Different levels of mucus inflammatory mediators in nasal polyposis with and without aeroallergen sensitivity
Abstract
Objectives
Biomarker levels in nasal secretions can reflect the inflammatory status of nasal mucosa and evolution of sinus disease. The aim of this study was to evaluate the relationship between local inflammatory mediator production and clinical characteristics of patients with nasal polyposis (NP).
Methods
Thirty-one nonaeroallergen sensitized patients with NP (NANP), 29 aeroallergen sensitized patients with NP (ANP), and 30 subjects without inflammation of nasal mucosa as controls (C) entered this prospective, cross-sectional study. Clinical parameters (symptoms, endoscopic, and radiological findings) were assessed. The concentrations of heat shock protein 70 (HSP70), eosinophil cationic protein (ECP), tryptase, substance P and Clara cell protein 16 (CC16) were measured in the nasal secretion samples of all participants by ELISA method.
Results
Our results showed higher concentrations of HSP70, ECP, and tryptase in ANP than in NANP and C (p < .001 for all markers). On the other hand, levels of CC16 were significantly higher in C than in NANP and ANP groups (p < .001; p < .001, respectively). We found positive correlations between HSP70, ECP, tryptase, and substance P levels and nasal symptom score in patients with NP. Also, HSP70, ECP, tryptase, and substance P showed different levels of positive correlation among themselves, with HSP70 showing highest positive correlation with ECP. Finally, relatively strong negative correlations were found between the levels of CC16 and nasal symptoms, as well as between the CC16 levels and levels of other four mediators in nasal fluid.
Conclusion
HSP70, ECP, tryptase, and substance P might play a role in the pathogenesis of NP. The results suggest that chronic inflammation in NP involves a self-sustaining local release of HSP70, ECP, and tryptase, independent of aeroallergen stimulation of the mucosal layer, although the production of these mediators is higher in aeroallergen sensitized NP patients.
1 INTRODUCTION
The chronic inflammation in a majority of Caucasian patients with chronic rhinosinusitis with nasal polyps (CRSwNP), also known as nasal polyposis (NP), is characterized by domination of type 2 (T2) immune response and intense infiltration of the nasal mucosa with eosinophils, mast cells, and other inflammatory cells.1, 2 Those cells are sources of inflammatory mediators which induce the damage of the epithelial cells and edema in the lamina propria, leading to remodeling and polypoid degeneration of the nasal and paranasal sinus mucosa.1, 2 Previous investigations demonstrated that prolonged aeroallergen stimulation of the nasal mucosa have influence on inflammatory mediator production and that “allergic” and “nonallergic” NP patients have different patterns of inflammatory response in polyp tissue and nasal secretions.3-5 The levels of main T2 cytokines, such as IL-4, IL-5, IL-10, and IL-13 were increased more significantly in the “allergic” polyp tissues and nasal secretions compared to the “nonallergic” ones.3-5
Recent studies demonstrated that apoptotic and necrotic cells release damage-associated molecules, such as heat shock proteins (HSPs), that promote early innate and adaptive immune responses.6, 7 HSP70 is the best-studied damage-associated molecule. It stimulates the maturation of dendritic antigen-presenting cells and activates the nuclear factor kappa B (NF-κB) pathway, which modulates production of several cytokines.6, 7 Several studies have indicated an important role of HSP70 in the pathogenesis of allergic diseases.8 The sputum and plasma levels of HSP70 are increased in patients with asthma and related to disease severity.9 The concentrations of HSP70 in nasal secretions are increased in patients with NP and correlate positively with the severity of sinus disease and the levels of IL-4 and IL-5 in nasal fluid.6, 7
Many biomarkers of inflammation can be detected in nasal secretion samples and they can reflect the inflammatory status of the nasal mucosa and progression of mucosal disease. In the present study, we compared the levels of HSP70, eosinophil cationic protein (ECP), tryptase, substance P, and Clara cell protein 16 (CC16) in nasal secretions of nonaeroallergen sensitized patients with nasal polyposis (NANP), aeroallergen sensitized NP patients (ANP), as well as in subjects without inflammation of nasal mucosa and evaluated their relationship with clinical severity of NP. To the best of our investigation, we evaluated for the first time the correlations between HSP70 levels in nasal secretions and the concentrations of ECP and tryptase, the main indicators of eosinophil and mast cells activity, anti-inflammatory CC16, and substance P, the main indicator of neurogenic inflammation in the nasal mucosa.
2 MATERIALS AND METHODS
2.1 Ethical consideration
This prospective, cross-sectional study was performed in accordance with the Helsinki Declaration. The protocol and methods of the study were approved by the Ethics Committee of the Military Medical Academy Faculty of Medicine (IRB Approval 05/2019). A written informed consent was obtained from all patients. The study was performed at the Department of Otorhinolaryngology of the Military Medical Academy in Belgrade, Serbia, between September 2016 and August 2020. The STROBE reporting method was used to present the results of the study.
2.2 Study population
Sixty patients with NP, 31 NANP, and 29 ANP, selected for endoscopic surgical treatment, were recruited for participation in this investigation. Thirty subjects selected for nasal septum surgery, without medical history of inflammatory disease and endoscopic findings of nasal/paranasal sinus inflammation, were included as controls.
The diagnosis of NP was based on the guidelines established by the Rhinosinusitis Task Force, endorsed and updated in 2007 by the American Academy of Otolaryngology, Head and Neck Surgery (AAO-HNS).10, 11 Presence of bilateral NPs on nasal endoscopy and found bilateral areas of opacification in the ethmoidal labyrinths on the computed tomography (CT) were the criteria for inclusion in this research. The diagnosis was confirmed by histopathological examination of surgically removed tissues.
Exclusion criteria: <18 years or > 65 years, choanal polyps, nasal hamartomas, fungal sinusitis, bronchial asthma, nonsteroidal anti-inflammatory drug-exacerbated respiratory disease (N-ERD), systemic diseases affecting the nasal cavity and sinuses (granulomatosis with polyangiitis, primary ciliary dyskinesia, Churg–Strauss syndrome, cystic fibrosis, etc.), cigarette smoking, prior nose/sinus surgery, inflammations of the respiratory tract, the use of intranasal or systemic corticosteroids, antihistamines, and antibiotics during 1 month before the start of this investigation.
2.3 Allergy determination
According to the AAO-HNS guideline,12 all participants were evaluated for allergy/aeroallergen sensitivity, according to the medical histories, previous symptoms of allergic rhinitis, as well as skin-prick tests. Determination of the total serum immunoglobulin E (IgE) concentration was introduced as an additional criterion, since in “allergic” patients with NP, the symptoms of CRS overlap with the simptoms of chronic rhinitis. A commercial package of common aeroallergens, including house dust mites (Dermatophagoides farinae and Dermatophagoides pteronyssinus), pollens (birch, olive tree, mugwort, Parietaria, Plantago, grass, common ragweed [Ambrosia elatior]), animal danders (cat, dog, horse), molds (Alternaria, Cladosporium) (Soluprick, ALK-Abelló A/S, Hørsholm, Denmark) was used for skin-prick tests. A negative (0.9% sodium-chloride) and a positive (histamine-dihydrochloride) controls were performed for all participants. The test was considered positive if the diameter of the protrusion on the skin was greater than 3 mm comparing to the control. For the measurement of IgE serum concentration on the ELISA reader (Spectra III, Austria), we used the human commercial ELISA kit (Elitech Diagnostics, Salon-de-Provence, France). The patients with total serum IgE levels higher than 100 IU/mL were considered as ANP.
2.4 Symptoms, endoscopic, and CT examination
The same rhinologist examined all the patients. All participants assessed their symptoms (nasal obstruction, rhinorrhoea, hyposmia, sneezing, and itching) by a 3-point scale. The symptoms were scored from 0 to 3, that is: 0 for no symptoms, 1 for mild symptoms, 2 for moderate symptoms, and 3 for severe symptoms. The maximum total symptom score is 15, as previously described.13
The evaluation of the endoscopic scores (NP size scores) was performed in all patients with NPs in a sitting position with a rigid endoscope (0° and 30°; Karl Storz Endoscope, Tuttlingen, Germany), according to Lildholdt et al.14: 0 for no NPs; 1 for mild NP (small NPs that do not reach the upper edge of the inferior turbinate); 2 for moderate NP (medium-sized NPs between the upper and the lower edges of the inferior turbinate); 3 for severe NP (large NPs that reach the lower edge of the inferior turbinate). Maximum endoscopic score is 6, including the right and left sides. The findings from the CT scans were evaluated in all NP patients, according to the Lund–Mackay score.15
2.5 Sampling of nasal secretions and measurement of biochemical parameters
We used the so-called absorption technique for the collection of nasal secretion samples of all 90 subjects (i.e., 60 NP patients and 30 subjects without nasal inflammation). Cotton wool sticks, 10 mm long and 4 mm wide, produced by the Institute of Virology, Vaccines and Sera, “Torlak,” Belgrade, Serbia, were inserted under the endoscopic guidance into the anterior part of the one nasal middle meatus, on the superior surface of the inferior turbinate, by use of bayonet forceps, as previously described.16 Based on previous studies, it has been estimated that 5 min is enough to completely soak the cotton fibers in the nasal secretions.4, 16 After that, all the samples were placed in a 2 ml Eppendorf tube containing 1 ml of transfer medium (phosphate-buffered saline with gentamycin 50 μg/ml, penicillin G 340 IU/ml, fungizone 500 μg/ml) for 30 min to allow diffusion of mediators into the medium and then stored at 4°C for a maximum of 2 h until processed. Nasal fluid samples were centrifuged at 1000g for 10 min to separate the cellular components. After centrifugation, supernatants were portioned and stored at −70°C, for no more than 2 months, pending mediator determination. Levels of inflammatory mediators were measured in all samples using commercial human ELISA kits (for HSP70, MyBioSource, Inc., San Diego, CA, USA; for CC16, Elabscience Biotechnology, Ltd., Houston, TX, USA; for ECP, Aviscera Bioscience, Inc., Santa Clara, CA, USA; for tryptase, Abbexa Ltd., Cambridge, UK; for substance P, RayBiotech, Norcross, GA, USA). The concentrations of mediators were expressed in picograms/milliliters (pg/ml). The sensitivities of detection, assay ranges and coefficients of variation for biochemical parameters are presented in Table 1.
| Mediator | Sensitivity of detection (pg/mL) | Assay range (pg/ml) | Coefficient of variation (%) |
|---|---|---|---|
| HSP70 | 10 | 125–4000 | <15 |
| CC16 | 37.5 | 62.5–4000 | <10 |
| ECP | 50 | 156–10,000 | 4–6 |
| Tryptase | 3.1 | 7.8–500 | <10 |
| Substance P | 100 | 100–1,000,000 | <10 |
- Abbreviations: CC16, Clara cell protein 16; ECP, eosinophil cationic protein; HSP70, heat shock protein 70.
2.6 Strength of the study and sample size calculation
Subjects were divided into three groups depending on clinical status. The strength of the study should have been at least 80% (0.8), and the probability of error of the first type (α) .05. Based on the data from the literature (Tsybikov et al.),6 a much higher level of HSP70 could be expected in patients with nasal polyposis compared to control subjects (0.21 ± 0.10 μg/ml vs 0.05 ± 0.03 μg/ml; p < .001). Also, based on data from the same article,6 it could be assumed that the values of standard deviations (SD) will be relatively high. Therefore, a moderate effect size (0.34) was chosen to calculate the group size. Approximatelly 90 participants (30 in each group) were required to demonstrate statistical significance at the p < .05 level between groups, with a study strength of 80%. The calculation was performed by the analysis of variance test (ANOVA, fixed effects, omnibus, one-way) and with the use of the available commercial software (GPower 3.1.).
2.7 Statistical analysis
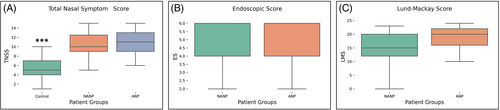
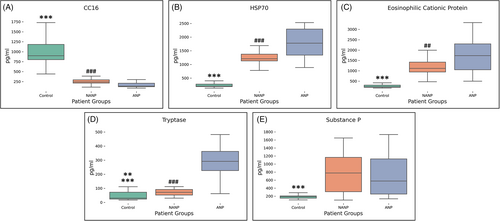
The data normality was tested using Kolmogorov–Smirnov and Shapiro–Wilk tests. Kruskal–Wallis H test was used to explore the differences between patient groups relative to the values of their clinical parameter scores and inflammatory mediators, followed by post hoc testing using Mann–Whitney U test. Correlation testing between these parameters was done using Spearman's rank correlation coefficient. A p value less than ≤.05 were considered statistically significant. Results in the Figures 1 and 2 and Table 2 are represented as medians with interquartile ranges (25th and 75th percentiles).
| Parameters | Controls | NANP | ANP |
|---|---|---|---|
| Participants (n) | 30 | 31 | 29 |
| Male/female ratio | 16/14 | 18/13 | 14/15 |
| Age (years)* | 43.25 (27.00–62.00) | 46.15 (28.00–63.00) | 44.20 (25.00–61.00) |
| Positive history of allergies | 0.00 | 0.00 | 29 |
| Positive skin prick test to aeroallergens | 0.00 | 0.00 | 29 (100%) D. farinae: 23 (79.3%) Plantago: 22 (75.8%) Ambrosia elatior: 21 (72.4%) D.pteronyssinus: 20 (68.9%) Parietaria: 18 (62.0%) |
| Total serum IgE* (IU/ml) | 32.44 (25.80–43.85) | 68.30 (51.45–78.35) | 138.35 (126.75–162.60) |
| Nasal symptom score* | 5.00 (4.00–7.00) | 10.00 (9.00–13.00) | 11.00 (9.00–13.00) |
| Endoscopic score* | 0.00 | 5.00 (4.00–6.00) | 6.00 (4.00–6.00) |
| Lund–Mackay score* | 0.00 | 15.00 (12.00–21.00) | 20.00 (15.00–22.00) |
| HSP70 (pg/ml)* | 204.45 (184.53–270.70) | 1208.20 (1123.80–1429.20) | 1778.50 (1313.80–2301.30) |
| CC16 (pg/ml)* | 899.00 (793.30–1223.15) | 242.00 (211.60–294.40) | 135.30 (119.00–217.43) |
| ECP (pg/ml)* | 223.25 (197.65–308.77) | 1118.80 (901.80–1499.20) | 1733.60 (1048.20–2432.55) |
| Tryptase (pg/ml)* | 30.75 (22.97–77.65) | 71.60 (48.80–95.00) | 291.60 (224.95–364.00) |
| Substance P (pg/ml)* | 186.88 (146.52–207.69) | 777.25 (297.30–1171.80) | 576.74 (251.85–1135.85) |
- Abbreviations: ANP, aeroallergen sensitized patients with nasal polyposis; CC16, Clara cell protein 16; D, Dermatophagoides; ECP, eosinophil cationic protein; HSP70, heat shock protein 70; IgE, immunoglobulin E; NANP, nonaeroallergen sensitized patients with nasal polyposis.
- * Results are presented as medians with 25th and 75th percentiles.
3 RESULTS
A total of 90 patients were included in the present study. The experimental group was comprised out of 60 (66.7%) patients who were diagnosed with NP, while the control group (C) included 30 (33.3%) participants with healthy nasal mucosa. In the group of patients with NP, 29 patients (32.2%) were ANP patients, while 31 patients (34.4%) were NANP patients. Thus, a total of three groups of participants were compared in the present study: C, ANP and NANP group.
Regarding the results of skin prick test to aeroallergens in ANP group, 79.3% patients were found to be sensitized to Dermatophagoides farinae, 75.8% sensitized to Plantago, 72.4% to Ambrosia elatior, 68.9% to Dermatophagoides pteronyssinus, and 62.0% to Parietaria. The concentrations of total serum IgE were higher in ANP than in NANP patients (p = .002) and in both ANP and NANP groups than in C subjects (p < .001; p = .005, respectively). The numerical data of demographic, clinical, and biochemical parameters are presented in Table 2.
Analysis and comparison of clinical parameters such as Total Nasal Symptom Score (TNSS), size of nasal polyps (endoscopic score, ES) and Lund–Mackay score (LMS), showed a significant statistical difference only for TNSS. Regarding TNSS, statistical difference was detected between all three groups of patients (χ2[2] = 46.326, p < .001), with a mean rank of 19.1 for C, 57.98 for NANP and 59.47 for ANP groups. Post hoc testing showed significant difference between C and NANP groups of patients (U = 42.5, p < .001), as well as between C and ANP groups (U = 65.5, p < .001). No difference was showed between NANP and ANP groups (U = 414, p = .597) (Figure 1A). Likewise, ES and LMS values showed no statistical difference between NANP and ANP groups (U = 358.5, p = .134 and U = 337, p = .095, respectively) (Figure 1B, C).
All three groups of patients showed significant statistical difference in the values of inflammatory mediators: CC16 (χ2[2] = 66.083, p < .001) with a mean rank of 75.5 for C, 38.97 for NANP and 21.45 for ANP groups (Figure 2A); HSP70 (χ2[2] = 65.332, p < .001) with a mean rank of 15.5 for C, 52.52 for NANP and 69.03 for ANP groups (Figure 2B); ECP (χ2[2] = 59.439, p < .001) with a mean rank of 16.35 for C, 54.05 for NANP and 66.52 for ANP groups (Figure 2C); tryptase (χ2[2] = 51.852, p < .001) with a mean rank of 24.23 for C, 40.82 for NANP and 72.5 for ANP groups (Figure 2D); substance P (χ2[2] = 36.223, p < .001) with a mean rank of 22.27 for C, 59.71 for NANP and 54.34 for ANP group (Figure 2E). Post hoc analysis for each of the inflammatory parameters showed significant difference between C and NANP groups of patients (CC16: U = 0, p < .001; HSP70: U = 0, p < .001; ECP: U = 25.5, p < .001; tryptase: U = 235, p = .007; substance P: U = 89, p < .001), C and ANP groups (CC16: U = 0, p < .001; HSP70: U = 0, p < .001; ECP: U = 0, p < .001; tryptase: U = 27, p = .008; substance P: U = 114, p < .001) and between NANP and ANP for all mediators except substance P (CC16: U = 187, p < .001; HSP70: U = 202, p < .001; ECP: U = 275, p = .006; tryptase: U = 74.5, p < .001; substance P: U = 385, p = .340) (Figure 2).
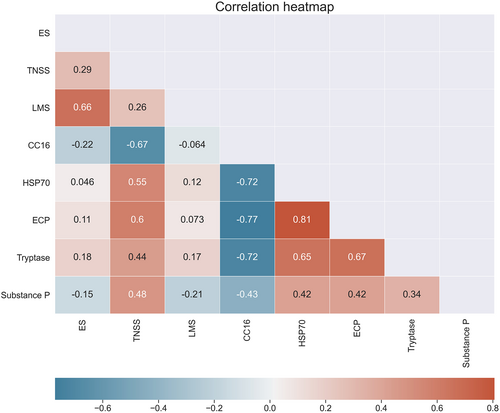
Relationship between inflammatory mediators and clinical parameter scores of patients included in the present study shows a moderate positive correlation between TNSS and HSP70, ECP, tryptase, and substance P, while a moderate negative correlation was detected with CC16 (rs = −0.67). Likewise, CC16 shows a moderate to high negative correlation with other four inflammatory mediators. HSP70, ECP, tryptase, and substance P show different levels of positive correlation among themselves, with HSP70 showing highest positive correlation with ECP (rs = 0.81) (Figure 3).
4 DISCUSSION
Allergy testing identified only five aeroallergens among ANP patients: two forms of mites, as perennial allergens, and three plants: Plantago, common ragweed (Ambrosia elatior) and Parietaria. Serbia is located at the cross-roads of the Mediterranean and Central European climate zones, so that only a few plants that bloom for several months are represented. Pollination of the Plantago lasts 8 months, from March to October. Parietaria blooms for 7 months, from April to October. An interesting detail is related to common regweed (Ambrosia elatior). This plant has pollination period from July to October, and as many as 72.4% of patients from the group of “allergic” NP were sensitized to it. This plant is one of the strongest allergens in Serbia, strong enough to trigger inflammation that can lead to remodeling of the nasal mucosa despite exposure for no more than 4 months during the year.
The results of our study showed increased nasal fluid concentrations of HSP70, ECP, tryptase, and substance P in patients with NP than in subjects without inflammation of the nasal mucosa. In addition, the concentrations of HSP70, ECP, and tryptase was found to be significantly higher in ANP than in NANP patients, suggesting the presence of increased level of chronic inflammation and eosinophil/mast cell activity in “allergic” form of NP. We, also, found a moderate positive correlation between the severity of nasal symptoms and the levels of these inflammatory mediators in nasal secretions. On the other hand, according to our results, the correlations are much weaker between the objective parameters of severity of sinus disease (endoscopic score, Lund–Mackay CT score) and the levels of inflammatory mediators (Figure 3). In previous investigations by Tsybikov6 and Min,7 the authors found significant statistical association between the levels of HSP70 in nasal secretions and the Lund–Mackay score, suggesting that HSP70 can be used as a biomarker that represents the severity of CRSwNP. These differences in the results of our and previous studies can be explained by the limited number od participants in our study but also by the fact that objective parameters (endoscopic score, Lund–Mackay score) represent the current condition in the nasal area, which can be subject to change relatively quickly. On the other hand, the long-term progression of inflammation of the nasal and sinus mucosa goes hand in hand with the production of inflammatory mediators, which may be consistent with the gradual progression of nasal symptoms as subjective indicators.
According to our results, HSP70, ECP, tryptase, and substance P show different levels of positive correlation among themselves, with HSP70 showing highest positive correlation with ECP. Although the exact role of HSP70 in the pathogenesis of NP is not well known, this mediator could play a wide range of immunological effects in this disease. The main sources of HSP70 in NP are nasal epithelial cells, but, during the chronic inflammation, many other activated and necrotic cells, including mast cells and eosinophils become the sources of this molecule.6, 7 After the contact with HSP70, antigen-presenting cells release several proinflammatory cytokines, including IL-1β, IL-6, IL-12, and tumor necrosis factor alpha (TNF-α).6 Lin et al.17 in an immunohistochemical study found higher expression of HSP70 in eosinophilic form of NP than in noneosinophilic form. By activation of NF-κB pathway, HSP70 stimulates the production of several cytokines and chemokines, especially eotaxin-1 and eotaxin-2, which attract eosinophils to the site of inflammation. Moreover, NF-κB has a role of anti-apoptotic molecule in eosinophils and may be responsible for the recruitment and enhanced survival of these cells in NP.18 Above-mentioned findings could be important for explanation of high positive correlation between the HSP70 and ECP concentrations in nasal secretions in our NP patients.
Products of activated eosinophils, especially enzyme ECP, have crucial role in damage of nasal mucosa in all studied forms of chronic upper airway inflammatory disorders.19 Experimental studies have shown cytotoxic effects of ECP on respiratory epithelium and lamina propria, resulting in lysis of epithelium and edema of subepithelium.19, 20 Our previous investigation demonstrated positive correlation between ECP levels in nasal secretions and eosinophil counts in nasal mucosa of patients with perennial allergic rhinitis and NP.16 After the intranasal corticosteroid therapy, the levels of ECP in nasal fluid significantly decrease.16 So, ECP levels in nasal secretions can be used to monitor the activity of eosinophilic inflammation in different forms of sinonasal diseases and to assess the efficacy of treatment. Activity of mast cells is well described in the pathophysiology of allergic rhinitis, but is reported to play a relevant role in NP.19 Tryptase is a proteolytic enzyme, located in the secretory granules of mast cells and it is released during the process of degranulation together with histamine. However, tryptase has a much longer plasma half-life compared with histamine, implying that tryptase is considered superior to histamine in order to study mast cell function.19, 20
The presence of neuropeptide-containing sensory nerves in human nasal mucosa is well established, but information regarding the neuropeptide production in NP is poorly available in the literature. As the main neuropeptide in the nasal mucosa, substance P has been reported to induce vasodilatation, vascular permeability and mucus secretion.21 The results of an immunohistochemical study by Kühn and Arnold indicated that substance P have an equal distribution in NPs and healthy nalas mucosa.22 Our results, however, suggest higher level of substance P production in patients with NP than in subjects without nasal inflammation. We found no statistical difference between substance P levels in NANP and ANP, suggesting that this neuropeptide maybe has not a crucial role in the pathogenesis of “allergic” form of NPs. On the other hand, according to our results, concentrations of substance P are in positive correlations with the levels of HSP70, ECP, and tryptase in nasal secretions. These correlations can be explained by the fact that ECP and tryptase expose the basement membrane of the nasal mucosa during epithelial damage. In that way, the ends of the sensory fibers become more stimulated to the production of substance P, which is released in the nasal secretions in larger amounts.
The results of the present study show a moderate negative correlation between CC16 and nasal symptom score. Likewise, CC16 shows a moderate to high negative correlation with other four inflammatory mediators. CC16 is a small anti-inflammatory protein, secreted primarily in the nonciliated Clara cells of the respiratory epithelium.23 This protein induces inhibition of enzymes phospholipase A2 and transglutaminase in the airways of patients with allergic rhinitis and CRS, as well as in subjects exposed to air pollutants.23 Our previous investigations showed that inflammation in patients with NPs and allergic rhinitis decreases the levels of CC16.16, 23 We, also, found that the concentration of CC16 in nasal secretions was negatively correlated with the levels of ECP and eotaxin-2 in patients with NP and perennial allergic rhinitis.16, 23, 24 The results of our current study show a negative correlation between CC16 and HSP70, ECP, tryptase, and substance P in nasal secretions, which indicates a significant sensitivity of Clara cells in the nasal respiratory epithelium to a number of pro-inflammatory mediators that can damage the epithelium. After topical corticosteroid use, the concentrations of CC16 increased in both allergic rhinitis and NP patients, suggesting that CC16 measured in nasal secretions could be a reliable marker for assessment of the recovery function of sinonasal mucosa during local corticosteroid therapy.16, 24
The relatively small number of participants is a limitation of this study and the results would need to be confirmed in larger multicentric investigations. Due to the financial reasons, we did not perform the immunohistochemical investigation regarding the expression of these inflammatory mediators in the nasal mucosa/NP tissue. Although a number of authors in their studies used the dilution technique to collect nasal secretions, we opted for the absorption technique, in line with the results of our previous studies.4, 16, 23, 24 Unlike the dilution technique, the absorption technique lacks the effect of diluting nasal secretions. Accordingly, the concentrations of inflammatory mediators in our samples were higher and the composition of the nasal secretions collected by the absorption technique reflects the state of inflammation in the nasal mucosa far more faithfully. In this way, a positive correlation between the concentrations of examined mediators and the intensity of nasal symptoms could be explained.
5 CONCLUSIONS
Our results suggest that HSP70, ECP, tryptase, and substance P might play a role in the pathogenesis of NP, as well as a possibility that those mediators can be used as biomarkers that reflect the disease symptom severity. The present findings suggest that chronic mucosal inflammation in NP involves a self-sustaining local release of HSP70, ECP, and tryptase, independent of aeroallergen stimulation of the mucosal layer, although the production of these mediators is higher in NP patients who are sensitized to aeroallergens. Positive correlations between concentrations of HSP70, ECP, tryptase and substance P, and negative correlation between CC16 and other four mediators in nasal secretions indicate the intense and orchestrated activity of proinflammatory and anti-inflammatory mediators, in conjunction with neurogenic inflammation in pathogenesis of chronic inflammatory process in NP.
ACKNOWLEDGMENTS
This investigation was conducted as a part of scientific project of the Faculty of Medicine of the Military Medical Academy, Belgrade, Serbia (MFVMA02/19–21/).
CONFLICT OF INTEREST
The authors declared no conflicts of interest.




