Chitosan-Based Hydrogels for Infected Wound Treatment
Abstract
Wound infections slow down the healing process and lead to complications such as septicemia, osteomyelitis, and even death. Although traditional methods relying on antibiotics are effective in controlling infection, they have led to the emergence of antibiotic-resistant bacteria. Hydrogels with antimicrobial function become a viable option for reducing bacterial colonization and infection while also accelerating healing processes. Chitosan is extensively developed as antibacterial wound dressings due to its unique biochemical properties and inherent antibacterial activity. In this review, the recent research progress of chitosan-based hydrogels for infected wound treatment, including the fabrication methods, antibacterial mechanisms, antibacterial performance, wound healing efficacy, etc., is summarized. A concise assessment of current limitations and future trends is presented.
1 Introduction
The breakdown of the skin integrity results in wound formation, while the human body initiates a series of complicated and intersecting dynamic processes to repair and regenerate damaged or lost tissue, known as wound healing.[1, 2] There are certain circumstances in which the wounds do not follow the healing process and may take longer to heal, such as the size and depth of wounds, infection, poor health, and old age. Among the various reasons that wounds fail to heal properly, infection is the most common and potentially preventable obstacle to healing. Besides impeding the healing process, wound infection can also lead to severe complications, such as septicemia, osteomyelitis, and even death, and is thus the primary cause of the failure of acute wound treatment and the development of nonhealing wounds.[3-8] Although traditional infection control methods relying on antibiotics are effective, they have led to drug-resistant bacteria emergence. Therefore, it is urgent to explore a strategy to prevent bacterial infection as well as to promote wound healing.
Wound dressings have been used to cover wound sites and protect wounds from external infections. Moreover, wound dressings can act as a template to guide skin cell recombination and then infiltrate and integrate host tissues, leading to a significant improvement in wound healing.[9, 10] There are three main types of commercially available antibacterial wound dressings: hydrogels, films or membranes, and foams.[11] Among these antibacterial wound dressings, hydrogels have emerged as the most competitive candidate for wound treatment, attracting widespread interest from researchers because of the following advantages: 1) possess excellent functions due to the inherent properties of chitosan; 2) can preserve a moist wound environment; 3) can absorb wound exudate, promote fibroblast proliferation and keratinocyte migration; 4) can protect the wound from infection by preventing microorganisms from reaching the wound sites due to the compact size of the hydrogel network structure; and 5) can provide the necessary elasticity and flexibility because of the high water content.[12]
To date, many synthetic polymers (e.g., polyethylene glycol [PEG], polyvinyl acetate [PVA], polyacrylamide [PAM]) and natural polymers (e.g., chitosan, hyaluronic acid) have been used to form hydrogel wound dressings.[13] Among these materials, chitosan has drawn significant attention due to its easy availability from natural sources, low-cost extraction, biocompatibility, and biodegradability.[14] Chitosan, especially, possesses inherent antibacterial activity and the property to promote wound healing. There have been numerous commercially available chitosan-based wound dressings, such as Chitoseal Abbott, Tegasorb 3M, Chitoflex HemCon, etc.[12, 14]
This review emphasizes chitosan-based hydrogels and their applications in the treatment of wound infection. First, chitosan and its wound-healing mechanisms are described. The second section discusses different kinds of chitosan-based hydrogel preparation methods. Finally, a concise assessment of current limitations and future trends is presented. The abbreviations are summarized and listed in Table S1 (Supporting Information).
2 Antimicrobial and Wound Healing Mechanisms of Chitosan
2.1 Structure Basis
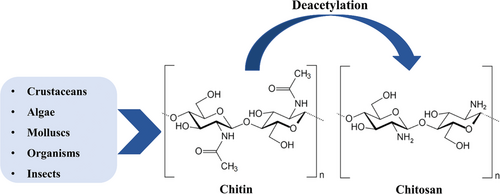
Chitosan is a linear naturally derived polysaccharide consisting of 2-amino 2-deoxy-d-glucopyranose and N-acetyl-2-amino-2-deoxy-d-glucopyranose. β-(1-4)-Glycosidic bonds link the repeating units.[15-17] Chitosan is synthesized by deacetylation of chitin, as shown in Figure 1.[18] The structure of chitosan is similar to chitin, except at the C2 position, which is an amine group in chitosan and an N-acetyl amine in chitin. Since the pKa is around 6.5, a large amount of protonated amine groups account for the solubility of chitosan in an acid solution.[19, 20]
2.2 Antimicrobial Activity
The emergence of drug-resistant microorganisms promotes the development of antibiotic alternatives.[18] Among the numerous alternatives, chitosan shows great potential in antimicrobial application due to its inherent antimicrobial activity. There are several accepted mechanisms, as shown in Figure 2, including the formation of dense films on the surface of cells, disruption of the cell wall or membrane, interaction with microbial Deoxyribonucleic acid (DNA), and chelation of nutrients.[21]
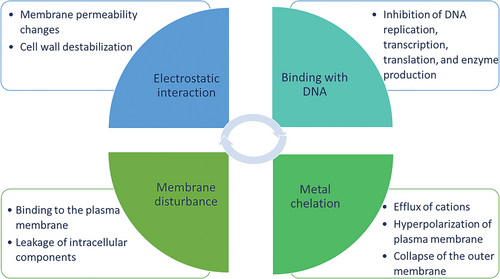
The cell wall of Gram-positive (G+) bacteria contains teichoic acid, which gives the bacteria negative charges on the surface, whereas the cell wall of Gram-negative (G-) bacteria is negative owing to the lipopolysaccharide in the outer membrane.[22] Under acidic conditions, the amine groups in chitosan are protonated and can form ammonium groups with positive charges. The electrostatic interaction of chitosan and negative charges on the surface of microorganisms alters the cell permeability and causes cell membrane lysis. A similar situation occurs with fungal and viral envelopes because of the negatively charged compounds.[21, 23, 24] Chelating properties towards metal ions and nutrients also contribute to the inhibitory activity of chitosan against microorganisms. Moreover, chitosan can deposit and form a film on the microorganism cell surface, thus inhibiting cell growth.[21]
2.3 Effects on Wound Healing
Chitosan has been proven to accelerate wound healing by improving the functions of inflammatory cells.[25-35] Inflammation is the first protective immune response to infection or injury of the human body. The immune-modulating properties of chitosan have been widely reported. Chitosan can inhibit the activation of NF-κB (Nuclear factor kappa-B) and Lipopolysaccharide (LPS) binding, reduce the production of pro-inflammatory cytokines, and inhibit Tumor necrosis factor-α (TNF-α).[26, 27] Chitosan is also an excellent candidate for an effective hemostatic agent. The hemostatic property of chitosan is related to the presence of positive charges in the structure. Chitosan with positive charges can interact with the blood cell membrane with negative charges, encouraging platelet adhesion, activation, and aggregation at sites of vascular injury.[36-38] Especially, a number of researchers have reported that chitosan has shown negligible hemolytic properties (below 2%) according to the American Society for Testing and Materials (ASTM F756-17), making it widely used for wound healing.[39, 40]
3 Chitosan-Based Hydrogels for Infected Wound Treatment
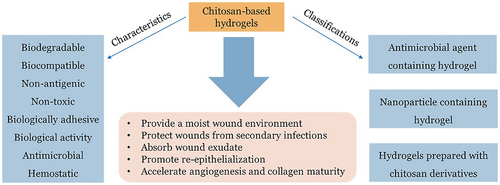
Chitosan-based hydrogels have demonstrated significant advantages in the treatment of infected wounds due to a variety of excellent properties (as shown in Figure 3). Biodegradation, biocompatibility, non-antigenicity, bioadhesion, and hemostatic activity are advantageous characteristics for their widespread use as wound dressings. Moreover, they can maintain a moderately moist healing environment, absorb wound exudate, and improve the local microenvironment of the wound which promotes tissue regeneration through re-epithelialization and granulation, thereby promoting wound healing. Most importantly, the excellent bioactivity of chitosan enables chitosan-based hydrogels to accelerate angiogenesis and collagen maturation, improve inflammatory cell functions, and protect wounds from microbial infection, which play key roles in the wound healing process, especially in the healing of infected wounds. However, due to the poor solubility, the antibacterial properties of hydrogels prepared only with chitosan and cross-linking agents are not satisfactory. The potential toxicity of chemical cross-linking agents also limits their application in the treatment of infected wounds. Herein, targeting antibacterial application chitosan-based hydrogels are classified into three widely studied categories (as shown in Figure 3): i) antibacterial agent-containing hydrogels, ii) nanoparticle-containing hydrogels, and iii) antibacterial hydrogels prepared with chitosan derivatives.[41] In addition to these categories, we also reviewed gaseous signaling molecules releasing chitosan-based hydrogels for infected wound healing. The preparation methods, characteristics, and functions of these kinds of chitosan-based hydrogels are discussed in this section.
3.1 Antimicrobial Components Containing Chitosan-Based Hydrogel
Antibiotics were developed in 1928 and have been widely used in controlling infections. Based on the mechanisms, antibiotics can be classified into inhibitors of the synthesis of DNA, protein, cell wall, mycolic acid, folic acid, and Ribonucleic acid (RNA).[42] The most commonly used antibiotics in wound healing include Ciprofloxacin HCl (CIP), Vancomycin, Gentamicin, and Moxifloxacin.[43] However, the emergence of drug-resistant microorganisms and side effects limit the use of antibiotics in controlling wound infections. To overcome this issue, minimizing the antibiotic dosage and delivering them directly to wound sites are promising and effective ways to reduce systemic toxicity and the emergence of drug-resistant microorganisms. Moreover, antimicrobial peptides (AMPs) and biological extracts have been developed as antibiotic alternatives due to the lower likelihood of the emergence of drug-resistant microorganisms. Based on their sources, AMPs are classified as amphibian-derived antimicrobial peptides, mammalian antimicrobial pesticides, microorganisms-derived antimicrobial peptides, and insect-derived antimicrobial peptides.[44] As reported in the literature, the mechanisms of AMPs against microbes include direct killing and immune modulation.[45] Secondary metabolites such as glycosides, tannins, coumarins, and saponins are sources of plant-derived antibacterial drugs that have been proven to be highly effective in the treatment of bacterial infections.[46]
Hydrogels possess a 3D structure that allows them to encapsulate antimicrobial agents, exchange them with absorbing wound exudates, protect them in hostile environments, and control their release.[47] CS-based hydrogels can exhibit easy drug loading and release, enhanced mechanical strength and antimicrobial activity.[48, 49] Some of the antimicrobial agents containing chitosan-based hydrogels are summarized in the following sections.
Ciprofloxacin HCl belongs to the class of drugs called quinolone antibiotics and is widely used to treat G+ and G− bacterial infections. The antibacterial mechanism of CIP is to block bacterial DNA replication by binding to DNA cyclase, leading to do double-strangle strand breakage of bacterial chromosomes.[41, 50, 51] Sharma et al. cross-linked chitosan polyethene polyethylene glycol acetaldehyde to form a hydrogel. Due to the dynamic reversible imine linkages, the hydrogel possessed self-healing, injectable, and biodegradable properties. The hydrogels demonstrated mechanical resilience and cytocompatibility with mammalian cells. In addition, the hydrogels exhibited good antibacterial activity against Escherichia coli.[52]
Vancomycin is an antibacterial agent of glycopeptides that is mainly used for controlling G+ bacterial infections. Vancomycin binds to the acyl-d-ala-d-ala moiety of the growing peptidoglycan cell wall, preventing double-strand cell wall synthesis in susceptible bacteria. With the development of bacterial resistance to methicillin-resistant Staphylococcus aureus, the use of vancomycin is gradually returning on a large scale.[53, 54] Naeimi et al. reported porous chitosan (CS), PVA, and PEG hydrogels prepared by lyophilization as vancomycin carriers for wound healing. The obvious inhibition zone confirmed the excellent antibacterial effect of vancomycin-loaded hydrogels.[55]
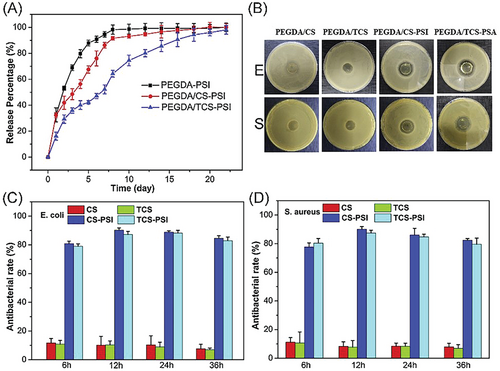
AMPs are a class of small peptides with broad antimicrobial activity against a variety of microorganisms. The antimicrobial activity of AMPs relies on the interaction with the DNA of microorganisms which inhibit the synthesis of RNA and proteins. The emergence of multi-drug-resistant microorganisms promoted the development of AMPs.[44, 56, 57] Rezaei et al. developed an antimicrobial peptide-loaded chitosan hydrogel by cross-linking chitosan with β-glycerol phosphate disodium salt pentahydrate. The authors discovered that the hydrogel containing 16 µg mL−1 of AMP was a promising dressing for XDR clinical isolates-infected full-thickness skin wound treatment.[58] A dual cross-linked polyethylene glycol diacrylate/chitosan (PEGDA/CS) hydrogel was designed and reported by Huang et al. The gelation of the hydrogel was triggered by UV irradiation. The hydrogel exhibited high cell compatibility, mechanical properties, and adhesiveness, and can adapt to irregularly shaped wound defects. Moreover, the hydrogel sustainably released antimicrobial peptides and showed a greater than 80% antibacterial rate (as shown in Figure 4), which significantly accelerates wound healing in full-thickness skin defect models by promoting angiogenesis and reducing the inflammatory response.[59]
Kiti et al. developed a curcumin-β-cyclodextrin (CMx) inclusion complex loaded sodium alginate/chitosan (CMx-loaded SA/CS) bilayer hydrogel that inhibited both G+ (S. aureus) and G− bacteria (E. coli) with a reduction percentage of 73.95% and 71.59%, respectively.[60] The indirect cytotoxicity evaluation indicated that the hydrogel was proven nontoxic to NCTC clone 929 and Normal human dermal fibroblasts (NHDF) cells.
3.2 Inorganic Nanoparticles Containing Chitosan-Based Hydrogel
Nowadays, a variety of nanoparticles (NPs) with unique physicochemical and biological properties have been designed and discovered.[61, 62] NPs are considered to be promising materials for improving the biological and mechanical properties of hydrogels.[63-65] 3D network hydrogel systems retain large amounts of water while maintaining their structure and provide a suitable platform for the contained nanoparticles to achieve superior biomedical applications.[64, 66-70] The current interest in biomedical applications is that a hydrogel system must show antimicrobial activity against exposed pathogens without manifesting biotoxicity to the tissue body.[71] In this section, metal NPs, and metal oxide NPs are presented according to the types of NPs incorporated into the hydrogels. In addition, the design and fabrication of antimicrobial nanocomposite hydrogels from chitosan are highlighted.
Silver (Ag) has been used as an antibacterial agent since ancient Egypt. In 425 bc, the Persians used silver to carry water to prevent water pollution in warfare, and Hippocrates used silver powder to treat wound ulcers.[69] Silver is now widely used to kill bacteria, prevent bacterial film production, and prevent bacterial infection in biomedical applications, such as antimicrobial coatings and wound healing.[72] With the rapid development of nanotechnology and nanoscience, Ag NPs have been prepared on a large scale and used as broad-spectrum antibacterial agents.
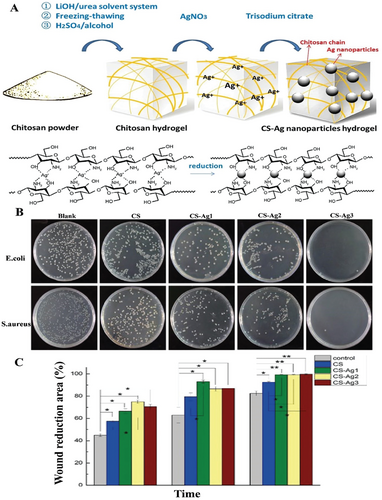
Nowadays, the antibacterial mechanism of Ag NPs is normally classified into six types: induction of photocatalytic damage to bacterial proteins, induction of bacterial genotoxicity, inhibition of bacterial respiratory chain, interference with the synthesis and folding of bacterial proteins, cell membrane collapse, and induction of bacterial oxidative stress to generate reactive oxygen species (ROS).[73] CS naturally has excellent antimicrobial properties and metal coordination and can work in synergy with Ag NPs to control NPs release and improve mechanical strength and antimicrobial activity.[48, 49] Xie et al. used LiOH/urea solvent system Ag NPs to prepare CS-Ag hydrogels, and the hydrogels showed high compressive strength and antibacterial efficacy (as shown in Figure 5).[48] Li et al. used N-[(2-hydroxy-3-trimethylammonium) propyl] CS chloride (HTCC) chain, N, N′-methylene bisacrylamide (BIS) cross-linked polyacrylamide network with Ag NPs encapsulated to construct a semi-interpenetrating hydrogel. With the synergistic effect of quaternary ammonium salts and Ag NPs, the hydrogel has good biocompatibility, antibacterial property, and mechanical properties.[44] Guo et al. used freeze-thawing and sodium citrate cross-linking to fabricate a silver-loaded PVA/CS/Gel hydrogel for wound dressing. The water absorption, moisture retention, and antibacterial activity were improved significantly.[74]
Gold NPs are also known for their excellent antimicrobial properties[75] and high-water solubility. Similar to the use of silver NPs, antimicrobial hydrogels work by encapsulating gold NPs in the hydrogel network systems. The antimicrobial mechanism of Au NPs has been studied for many years. On the one hand, Au NPs have antibacterial effects and can attach to bacterial membranes, causing bacterial contents to leak or penetrate the outer membrane and peptidoglycan layers, resulting in bacterial death.[76] On the other hand, Au NPs also reversed bacterial resistance to some extent when bound to non-antibiotics or antibiotic molecules.[76] Regiel-Futyra et al. reported a CS-based synthesis of Au NPs with total bactericidal efficacy against two antibiotic-resistant strains (Staphylococcus aureus and Pseudomonas aeruginosa) that formed biofilms. Importantly, gold has no significant cytotoxic effects on human cells in the 10–20 nm range.[77]
In addition to these commonly used metal nanoparticles for antimicrobial applications, copper (Cu) and cerium (Ce) NPs are also known as antibacterial agents. Villanueva et al. demonstrated a starch gel containing silicon-coated copper nanoparticles with antibacterial activity.[78] The Si-Cu hydrogels exhibited excellent antibacterial activity against Gram-negative and Gram-positive bacteria. Madzovska et al. developed Cu-SA-based hydrogels by electrostatic extrusion which showed significant inhibition against E. coli and MRSA at high Cu (II) content (≈100 × 10−6 m).[79] Furthermore, the Cu-SA hydrogels had an immediate bactericidal effect on S. aureus and E. coli. Yao et al. developed a thermos-reversible antibacterial Ce@LTA-NPs-F127/CS hydrogel. The hydrogel showed antibacterial activity against S. aureus and E. coli. Furthermore, the hydrogel can also accelerate wound healing in diabetic rats by remodeling the microenvironment of the wound site and increasing neovascularization.[80]
Examples of metallic oxide antimicrobial materials include, but are not limited to, nickel oxide (NiO), titanium dioxide (TiO2), and zinc oxide (ZnO). Unlike metal nanoparticles, photocatalysis is the major antibacterial mechanism of metal oxides.[81] In short, a large number of free radicals, including oxygen radicals and hydroxyl radicals, are generated on metal oxide nanoparticle surfaces under UV irradiation. When free radicals encounter microorganisms, the organic components in the microorganisms will be quickly oxidized into carbon dioxide. Therefore, metal oxide nanoparticles can kill microorganisms in a relatively short period. Among all metal oxides, ZnO is the most extensively used antibacterial agent. ZnO NPs exhibit antibacterial activity and non-cytotoxicity at appropriate concentrations and are widely used in many cosmetic materials.[82] Mohandas et al. developed a CS hydrogel/ZnO NPs composite bandage. Compared to KALTOSTAT and nZnO-free control bandages, composite bandages containing nZnO showed faster clotting ability and controlled degradation characteristics. The composite bandage showed antibacterial activity against C. albicans, E. coli, and S. aureus.[83, 84]
Although numerous studies have focused on explaining the antibacterial mechanisms and efficacy of metal as well as metal oxide nanoparticles, the existing literature is still inadequate and controversial.[85] The antimicrobial properties of the metals, including their alloys as well as derivative nanoparticles, must be further explored.
3.3 Antibacterial Hydrogels Prepared with Chitosan Derivatives
Although chitosan possesses unique biological activities such as biocompatibility, biodegradability, hemostasis, and antibacterial activity, its mechanical properties and poor water solubility limit its application in the biomedical field. Chemical modification of chitosan is a commonly used method to improve its bulk properties while retaining its biological functions.[86] There are three main functions of functionalization: 1) to improve the solubility, 2) to improve or maintain the biological activities, and 3) to introduce functional groups into the structure so that it can be used to form hydrogels without the use of potentially toxic small molecular cross-linkers. The active groups of chitosan include hydroxyl groups at C3 and C6 positions, and amine groups at the C2 position.[18, 87] These active groups of chitosan allow for chemical cross-linking and modifications (as shown in Table 1).
| Reactions | Active groups | Ref. |
|---|---|---|
| Alkylation | NH2, OH | [88-92] |
| Oxidation | OH (The hydroxyl groups at the C6 position can be oxidized to aldehyde or carboxyl groups, and the hydroxyl groups at the C3 position can be oxidized to carbonyl groups)[93] | |
| Acylation | NH2, OH | [97, 98] |
| Chelation | NH2, OH | [99] |
| Cross-linking | NH2, OH | [100] |
| Etherification | OH | [101] |
| Carboxylation | NH2, OH | [102] |
The functionalization of chitosan and hydrogel fabrication with chitosan derivates for antibacterial applications have been explored by numerous research groups. Antibacterial applications involving chitosan derivatives are shown in Table 2.
| Chitosan derivatives | Microbe |
|---|---|
| Dodecenyl succinylated phthaloyl chitosan | B. subtilis, S. aureus, E. coli[103] |
| N, N, N-trimethyl chitosan-polylactide | S. aureus[104] |
| Chitosan-sulfonamide derivatives | S. aureus, E. coli [105] |
| N-succinyl chitosan | E. coli and S. aureus[106] |
| Carboxymethylated chitosan | E. coli[107] |
| Aldehyde chitosan | E. coli[108] |
| N-Aminorhodanine modified chitosan/copper ions | Staph. strain[109] |
| Carboxymethyl chitosan/ZnO nanocomposite | S. aureus, E. coli[110] |
| Hydroxypropyltrimethylammonium chloride CS | S. aureus, Methicillin-resistant Staphylococcus aureus, Staphylococcus epidermidis[111] |
| Poly(aminoethyl) modified chitosan | E. coli[112] |
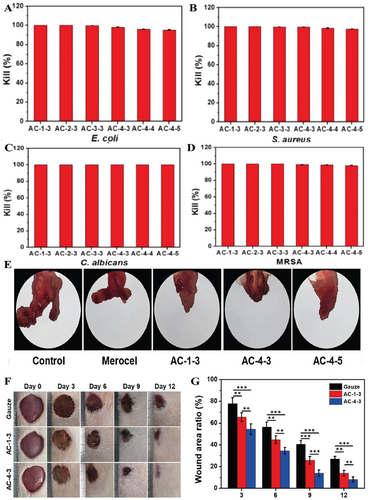
Here are some detailed examples. Fan et al. used N-(3-chloro-2-hydroxypropyl) trimethyl ammonium chloride to synthesize quaternary ammonium chitosan and fabricated a quaternary ammonium chitosan/polyvinyl alcohol/polyethylene oxide hydrogel using gamma radiation. The polymers were perfectly compatible and interacted via hydrogen bonds. The hydrogel showed antibacterial performance against E. coli and S. aureus.[113] Zhang et al. used chitin to synthesize poly(aminoethyl) modified chitosan (PAEMCS) successfully and then fabricated the hydrogels with PAEMCS and dipotassium hydrogen phosphate (DHP). The hydrogels exhibited good cytocompatibility, antibacterial activity, and hygroscopicity. The minimum inhibition rate of the hydrogels against E. coli, S. aureus, and S. epidermidis was above 50%.[114] Zheng et al. functionalized quaternized chitosan (QCS) with catechol groups and obtained catechol-modified quaternized chitosan (QCS-C). Then an injectable hydrogel with antibacterial, thermos-sensitive and tissue adhesive properties was fabricated by incorporating QCS-C into poly(d, l-lactide)-poly(ethylene glycol)-poly(d, l-lactide) (PLEL) hydrogel. The hydrogel showed a killing ratio above 95% for S. aureus and E. coli.[115] Deng et al. synthesized adenine-modified chitosan (AC) and designed a hydrogel (AC hydrogel) system using the heating/cooling process. The AC derivatives had no cytotoxicity against L929 cells. The results of antibacterial activity evaluation showed that 100% of C. albicans, more than 97.4 ± 0.3% of S. aureus, and 95.3 ± 0.4% of E. coli were killed by AC hydrogels. AC hydrogels exhibited the ability to promote cell proliferation and an outstanding hemostatic effect. The hydrogels also showed excellent wound healing efficacy in the in vivo experiment (as shown in Figure 6).[116]
3.4 Gaseous Signaling Molecules Releasing Chitosan-Based Hydrogel
Gaseous signaling molecules play important roles in wound healing.[117, 118] Chitosan-based hydrogels capable of releasing gaseous signaling molecules have attracted attention in the treatment of infected wounds. Carbon monoxide (CO) and nitric oxide (NO) are the most widely concerned gaseous signaling molecules. NO has a biphasic effect on wound healing. Low levels of NO can positively affect cytokine expression. High levels of NO can suppress skin inflammation.[119] Hasan et al. prepared a NO-releasing hydrogel using oxidized bacterial cellulose (BCTO), chitosan, and linear polyethyleneimine diazeniumdiolate (PEI/NO) for wound infection. The hydrogel showed sustained release over four days and excellent antibacterial performance against S. aureus, P. aeruginosa, MRSA, and polymicrobial infection.[120] CO generated can enhance the proliferation, differentiation, and polarization of macrophages, resulting in anti-inflammatory effects. However, the use of exogenous CO is limited because it is difficult to quantify and may increase plasma carboxyhemoglobin to toxic levels.[121] At present, there are few reports on the use of chitosan-based hydrogels combined with CO for the healing of infected wounds. This will be an area of research worthy of attention and a challenge for researchers.
4 Conclusion and Perspective
In this review, the structural basis of chitosan, its antibacterial mechanism and its effects on wound healing were described. The characteristics of hydrogel dressings were briefly discussed. Chitosan-based antibacterial hydrogels were classified as hydrogels containing inorganic nanoparticles, hydrogels containing antibacterial agents, and hydrogels prepared with chitosan or its derivatives. The fabrication, characterization, wound-healing effects, and antibacterial activity of the hydrogels, as well as typical examples were discussed.
Hydrogels have important advantages, such as excellent biocompatibility, controllable release, and local drug delivery, all of which are necessary for antibacterial biomaterials. Hydrogels with antimicrobial properties have become an alternative to traditional antibiotics in infected wound treatment. The most important requirements for hydrogels used in wound healing are biocompatibility and biodegradability. Therefore, as a biomaterial that is degradable, biocompatible, antibacterial, and promotes wound healing and hemostasis, chitosan has great potential in the development of antibacterial hydrogels and will be widely used in the treatment of infected wounds in the future.
At present, most researchers have been studying the combination of hydrogels composed of hydrophilic polymers with various bactericidal substances, such as antibiotics, nanoparticles, etc. Although nanoparticles and antimicrobial agents show effectiveness in infected wound healing, the potential toxicity, adverse effects, and possible emergence of drug-resistant microorganisms are the limitations for clinical applications.
Chitosan has poor solubility and can only be dissolved under acidic conditions, which limits its clinical application to a certain extent. Advanced material science and techniques allow desired biomolecules to be obtained, which can further improve the physiochemical and biological properties of polymers and even resulting hydrogels. Therefore, the properties of chitosan, such as solubility, functional group density with antibacterial activity, and cross-linking abilities, will be improved through chemical functional modifications. Chitosan-based antibacterial hydrogels, particularly those prepared with functionalized chitosan, have a promising future in the treatment of infected wounds. The unique combination of chitosan-based antibacterial hydrogels and other antibacterial biomaterials or gaseous signaling molecules merits additional investigation.
5 Abbreviations
The abbreviations in this chapter are summarized and listed in Table 3.
| Abbreviations | Meanings |
|---|---|
| PEG | Polyethylene glycol |
| PVA | Polyvinyl acetate |
| PAM | Polyacrylamide |
| NF-κB | Nuclear factor kappa-B |
| LPS | Lipopolysaccharide |
| TNF-α | Tumor necrosis factor-α |
| DNA | Deoxyribonucleic acid |
| RNA | Ribonucleic acid |
| AMPs | Antimicrobial peptides |
| 3D | 3D dimensions |
| G+ | Gram-positive |
| G- | Gram-negative |
| CIP | Ciprofloxacin HCl |
| E. coli | Escherichia coli |
| XRD | Extensively drug-resistant |
| PEGDA | Polyethylene glycol diacrylate |
| UV | Ultraviolet |
| CMx | Curcumin-β-cyclodextrin |
| SA | Sodium alginate |
| S. aureus | Staphylococcus aureus |
| B. subtilis | Bacillus subtilis |
| C. albicans | Candida albicans |
| CTS/CS | Chitosan |
| HEK293 cells | Human embryonic kidney cells 293 |
| NPs | Nanoparticles |
| Ag | Silver |
| ROS | Reactive oxygen species |
| LiOH | Lithium hydroxide |
| Au | Gold |
| Cu | Copper |
| Ce | Cerium |
| NiO | Nickel oxide |
| TiO2 | Titanium dioxide |
| ZnO | Zinc oxide |
| HTCC | N-[(2-Hydroxy-3-trimethylammonium) propyl] CS chloride |
| BIS | N, N′-Methylene bisacrylamide |
| Gel | Gelatin |
| Si | Silicon |
| AC | Adenine-modified chitosan |
| NHDF | Normal human dermal fibroblasts |
| PAEMCS | Poly(aminoethyl) modified chitosan |
| DHP | Dipotassium hydrogen phosphate |
| QCS | Quaternized chitosan |
| QCS-C | Catechol-modified quaternized chitosan |
| PLEL | Poly(d, l-lactide)-poly(ethylene glycol)-poly(d, l-lactide) |
| CO | Carbon monoxide |
| NO | Nitric oxide |
| BCTO | Oxidized bacterial cellulose |
| PEI/NO | Polyethyleneimine diazeniumdiolate |
Acknowledgements
This work was supported by the China Scholarship Council (CSC202009110089) and Science Foundation Ireland (SFI) (Funding Agency Ref. No. 19/FFP/6522).
Open access funding provided by IReL.
Conflict of Interest
The authors declare no conflicts of interest
Biographies

Xiaoyu Wang is a Ph.D. student under the supervision of Prof. Wenxin Wang at Charles Institute of Dermatology, School of Medicine, University College Dublin (UCD). Her research focuses on the functionalization of biomaterials and exploring their applications in the biomaterial field.

Qian Xu is a Postdoctoral Research at Charles Institute of Dermatology, School of Medicine, University College Dublin (UCD). Dr. Xu's has extensive expertise in design of functional biomaterials for wound healing, tissue engineering, and stem cell in medicine. Her scientific contribution and achievements have been recognized both nationally and internationally including over 30 peer-reviewed scientific journal papers (Chemical Science, Angewandte Chemie, Acta Biomaterialia, etc.). She owns ten patents (three awarded).

Wenxin Wang is a Full Professor in Skin Research and Wound Healing at the Charles Institute of Dermatology, School of Medicine, University College Dublin (UCD). His research focuses on the development of multifunctional polymeric biomaterials, stem cell and gene therapy for skin wound healing. His scientific contribution and achievements have been recognized both nationally and internationally including over 220 peer-reviewed scientific journal papers (Nature Communications, Nature Reviews Chemistry, Science Advances, Angewandte Chemie, and Nano Letters), 5 books chapters, 34 patents, 149 conference abstracts and presentations, and 116 invited lectures and keynote presentations.