effect on the polymer jet. This effect tends to enhance the mechanism of solvent evaporation thus favoring a slight reduction of the ultimate diameter of the fibers, preserving a good uniformity of flake dispersion into the fiber bulk, in agreement with previous similar studies.[27, 28] However, MXene concentrations above the optimal concentration can negatively affect the jet stability in the electrospinning process by increasing the viscosity in the solution. Besides, it can reduce the suitability of nanofibers for tissue engineering applications since the lower ratios are known to provide an ideal balance. PCL nanofibers loaded with MXene (up to 0.5 wt%) are known to show greater biocompatibility compared to structures embedded with higher MXene ratios.[29]
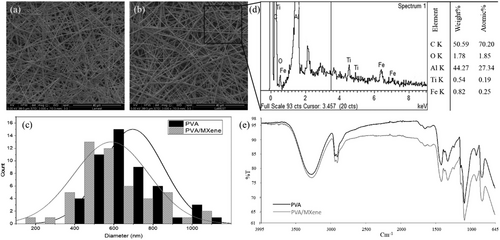
The presence of MXene within the electrospun nanofibers was confirmed by detecting the Ti element in the EDS spectra (Figure 3d), consistent with earlier reports.[30] The spectrum analysis provided a detailed breakdown of the elemental composition, with weight and atomic percentages of titanium recorded at 0.54% and 0.19%, respectively.
The FTIR spectra of PVA and PVA/MXene are shown in Figure 3e, covering the frequency range of 4000–650 cm−1. The spectra display almost identical peaks with slight shifts, indicating primarily physical interactions between PVA and MXene nanoparticles. The main absorption peaks for PVA and PVA/MXene occur at ≈3277 cm⁻¹ (O─H stretching), 2909 cm⁻¹ (C─H stretching), 1420 cm⁻¹ (CH2 bending), 1088 cm⁻¹ (C─O─C stretch), and 911 cm⁻¹.[31] However, shifted stretching of pure PVA at 3274 and at 832 cm−1 likely results from MXene's surface functional groups interacting with PVA and altering the vibrational properties of the polymer matrix.[5, 13, 32] The comparison of these spectra indicates the successful integration of MXene into the PVA matrix, leading to modified vibrational characteristics.
The electrical properties of the investigated films, deposited on Si++/SiO2 substrates equipped with gold electrodes (Figure 4a,b), were assessed in the air by performing two-probe current-voltage (IV) measurements. Figure 4c shows an optical microscopy image of a PVA/MXene layer covering the interdigitated gold contacts. In the IV tests, the applied voltage was swept from 0 to + 30 V and then backward to highlight any possible hysteretic effects. The voltage step was fixed at 200 mV with an overall scan rate of 1 V s−1. Figure 4d (left panel) shows two IV traces recorded for PVA/MXene active channels. These curves exhibit a clockwise hysteresis, indicating that the current values acquired during the forward scan (from 0 to 30 V) were larger than those recorded during the backward scan (from 30 to 0 V). This behavior is typical of many non-crystalline conducting systems and is mainly associated with charge-trapping mechanisms, which tend to immobilize carriers during the measurement acquisition.[20] Figure 4d (right panel) presents a direct comparison between a set of IV curves measured for devices based on PVA/MXene and pure PVA fibers. This plot makes clear that the integration of MXene flakes in the PVA fibers tends to enhance the electrical properties in comparison with the pure PVA system.
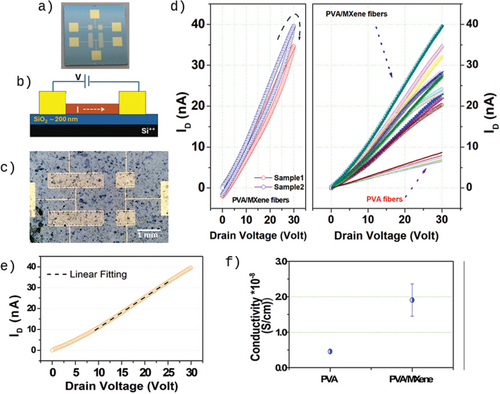
In Figure 4e, we detail the forward scan of an IV curve recorded for a PVA/MXene active channel. While the current exhibits a diode-like (sub-linear) behavior in the low voltage region, indicating the presence of non-ideal charge injection mechanisms, it follows a linear behavior when the applied voltage exceeds 10 V. This latter region was used to extract the conductivity (σ) values by fitting the measurement linearly based on Ohm's law. In Figure 4f, the average conductivity values estimated for PVA/MXene and pure PVA fibers are plotted with error bars representing the standard deviations. The MXene integration in PVA fibers resulted in a fourfold increase in conductivity (σ = (1.90 ± 0.45) × 10−8 S cm−1) compared to the pure PVA system (σ = (0.46 ± 0.05) × 10−8 S cm−1).
Typical stress versus strain curves for PVA and PVA/MXene are shown in Figure 5a. Both materials presented a maximum point before fracturing located at an elongation of ≈50% and 200% for PVA/MXene and PVA, respectively. Figure 5b,c shows the stretching occurring during the tensile test for PVA and PVA/MXene, respectively. While PVA continuously elongates reducing its cross-section area up to rupture (Figure 5b), PVA/MXene displayed a cross-section reduction up to a lower elongation level as a crack started to slowly propagate through the specimen (Figure 5c)
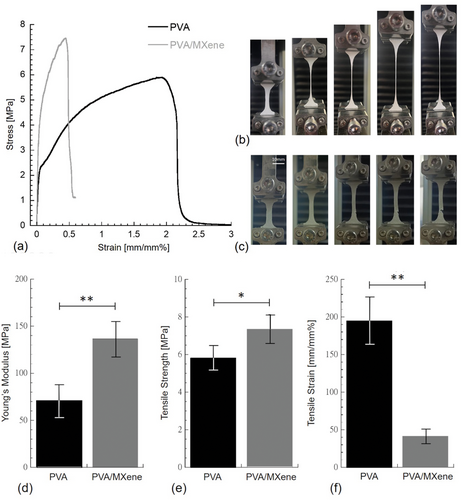
Mechanical properties of PVA and PVA/MXene samples were computed through the stress versus strain diagrams, and they were reported in Figure 5d–f. The incorporation of MXene flakes into PVA significantly improved both the stiffness and strength of PVA (Figure 5d,e, respectively); however, a significant decrease in ductility was observed (Figure 5f).
Young's modulus (i.e., the stiffness) of PVA (71.42 ± 16.56 MPa) was significantly lower (p < 0.01) than that of PVA/MXene (136.87 ± 19.63 MPa). Mean Young's modulus measured for PVA was consistent with values reported in the literature,[33, 34] while a similar stiffness increase of ≈90% was also reported for the storage modulus at room temperature as MXene was incorporated into PVA.[30]
The tensile strength of PVA/MXene (7.35 ± 0.78 MPa) was significantly higher (p < 0.05) than that of PVA (5.34 ± 0.67 MPa). The tensile strength mean value measured for PVA was consistent with those reported in the literature,[33, 35] but higher strength values for PVA were also recorded.[34] The above results suggest that the incorporation of MXene flakes into PVA effectively increased the strength by acting as a reinforcement agent; however, the stiffness increase of ≈90% suggested that MXene increased cross-linking density. Maximum strain (i.e., ductility) of PVA/MXene (41.31 ± 9.69 mm mm−1%) was significantly lower (p < 0.01) than that of PVA (194.87 ± 31.43 mm mm−1%). The maximum strain recorded for PVA is consistent with maximum strain values reported in the literature,[30, 35] and similar ductility reduction was observed as chitosan nanoparticles were incorporated into PVA.[36]
Despite the significant reduction in ductility observed when MXene is incorporated into PVA (Figure 5f), this does not hinder the application of wearable MXene-based strain sensors for mimicking human skin, as their performance has only been explored up to 20% strain under tensile conditions.[37] Conversely, the observed reduction in ductility does not constrain its application in nerve tissue engineering, as the maximum strain of peripheral nerve fascicles is less than 1%.[38] Surface functional groups on MXenes, such as ─OH, ═O, and ─F, have been shown to significantly impact mechanical and electrical properties by modulating interfacial bonding, interlayer spacing, and charge transport.[39-42] While this study focused on optimizing MXene dispersion within PVA nanofibers, future work could systematically investigate the effects of termination engineering on MXene-polymer composites, particularly for applications requiring tailored properties.
Previous studies indicated significant differences in the cytotoxic properties of MXene-based materials depending on dosage, type of material composition, and cell line tested. The low dosages of MXenes are mostly defined as nontoxic. Nevertheless, when applying high concentrations, this may develop toxic effects as a result of generating reactive oxygen species (ROS) and interaction with cell membranes.[43] Besides, MXene-polymer composites, such as MXene-PVA hydrogels, showed excellent anti-microbial and photothermal properties, especially for wound dressing and localized therapeutic applications.[44] To evaluate in vitro biocompatibility of PVA/MXene fibrous membranes, L929 fibroblasts were used (Figure 6). The viability of cells seeded onto PVA and PVA/MXene fibers exhibited a consistent increase throughout the assay period, suggesting favorable biocompatibility. This effect can be attributed to the high hydrophilicity of PVA[45, 46] as previously reported in similar studies addressing various biomedical applications such as wound dressing, tissue regeneration, and drug delivery.[47-49] Notably, a significant rise in metabolic activity was observed for cells in contact with PVA/MXene fibers as early as 3 days, and this trend continued up to 7 days. This is consistent with previous experimental studies from Asaro et al.[50] In contrast, a significant increase in viability was detected only after 7 days for PVA fibers, indicating that MXene integration enhances early cell proliferation. Such enhanced biocompatibility can be attributed to the surface functional groups of MXene, including hydroxyl, oxygen, and fluorine, which are known to improve hydrophilicity and cellular interactions.[51, 52] The electrical conductivity of Ti3C2Tx can mimic the natural electrical gradients found in electroactive tissues, such as muscle and nerve, promoting cell alignment and differentiation along these gradients. Instead, the unique surface functional groups present on Ti3C2Tx facilitate protein adsorption, a vital step in cell-material interaction, as adsorbed proteins provide adhesion sites for cells via integrin-mediated attachment. Such interactions influence cell morphology, migration, and signaling pathways. These preliminary findings will be foundational for future biological studies aimed at in vitro validation of the electrical properties of PVA/MXene fibers and their effects on cell proliferation and differentiation.[53] This research is particularly relevant for mimicking the unique microenvironment of electroactive tissues such as muscle and neural tissue.
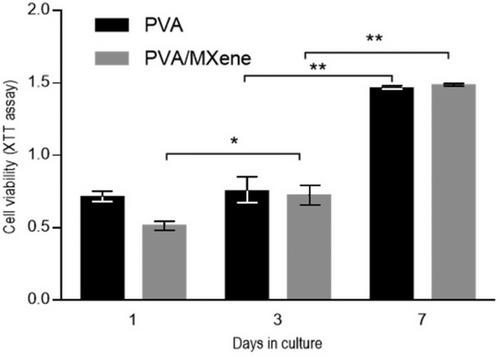
Studies show that these MXene-PVA composites exhibit similar electrical properties as graphene-PVA and CNT-PVA systems, but significantly increase biocompatibility, as illustrated by fibroblast viability in this study. The unique 2D structure and high surface area of MXenes enable the development of nanofibers with a high surface-to-volume ratio and uniformly distributed pores. The resulting increase in cell-material interaction implies that MXene-PVA composites are promising candidates for biomedical applications. In comparison, while graphene-PVA and CNT-PVA systems offer notable mechanical and electrical performance, their hydrophobicity and potential cytotoxicity, respectively, may limit their broader applicability in biological environments. Compared to conducting polymers such as PEDOT: PSS, the combination of electrical, mechanical, and biocompatible properties of PVA/MXene nanofibers positions them as promising candidates for electrostimulation devices. In addition, their nanoporous structure promotes cell adhesion and growth, making them suitable for facilitating tissue regeneration.
MXene-PVA nanofibers exhibit unique properties, making them very promising for biomedical applications especially where electrically conductive is crucial. For example, in wound healing, their conductive and hydrophilic nature could support electrostimulation therapy, which could further improve tissue regeneration by facilitating cell migration, angiogenesis, and collagen deposition. In tissue engineering, the nanofibers’ porous structure and high surface area provide a conductive microenvironment for cell attachment and differentiation, particularly for neural or muscle tissues requiring electrical cues. Furthermore, their excellent conductivity and mechanical stability position them as strong candidates for wearable sensors, enabling real-time monitoring of physiological parameters such as heart rate or muscle activity.
The long-term stability and biodegradability of MXene-PVA composites are key considerations for their applications in biomedical environments. PVA, being a synthetic polymer, is known for its biocompatibility and water solubility, but its degradation rate depends on factors such as molecular weight and crosslinking density. Meanwhile, MXenes, with their 2D structure and surface terminations (─OH, ═O, ─F), may experience chemical modifications or degradation under oxidative or hydrolytic physiological conditions. Studies have reported that MXenes can degrade through oxidative pathways, leading to structural changes that might affect their mechanical and electrical properties.[54] While PVA and MXene exhibit properties that make them attractive for green and biomedical applications, it is crucial to assess energy requirements, waste generation, and the overall environmental footprint when integrating these materials, considering their entire lifecycle from synthesis to disposal.[55]
4 Conclusion
This study underscores the transformative potential of MXene-incorporated PVA nanofibers as innovative materials for bioelectronic and biosensing applications. By incorporating MXenes, we achieved a fourfold increase in electrical conductivity and nearly a twofold improvement in mechanical stiffness, while maintaining excellent biocompatibility. Preliminary in vitro tests demonstrated that MXene-containing nanofibers not only support fibroblast viability but also enhance cell growth. The sub-micrometric fiber architecture, characterized by uniform pore distribution and a high surface-to-volume ratio, further supports the biological activity, distinguishing these materials from other forms, such as film and hydrogels. Despite these promising findings, the scalability of electrospinning for industrial applications remains a key challenge. Conventional methods often suffer from low throughput; however, emerging high-throughput techniques, such as multi-needle and free-surface electrospinning, provide viable pathways to upscale production. Further research should focus on optimizing these techniques to enable the commercial fabrication of MXene-loaded nanofibers. Additionally, further exploration of their electrical properties under physiological conditions and their functionalization with biorecognition elements (e.g., enzymes and antibodies) will advance their integration into biosensing platforms. The demonstrated combination of electrical, mechanical, and biocompatible properties sets the foundation for the development of electro-actuated patches, suitable for high-performance biomedical and wearable applications, including medical and military-grade devices.
Acknowledgements
Open access publishing facilitated by Universita degli Studi di Napoli Federico II, as part of the Wiley - CRUI-CARE agreement.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
N.Z.R., Z.U.D.B., V.I., and V.G. contributed equally to this work. The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.