Improvement of Poly(lactide) Ductile Properties by Plasticization with Biobased Tartaric Acid Ester
Abstract
Diethyl l-tartrate (DET) is used as a biobased plasticizer for poly(lactide) (PLA) formulations with improved ductile properties without compromising biodegradation. Different weight percentages (wt.%) of DET in the 0–50 wt.% range are added to PLA by melt compounding and subsequently processed by injection molding. The effect of wt.% DET on mechanical, thermal, thermo-mechanical, morphology, biodegradation, and crystallinity is studied. Addition of 20 wt.% DET leads to a noticeable increase in elongation at break up to values of 567%, which is quite an interesting result considering the extreme brittleness of PLA. These results are verified by field emission scanning electron microscopy (FESEM) images, where filament-like structures are observed, indicative of an effective plasticization. Differential scanning calorimetry (DSC) and dynamic mechanical thermal analysis (DMTA) show that the glass transition temperature of PLA is drastically decreased down to values of 23 °C for the sample with the highest amount of DET (50 wt.%), thus increasing its ductility and processability. Fourier-transformed infrared spectroscopy (FTIR) spectra show that there exists chemical interactions between PLA and DET. Finally, the biodegradability analysis proves that the developed blends are fully biodegradable, achieving complete disintegration after 49 days. It is observed that DET enhanced the disintegration rate of PLA.
1 Introduction
Poly(lactide) (PLA) is an aliphatic polyester that can be obtained from fermentation of starch-rich materials.[1] It has been raised as one of the most promising biopolymers since it combines balanced properties and processability, it is mostly obtained from natural resources, it can undergo biodegradation and, in addition, it is biocompatible and bioabsorbable. All these features, together with the potential of additives to tune its behavior, have contributed to a widespread use of PLA in a variety of applications such as the packaging industry,[2] textile, electronics,[3, 4] automotive industry,[5] and medical applications,[6] among others. It is also a standard material in 3D printing[7] and, due to its shape-memory behavior it has been proposed for several biomedical applications.[8]
Despite all these interesting features, PLA shows some drawbacks such as poor gas-barrier properties, relatively low thermal stability, and a high inherent brittleness. PLA is a low-toughness polymer with very low elongation at break (less than 10%).[9-11] Therefore, numerous research works have been focused on improving its flexibility.[12, 13] Plasticization is a quite common procedure to overcome its brittleness. A wide range of plasticizers have been proposed for PLA plasticization, including adipates,[14, 15] sebacates,[16] oligomers of lactic acid (OLA),[17] epoxidized vegetable oils,[18, 19] glycerol triesters,[20] citrates,[21] among others. Nonetheless, there exist some other methods to improve the ductility of PLA, such as the work of Kuang et al.[22] who modified the crystalline structure of PLA through a pressure-driven flow treatment without additives, modifying its structure; other method includes the nanofibrillation of PLA using polytetrafluoroethylene as a nucleating agent by means of a loop oscillatory push-pull molding method.[23]
In general, the effect of a plasticizer on PLA is an increase in the elongation at break. This is a consequence of a dramatic decrease in the glass transition temperature (Tg), even below room temperature as reported by Maiza et al.[21] for plasticized PLA formulations with 10–20 wt.% triethyl citrate (TEC), and acetyl tributyl citrate (ATBC). In general, a plasticizer content between 10–20 wt.% is needed to promote a significant decrease in Tg while over 20–30 wt.% plasticizer, phase separation usually occurs.[21, 24] This phase separation phenomenon has also been observed in plasticized PLA formulations containing polymeric plasticizers such as polyethylene glycol (PEG).[25]
With the aim of developing environmentally friendly PLA formulations, new plasticizers are continuously being developed. Tartrates or diesters of tartaric acid, have been proposed as “double green” plasticizers in PLA formulations since they can be bioderived and can undergo biodegradation. Zawada et al.[26] have reported the synthesis of different tartrate esters, and methylated tartrate esters by reacting tartaric acid with different chain length alcohols such as methanol, ethanol, n-butanol, 2-ethylhexanol, octanol, among others. They evaluated the efficiency of the synthesized tartrates as plasticizers for PLA and concluded that diethyl-L-tartrate (DET) and dibutyl-L-tartrate (DBT) gave the best plasticization properties to PLA according to the highest decrease in Tg provided by both tartrates. They also reported an exceptional increase in elongation at break from <10% up to values of around 500% with a DET content of 30 wt.%. They used a mixing time of 30 min prior to mechanical characterization. As they indicate, the actual residence time of PLA formulations under real manufacturing conditions is remarkably shorter. Despite tartrates have shown their plasticization efficiency, the processing conditions play a key role, especially when industrial conditions are used instead of laboratory conditions. The main aim of this work is to assess the effect of the real processing conditions by extrusion/injection molding on mechanical, thermal, thermomechanical and biodegradation properties of plasticized PLA formulations with varying the content of diethyl-L-tartrate (DET) in the 0–50 wt.% range.
2 Experimental Section
2.1 Materials
Bio-based PLA Purapol L130 grade was supplied by Corbion purac (The Netherlands, Amsterdam), with a melt flow index (MFI) of 16 g/10 min (at 210 °C/2.16 kg), a density of 1.24 g cm−3 and a melt peak temperature of 175 °C. Diethyl L-tartrate was purchased from Sigma Aldrich (Product Code: W237809) with a molar mass of 206.19 g mol−1.
2.2 Preparation of PLA/DET Formulations
PLA was initially dried at 40 °C for 48 h in a dehumidifying dryer before processing in order to remove any residual moisture. Afterward, according to Table 1, the corresponding amount of plasticizer (wt.%. DET) was mixed with PLA and compounded in a twin-screw extruder from Construcciones Mecánicas Dupra, S.L. (Alicante, Spain). This extruder has a screw diameter of 25 mm and a length-to-diameter ratio (L/D) of 24. The extrusion process was carried out at a rate of 22 rpm, using the following temperature profile (from the hopper to the die): 170—175–180–185 °C. The compounded materials were pelletized using an air-knife unit. Residence time was ≈1 min. Table 1 shows the compositions of the formulations developed in this work.
| Code | PLA (wt.%) | DET(wt.%) |
|---|---|---|
| PLA | 100 | 0 |
| PLA90DET10 | 90 | 10 |
| PLA80DET20 | 80 | 20 |
| PLA70DET30 | 70 | 30 |
| PLA60DET40 | 60 | 40 |
| PLA50DET50 | 50 | 50 |
The compounded pellets were injection molded in an injection molding unit from Mateu & Solé (Barcelona, Spain) Meteor 270/75. The temperature profile used was 155 °C (hopper), 160 °C, 165 °C, and 170 °C (injection nozzle). A clamping force of 75 tons was applied while the cavity filling and cooling times were set to 1 and 10 s, respectively. Standard samples for mechanical and thermal characterization with an average thickness of 4 mm were obtained.
2.3 Characterization of PLA/DET Blends
2.3.1 Theoretical Approach to Solubility of PLA and DET
where V [cm3 mol−1] stands for the molar volume, Fdi [(MJ/m3)1/2 mol−1] corresponds to the group contributions of the molar attraction constant with regard to the dispersion component, Fpi [(MJ/m3)1/2 mol−1] stands for the characteristic molar attraction constants related to the polar component, while Ehi [J mol−1] values are representative for the hydrogen bonding energy which are almost constant per structural group.
| Material | δd (MPa1/2) | δp (MPa1/2) | δh (MPa1/2) | δ (MPa1/2) | Ra (MPa1/2) | RED |
|---|---|---|---|---|---|---|
| Poly(lactide) (PLA) | 15.33 | 8.44 | 10.98 | 20.66 | - | |
| Diethyl L-tartrate (DET) | 16.00 | 5.78 | 17.76 | 24.59 | 7.40 | 0.69 |
As it can be observed in Table 2, diethyl L-tartrate presents good miscibility with PLA, as its RED value is lower than 1 (0.69). Nonetheless, this result is just a theoretical approach, and it will be corroborated by experimental results.
2.3.2 Mechanical Characterization
The tensile behavior of PLA/DET blends were obtained in a universal testing machine ELIB 50 from S.A.E. Ibertest (Madrid, Spain) according to ISO 527-1:2012. Cross-head speed was set to 5 mm min−1 and a 5-kN load cell was used. Shore D hardness was measured in a 676-D durometer from J. Bot Instruments (Barcelona, Spain) on rectangular samples with dimensions 80 × 10 × 4 mm3, according to ISO 868:2003. All mechanical tests were performed at room temperature, and at least 6 samples of each material were tested and the corresponding mechanical parameters were averaged.
2.3.3 Morphological Characterization
The surface of fractured samples from Charpy test was studied to see the morphology of the plasticized blends. The morphology was studied by field emission scanning electron microscopy (FESEM) in a ZEISS ULTRA 55 microscope from Oxford Instruments (Abingdon, United Kingdom). The samples were sputtered with a gold-palladium alloy in an EMITECH sputter coating SC7620 model from Quorum Technologies, Ltd. (East Sussex, UK). The microscope was worked at an acceleration voltage of 2 kV.
2.3.4 Thermal Analysis
Thermal decomposition of the PLA/DET blends was assessed by thermogravimetric analysis (TGA) in a LINSEIS TGA 1000 (Selb, Germany) thermobalance. Samples with a weight of 15–25 mg were placed into 70 µL alumina crucibles and subjected to a dynamic heating program from 35 °C to 700 °C at a heating rate of 10°C/min in nitrogen atmosphere.
2.3.5 Dynamical-Mechanical Thermal Characterization
Dynamical mechanical thermal analysis (DMTA) was carried out in a DMA1 dynamic analyzer from Mettler-Toledo (Schwerzenbach, Switzerland), working in single cantilever flexural conditions. The selected frequency was 1 Hz and the maximum flexural deformation or cantilever deflection was set to 10 µm. Rectangular samples with dimensions 20 × 6 × 2.7 mm3 were subjected to a dynamic temperature sweep from −150 °C to 100 °C at a constant heating rate of 2 °C min−1.
2.3.6 Fourier Transform Infrared Spectroscopy
The interactions of PLA and DET in the PLA/DET blends was analyzed by means of attenuated total reflection-Fourier transform infrared spectroscopy (ATR-FTIR) spectroscopy. Spectra were collected using a Bruker S.A Vector 22 (Madrid, Spain) coupled to a PIKE MIRacle single reflection diamond ATR accessory (Madison, Wisconsin, USA). Data were collected as the average of 20 scans between 4000 and 500 cm−1 with a spectral resolution of 2 cm−1.
2.3.7 Biodegradability
The biodegradability of the plasticized PLA/DET blends under controlled compost soil was assessed through the disintegration test following ISO 20 200. Samples sizing 2.5 × 2.5 cm2 were dried for 24 h at 40 °C. They were then weighted and buried in a bioreactor of dimensions 30 × 20 × 10 cm3 in a solid synthetic wet soil prepared with 40 wt.% sawdust, 10 wt.% corn starch, 30 wt.% rabbit-feed, 10 wt.% compost, 5 wt.% sugar, 4 wt.% of corn oil, and 1 wt.% of urea. This mixture was combined with distilled water in a 45:55 ratio (wt.%/wt.%). In these conditions, the samples were aerobically degraded at a constant temperature of 58 °C in an air circulating oven during 4 weeks. Measurements were taken by extracting samples from the reactor, washing them with distilled water, drying them for 24 h at 40 °C and finally weighting them. Several measurements were taken during the 4 weeks to observe the biodegradation profile of the samples over time.
3 Results and Discussion
3.1 Mechanical Characterization
Table 3 gathers the results concerning the mechanical performance of plasticized PLA/DET blends in terms of elastic modulus, tensile strength, elongation at break and Shore D hardness; while Figure 1 shows the stress – strain curves of all the samples. Neat PLA presents the typical behavior of a hard, brittle polymer with low toughness.[29] This is indicated by its low elongation at break (11.26%) and by its high tensile strength (68.34 MPa). When 10 wt.% DET is added into the polymer matrix, the elastic modulus decreases from 926 down to 837 MPa. The tensile strength decreases from 68.3 down to 53.3 MPa, and elongation at break also decreases down to 8.2%. This implies that 10 wt.% of plasticizer is not sufficient to effectively plasticize PLA, with a general worsening of the mechanical performance. This effect is known as anti-plasticization and it occurs when a little amount of plasticizer is added to some polymers.[30] Nonetheless, when the amount of plasticizer is increased to 20 wt.% the ductility of the blend impressively increases, presenting an elongation at break of 567.6%, maintaining a tensile strength of 26 MPa. This behavior is ascribed to the plasticizing effect of DET, which promotes a reduction in the attraction forces between PLA polymer chains, so their movement requires less energy, making it possible to obtain more elongation capacity.[31]Once 20 wt.% of DET is surpassed, the plasticizing effect is still surprising, with elongation at break values of 463% for 30 wt.% of DET or 399% for 50 wt.% of DET, although it is worse in comparison with 20 wt.% of DET. This could be related to PLA reaching saturation related to the plasticizer, a very similar phenomenon to the one observed by Ivorra–Martinez et al.[31] when plasticizing PLA with dibutyl itaconate (DBI). Therefore, 20 wt.% DET can be considered the optimal plasticizer concentration in order to achieve the best mechanical properties. As expected, tensile strength decreases as the amount of plasticizer increases, reaching a minimum value of 15.2 MPa for the sample with 50 wt.% DET. This is ascribed to the softening effect previously described, which gives more mobility to the polymer chains. Anyway, the tensile strength in plasticized formulations containing 20–30 wt.% DET, are close to that of polyethylene (around 20 MPa). Therefore, despite a decrease in tensile strength is observed, the obtained values allow using PLA in many applications that do not require high tensile strength such as the food packaging, thus broadening its potential applications.
| Material | Elastic modulus, E (MPa) | Tensile strength, σmax (MPa) | Elongation at break, εb (%) | Shore D hardness |
|---|---|---|---|---|
| PLA | 926 ± 15 | 68.3 ± 2.2 | 11.3 ± 0.8 | 82.4 ± 1.1 |
| PLA/10DET | 837 ± 39 | 53.3 ± 3.5 | 8.2 ± 0.5 | 82.0 ± 1.0 |
| PLA/20DET | 315 ± 29 | 26.0 ± 1.0 | 567.6 ± 8.3 | 78.2 ± 3.1 |
| PLA/30DET | 57 ± 8 | 21.7 ± 1.2 | 463.0 ± 41.5 | 57.2 ± 1.9 |
| PLA/40DET | 121 ± 11 | 18.6 ± 1.1 | 362.3 ± 22.8 | 62.2 ± 0.8 |
| PLA/50DET | 120 ± 1 | 15.2 ± 0.6 | 399.7 ± 39.9 | 57.6 ± 0.9 |
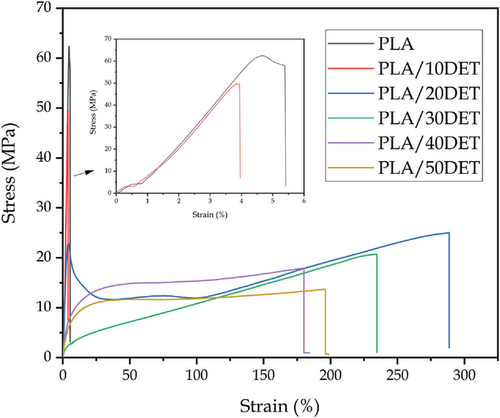
Regarding Shore D hardness, it follows a very similar trend to that of elastic modulus and tensile strength. Neat PLA presents a hardness of 82.4, and it can be seen how as the amount of plasticizer increases, this value is diminished. PLA/10DET presents a value of 82, while PLA/50DET presents a value of 57.6, which is a considerable decrease. This phenomenon is obviously related to the plasticizing effect exerted by DET, which makes the polymer chains more easily deformable as a result of an increase in their mobility. The results presented here seem to corroborate the good miscibility between PLA and DET calculated in the theoretical solubility section.
3.2 Morphological Characterization
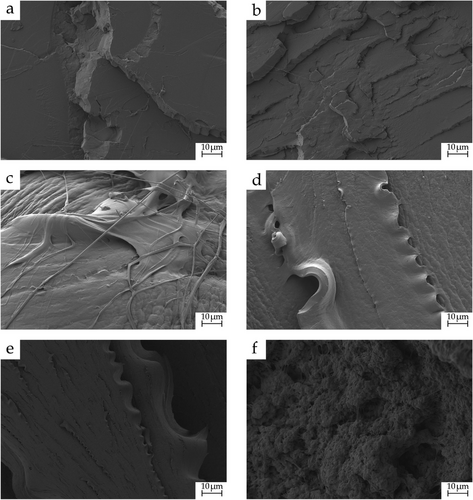
Figure 2 shows the FESEM morphology of PLA/DET blends at 500×. Figure 2a illustrates the morphology of neat PLA. The observed flat surface shows the typical brittle behavior of this polymer, which is indicative of a low ductility, and a fracture process with very low plastic deformation. Fracture in neat PLA occurs without plastic deformation which could be identified as wavy surface. As only a flat surface with different microcrack can be observed, it can be assessed that fracture of PLA occurs with almost negligible plastic deformation which is responsible for the low toughness.[31] Figure 2b (10 wt.% DET) also shows a surface with very little roughness, which is also indicative of a hard and brittle behavior, as it was also observed in the mechanical properties section. Interestingly, the rest of the samples (from 20 wt.% to 50 wt.% of DET) show clear signs of plasticization. This is noted by a rough surface with filament-like formations and a cavernous morphology, especially in Figure 2c. This perfectly matches the mechanical results reported before, as the sample with 20 wt.% DET was the optimal one in terms of plasticization, with an elongation at break of more than 500%. Nonetheless, the rest of the samples also showed great elongation at break, which is also reflected in the morphologies herein presented. In spite of the fact that PLA reached its saturation point related to diethyl L-tartrate, no phase separation was observed in the FESEM images, which is in accordance with the calculated solubility parameters that showed good miscibility between PLA and DET. A very similar result was observed by Ivorra-Martinez et al.[31] in plasticized PLA blends with dibutyl itaconate (DBI).
3.3 Thermal Characterization
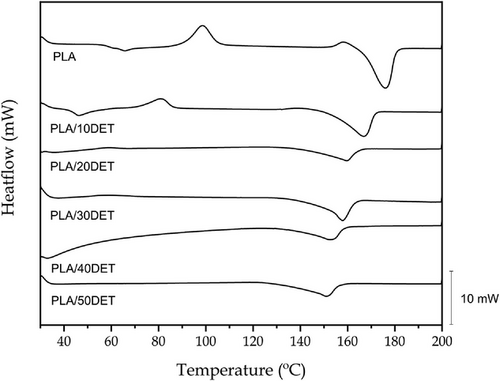
Figure 3 and Figure 4 show the DSC thermograms for the first heating cycle and the cooling cycle of the PLA/DET samples, respectively, while Table 4 gathers the most important thermal parameters regarding those thermograms. It can be seen that the glass transition temperature of PLA in the first heating cycle is 64.2 °C, which is a typical value for this rigid polymer.[32] When 10 wt.% DET is added to PLA, the glass transition is reduced down to 47.9 °C, which is ascribed to an enhanced chain mobility exerted by the plasticizer.[32] The glass transition cannot be observed in the rest of the samples because the test was done from 30 °C to 200 °C, and higher proportion of plasticizer lead to glass transition temperatures very close to 40 °C, which disables its proper analysis in these thermograms. The cold crystallization temperature (Tcc) of PLA is ≈98 °C, which is decreased down to values of 81 °C when 10 wt.% DET is added to it. This peak appears due to a rapid cooling after the injection process, which disables the crystalline phase of PLA to properly crystallize. The rest of the samples do not present this peak because they were analyzed from pelletized blends after the extrusion process, which implies a slow cooling, that allows the crystalline phase to form adequately.[33] This thermal transition will be better discussed in the second heating cycle, as the first cycle is made with the objective of removing all the thermal memory of the material. On the other hand, the melting temperature of PLA is located at 175 °C, and it can be observed how the plasticizer progressively decreases it down to a value of 150 °C for the sample with 50 wt.% of DET. This phenomenon was also observed by Ivorra-Martinez et al.[31] in PLA plasticized with dibutyl itaconate. The crystallinity degree is also increased from a value of 33% for neat PLA to values of 57% and 67% for the samples with 40 and 50 wt.% of DET, respectively. This fact is ascribed to a promotion of crystallization induced by the presence of the plasticizer, altogether to the thermal cycle, as the samples herein presented are not analyzed under the same conditions, while the first two samples have suffered a rapid cooling, the other four have undergone a slow cooling rate. Finally, the crystallization temperature (Tc) during cooling is a thermal transition that occurs very slowly, as it is demonstrated by the very wide temperature range of the peak of this transition. This allows to study all the samples in the same conditions in the second heating cycle, as all of the materials pass through the same cooling rate. The crystallization temperature of PLA is ≈62 °C, which is quite lower than the melting temperature. Nonetheless this is ascribed to the phenomenon previously mentioned, which makes this transition to occur between 100 and 50 °C, approximately. A similar phenomena happens in the plasticized samples.
| Code | Tg (°C) | Tcc (°C) | ΔHcc (J g−1) | Tm (°C) | ΔHm (J g−1) | χc (%) | Τc (°C) |
|---|---|---|---|---|---|---|---|
| PLA | 64.2 ± 0.8 | 98.7 ± 3.1 | 25.5 ± 0.3 | 175.5 ± 2.1 | 56.7 ± 0.3 | 33.5 ± 0.2 | 62.1 ± 0.9* |
| PLA/10DET | 47.9 ± 1.1 | 81.0 ± 3.2 | 11.1 ± 0.2 | 166.5 ± 3.4 | 44.9 ± 1.6 | 40.4 ± 1.1 | 51.2 ± 2.3* |
| PLA/20DET | - | - | - | 159.5 ± 1.6 | 21.4 ± 0.9 | 23.0 ± 1.6 | 81.9 ± 2.1* |
| PLA/30DET | - | - | - | 157.5 ± 2.3 | 35.1 ± 0.7 | 37.7 ± 1.5 | 80.1 ± 1.9* |
| PLA/40DET | - | - | - | 152.7 ± 3.2 | 32.0 ± 1.2 | 57.3 ± 2.7 | 67.9 ± 4.7* |
| PLA/50DET | - | - | - | 150.8 ± 2.9 | 31.5 ± 0.2 | 67.7 ± 3.4 | 61.1 ± 2.5* |
- * The crystallization temperature occurs in quite a wide range, the value shown in the table is the peak value of the crystallization band.
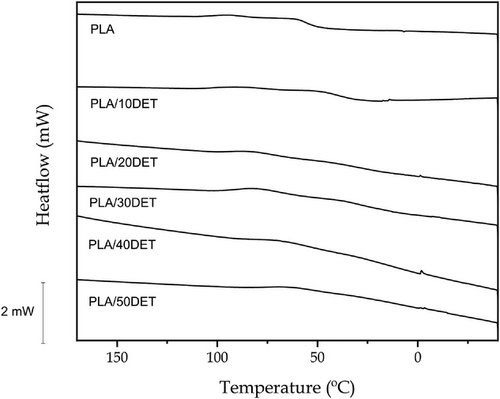
Figure 5 shows the DSC thermograms of the second heating cycle of PLA/DET blends, while Table 5 gathers the main thermal parameters related to the aforementioned thermograms. Neat PLA presents a glass transition temperature of 61.4 °C, which is a typical value for this polymer,[34] being indicative of quite a brittle material at room temperature. As it was expected, the incorporation of the plasticizer drastically decreased the glass transition temperature. The higher the DET proportion in the blend, the lower the glass transition temperature. This is ascribed to the plasticization effect of DET, which increases the mobility of the PLA polymeric chains.[31] Considering that the glass transition is related to the mobility of the amorphous phase of the polymer, this result was predictable, achieving a value of 23 °C for the sample with 50 wt.% of DET. The PLA used in this study is a semicrystalline polymer, as it can be deduced from the cold crystallization temperature peak, which appears at 103.6 °C. The incorporation of DET also favors crystallization, as it can be observed by the cold crystallization peaks in the PLA/DET samples. Addition of DET moves the cold crystallization peak down to values around 85 °C. This fact is also related to the enhanced chain mobility exerted by DET, which favors the rearrangement, promoting crystal formation. Choin et al.[35] observed a similar effect in PLA plasticized with polyethylene glycol monoacrylate (PEGA), reporting a cold crystallization peak of 85.2 °C, which is indeed very similar to the one observed in this study. Regarding the melting temperature, a notable decrease in this thermal transition is also observed for the plasticized blends. Neat PLA presents a melting temperature of 173.7 °C, whereas the sample plasticized with 50 wt.% of DET exhibits the lowest value, which is 152 °C. Finally, it can be observed that the crystallinity proportion of PLA increases with the content of DET, going from a crystallinity degree of 45.4% for neat PLA to 60.6% for PLA/50DET. These results are in accordance with those observed by Ivorra-Martinez et al.[31] who reported an increase in the crystallinity of PLA after introducing dibutyl itaconate (DBI) plasticizer on its structure. This is ascribed to the fact that DET promotes the formation of crystal growth nuclei form where the crystallization process is catalyzed.
| Code | Tg (°C) | Tcc (°C) | ΔHcc (J g−1) | Tm (°C) | ΔHm (J g−1) | χc (%) |
|---|---|---|---|---|---|---|
| PLA | 61.4 ± 1.2 | 103.6 ± 3.5 | 29.2 ± 0.1 | 173.7 ± 3.5 | 42.6 ± 0.1 | 45.5 ± 0.5 |
| PLA/10DET | 42.6 ± 1.5 | 88.5 ± 4.1 | 22.9 ± 0.4 | 167.5 ± 4.1 | 36.8 ± 1.2 | 43.6 ± 0.2 |
| PLA/20DET | 39.4 ± 2.1 | 87.0 ± 3.6 | 17.6 ± 1.1 | 166.0 ± 3.6 | 34.3 ± 1.7 | 45.9 ± 0.1 |
| PLA/30DET | 37.1 ± 1.7 | 83.6 ± 2.7 | 13.4 ± 0.5 | 161.2 ± 2.7 | 33.3 ± 1.2 | 50.9 ± 0.3 |
| PLA/40DET | 38.2 ± 1.7 | 85.2 ± 1.7 | 13.0 ± 0.5 | 163.1 ± 1.7 | 29.7 ± 0.5 | 52.9 ± 0.5 |
| PLA/50DET | 23.0 ± 1.7 | 85.3 ± 1.7 | 12.0 ± 0.5 | 152.0 ± 1.7 | 28.4 ± 0.5 | 60.6 ± 0.5 |
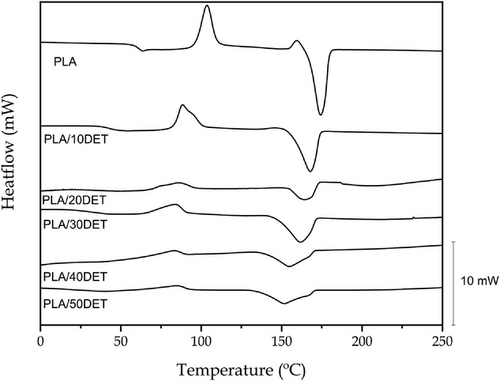
The thermal degradation of PLA/DET blends was also analyzed by means of thermogravimetric analysis (TGA). Figure 6 shows the evolution of the mass of all samples with the temperature and the first derivative (DTG), while Table 6 gathers the main thermal parameters that can be extracted from both thermograms. First, neat PLA shows the typical single-stage degradation profile of this polymer, as it is also observed in other studies.[36]
| Code | T5% (%) | Tdeg (°C) | Residual mass (%) |
|---|---|---|---|
| PLA | 287.6 ± 2.5 | 324.6 ± 3.1 | 0.2 ± 0.1 |
| PLA/10DET | 229.1 ± 1.8 | 349.1 ± 1.3 | 0.1 ± 0.1 |
| PLA/20DET | 184.6 ± 2.1 | 349.1 ± 2.9 | 0.2 ± 0.1 |
| PLA/30DET | 177.6 ± 1.9 | 345.6 ± 1.2 | 0.1 ± 0.1 |
| PLA/40DET | 178.3 ± 1.9 | 331.8 ± 1.2 | 0.1 ± 0.1 |
| PLA/50DET | 166.1 ± 1.9 | 335.6 ± 1.2 | 0.2 ± 0.1 |
The onset degradation temperature (temperature at which the polymer has lost its 5% of mass) of PLA stands at 287.6 °C and its maximum degradation rate temperature is 324.6 °C. The incorporation of the plasticizer into the polymer matrix clearly decreases the thermal stability of the blend in the temperature range between 200 °C and 300 °C. This fact is clearly demonstrated by the low onset degradation temperatures for the PLA/DET samples. As expected, the higher the DET proportion in the blend, the lower the onset degradation temperature and the faster the blend degrades, reaching values of 166.1 °C for the PLA/50DET blend. Diethyl L-tartrate has a boiling point of 280 °C, but its low molecular weight allows plasticizer removal at lower temperatures than PLA. This high boiling point of DET prevents from plasticizer removal during processing. On the other hand, the maximum degradation rate temperature for the plasticized samples does not vary a lot from neat PLA, showing values between 335 and 350 °C. This is probably due to the fact that the plasticizer has already been volatilized in this temperature range due to its low molecular weight. Finally, the residual mass in all the samples is practically zero, with no ash formation.
3.4 Dynamic-Mechanical Thermal Analysis
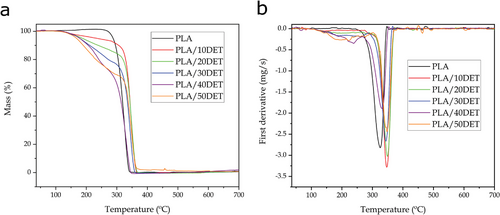
Figure 7 shows the storage modulus (E’) and the dynamic damping factor (tan δ) variation with temperature for the PLA/DET blends, while Table 7 gathers the main thermal parameters related to this characterization.
| Code | E’ at −50 °C (MPa) | E’ at 75 °C (MPa) | Tg (°C) |
|---|---|---|---|
| PLA | 1942.0 ± 33.8 | 2.6 ± 0.9 | 67.0 ± 0.2 |
| PLA/10DET | 1842.4 ± 29.8 | 20.7 ± 1.2 | 48.1 ± 0.1 |
| PLA/20DET | 1848.1 ± 27.0 | 55.4 ± 3.8 | 36.3 ± 0.9 |
| PLA/30DET | 1897.5 ± 30.7 | 82.1 ± 2.4 | 24.6 ± 0.7 |
| PLA/40DET | 1439.6 ± 28.0 | 56.0 ± 1.2 | 24.1 ± 0.1 |
| PLA/50DET | 2170.0 ± 36.1 | 54.3 ± 1.2 | 26.0 ± 0.2 |
Neat PLA shows its typical storage modulus decrease, which comes as a result of an α-relaxation process, attributed to the glass transition temperature. The glass transition is located in the sudden decrease of the storage modulus from values around 1940 MPa at −50 °C, to values around 3 MPa at 75 °C. The dynamic damping factor better indicates the position of the glass transition temperature, indicated by a peak maximum. In this case, the glass transition temperature of neat PLA is ≈67 °C, which is a similar value to the one observed in DSC and very similar to the one observed by Ivorra–Martinez et al.[31] The incorporation of DET drastically decreases the glass transition temperature down to values of 24 °C and 26 °C for the samples with the highest DET content. The plot of the storage modulus also shows a drastic decrease in E’, which occurs long before the sudden decrease in neat PLA. This is another indicative of a highly lower glass transition temperature. This fact is related to increased mobility of the polymeric chains as a result of the plasticizing effect. Interestingly, the higher the composition of the plasticizer, the wider the tan δ peak. This effect is especially noticeable in samples with 30 wt.% DET and above. This phenomenon implies that the polymer can show ductile properties even at temperatures below the glass transition, due to this transition occurring at a broader temperature range. This interesting phenomenon was also observed by Quiles–Carrillo et al.[37] in PLA formulations plasticized with acrylated epoxidized soybean oil (AESO).
3.5 Chemical Interactions in Poly(lactide) (PLA)-Diethyl l-tartrate (DET) Blends
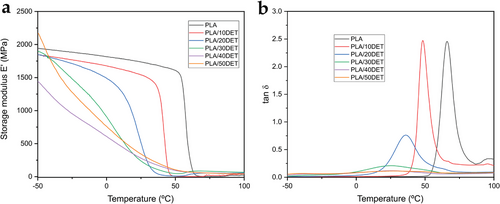
Figure 8 shows the FTIR spectra of PLA and all the PLA/DET blends. Neat PLA presents the typical spectra of this polymer. The main absorption bands of PLA are observed at 1750 and 1180 cm−1, which are ascribed to C≐O stretching bonds and the C—O–C stretching of PLA, respectively.[38] The band at 1452 cm−1 is related to C–H bonding in lactic acid fractions, while the low intensity peak located at 3000 cm−1 is due to the antisymmetric and symmetric stretching vibrations of CH2.[39] These peaks are present in all the samples, as PLA is the main component of all the blends. The incorporation of diethyl L-tartrate into the poly(lactide) polymeric matrix does not change the spectra of PLA in a great measure. Nonetheless, some slight changes can be appreciated. A low intensity band appears at 3450 cm−1, especially in the sample with 50 wt.% of DET, which is ascribed to the presence of hydrogen bonding between PLA and DET.[40] Additionally, the peak at 1180 cm−1 seems to decrease in intensity when DET is added into the blend. Considering that this peak is ascribed to the ester group, it could be related to certain chemical interaction between PLA and DET that reduces the availability of ester groups in the blends, as a result of their good miscibility, as it was predicted in the theoretical solubility analysis.
3.6 Biodegradability of Poly(lactide) (PLA)-Diethyl l-tartrate (DET) Blends
Figure 9 shows the evolution of the weight loss of PLA/DET samples under controlled compost soil over time, while Figure 10 gathers the visual appearance of the disintegration process of the materials. 90% of disintegration is considered as the disintegration goal for considering the samples as biodegradable. It can clearly be seen that all samples are completely biodegradable, as they lose the 100% of their mass after 49 days.
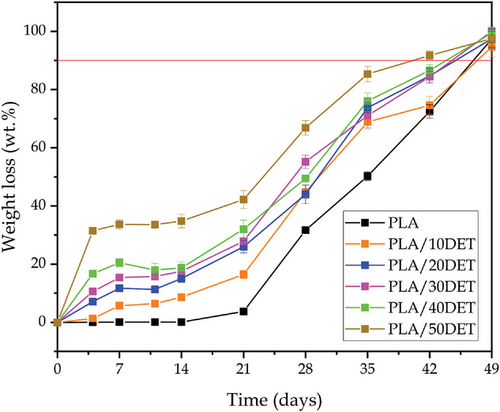
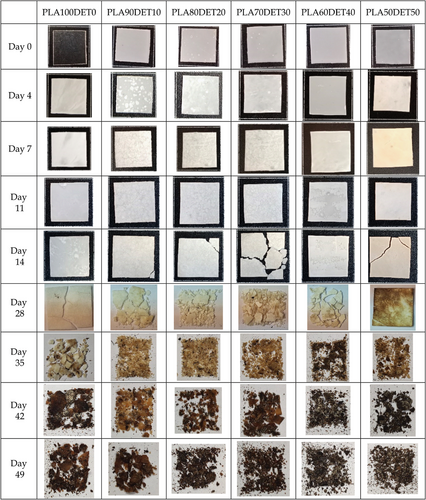
However, the disintegration rate changes with the plasticizer amount in the blend. The greater the amount of plasticizer in the blend, the faster the disintegration rate is. This fact is clearly reflected in Figure 9, where the sample with 50 wt.% of DET achieves 90% of disintegration between 35 and 42 days, while the rest of the samples achieve 90% of disintegration between 42 and 49 days. Nonetheless, plasticized samples disintegrate faster than neat PLA, as it is indicated by the graphs. This fact is ascribed to the hydrophilic nature of the plasticizer, which promotes hydrolysis in the polymer chains, resulting in the formation of small molecules that are easier for the microorganisms to attack, thus accelerating the disintegrating rate.[41] This can be corroborated in Figure 10, where it can be seen that at day 49 all samples are drastically disintegrated. Additionally, from day 14 to day 21 there is a drastic increase in weight loss, which is both seen in Figure 9, indicated by a sudden increase of weight loss in the graph, and in Figure 10, where day 28 is the first measurement in which the disintegration of the samples is clearly visible.
4 Conclusions
The work herein presented reports on the effective development of environmentally friendly plasticized poly(lactide) (PLA) blends with different contents of diethyl l-tartrate (DET). The developed blends have proved to possess an excellent ductility as a result of the plasticizing effect exerted by DET, reporting elongation at break values of nearly 600% for the sample with 20 wt.% of DET, which was the optimal PLA/DET composition. These results are quite impressive considering the extreme brittleness of PLA. FESEM images supported the mechanical findings, as typical plasticized morphologies were observed for all the plasticized samples. Furthermore, a theoretical solubility study demonstrated that PLA and DET show good miscibility and possess good compatibility. Regarding thermal properties, the glass transition temperature of PLA was drastically decreased from values of 63 °C down to values close to 40 °C, which implies a great enhancement in processability. Additionally, the melting temperature was also reduced down to 152 °C for the sample with 50 wt.% of DET as a result of its plasticizing effect, which increases the mobility of polymeric PLA chains. The thermogravimetric analysis of the samples illustrated the high volatility of DET, which made the PLA/DET blends less thermally stable than neat PLA, as the plasticizer starts to volatilize in the above 200–220 °C. FTIR spectra suggested that there were certain chemical interactions between PLA and DET, as the characteristic band for the ester group decreased in intensity. Finally, the herein presented blends proved to be completely biodegradable, as it was demonstrated by disintegration test under controlled compost soil conditions. All blends achieved a 90% of disintegration, which is the disintegration goal, at 49 days of incubation time. Moreover, it was observed that DET accelerated the disintegration rate of PLA. This makes for an enhancement in the environmentally friendly potential of these composites.
Acknowledgements
This research was a part of the grant PID2020-116496RB-C22 funded by MCIN/AEI/10.13039/501100011033. The authors also thank Generalitat Valenciana-GVA, grant number AICO/2021/025 and CIGE/2021/094 for supporting this work. J.G.-C. wants to thank Generalitat Valenciana-GVA, for his FPI grant (ACIF/2021/185) and grant FPU20/01732 funded by MCIN/AEI/10.13039/501100011033 and by ESF Investing in your future. I.D.-C. wants to thank Universitat Politècnica de València for his FPI grant (PAID-2019-SP20190013) and Generalitat Valenciana-GVA (ACIF/2020/233). J.I.-M. wants to thank FPU19/01759 grant funded by MCIN/AEI/ 10.13039/501100011033 and by ESF Investing in your future. V.M. wants to thank Generalitat Valenciana – GVA for funding a postdoc position through the APOSTD program co-funded by ESF Investing in your future, grant number CIAPOS/2021/67. Microscopy Services at UPV are also acknowledged by their help in collecting and analyzing images. Funding for open access charge: Universitat Politècnica de València.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




