Congenital aural atresia and first branchial cleft anomalies: Cholesteatoma and surgical management
Abstract
Objectives
To describe the prevalence and significance of first branchial cleft anomalies in children with congenital aural atresia.
Methods
Retrospective cohort study and case series. Patients were included if they had ICD-10 code Q16.0, Q16.1, Q16.9, Q17.2, or Q17.9 in their medical record and were seen at UCSF Benioff Children's Hospital from 2012 to 2020 for aural atresia. Children were categorized as having aural atresia and first branchial cleft anomalies if the presence of a first branchial cleft anomaly was noted in otolaryngology provider notes; otherwise, they were categorized as aural atresia alone. Patients with aural atresia and first branchial cleft anomalies were included in the case series.
Results
Among 125 children with congenital aural atresia, 5 (4%) were identified with first branchial cleft anomalies. In all cases, an epithelialized tract was noted to originate from the inferior aspect of the middle ear cleft, exiting the temporal bone in an inferomedial position adjacent to the stylomastoid foramen, with a cutaneous exit point inferior to the expected location of the native ear canal. There was no association with sex, microtia grade, or laterality of atresia; however, children with aural atresia and first branchial cleft anomalies were significantly more likely to have syndromes such as Goldenhar and Treacher Collins (p = .04) than those with aural atresia alone.
3/5 (60%) children with aural atresia and first branchial cleft anomalies presented with cholesteatoma compared with 1/120 (0.8%) children with aural atresia alone, a significant difference (p < .001). All four children over the age of two have undergone surgical management. In two of these, excision of the branchial cleft anomaly could be combined with atresiaplasty, with normal hearing results in both cases.
Conclusions
Aural atresia can be associated with comorbid anomalies of the head and neck. First branchial cleft anomalies can be suspected based on characteristic clinical appearance and confirmed with computed tomography showing a typical course through the temporal bone. When present in the context of congenital aural atresia, first branchial cleft anomalies are associated with a significantly increased risk for cholesteatoma, often necessitating surgical management with favorable hearing outcomes.
Level of evidence
4.
1 INTRODUCTION
Aural atresia, branchial cleft anomalies, and cholesteatoma are common conditions managed by otolaryngologists. Embryologically, there are three essential steps to the proper formation of the outer ear, involving the first and second branchial arches and the first branchial cleft: elongation, obliteration, epithelial plug formation, and recanalization.1 Elongation takes place at 4 weeks and is when the first branchial cleft elongates to form the dorsal and ventral clefts, with the dorsal cleft elongating toward the tubotympanic recess (first pouch); at the same time, the first and second arches form hillocks superior and inferior to the cleft, thereby creating the pinna. Obliteration takes place at 6 weeks; here, the ventral cleft is obliterated. At around 12 weeks, the dorsal cleft is replaced by a meatal plug made up of epithelial cells. At 28 weeks, the formation of a patent external auditory canal (EAC) is complete.
Failure at one or more steps of this process creates anomalous outer ears. If the first branchial cleft fails to elongate, then there is no EAC (aplasia). If the ventral cleft fails to obliterate, then there can be either duplication of the EAC or duplication and atresia (if there is a subsequent failure to recanalize). If the EAC fails to recanalize, then there is atresia or stenosis of the EAC.
Aural atresia describes the absence of a patent EAC. The condition can be acquired due to inflammation from external ear trauma or surgery, or it can be congenital. The incidence of congenital aural atresia is between 1:10,000 and 1:20,000,2, 3 is found more often in the right ear than the left, and occurs more often in males. Congenital aural atresia can be classified into stenosis, partial atresia, and total atresia.4 Stenosis is when the fibrocartilaginous or bony parts of the EAC are narrowed, whereas the tympanic membrane and middle ear are normal or have slight deformities; partial atresia is when only some parts of the EAC are present, there is a bony atretic plate, and the tympanic membrane and/or middle ear is underdeveloped; total atresia indicates that the fibrocartilaginous and bony parts of the EAC are completely absent, there is a bony atretic plate, the tympanic membrane is absent, and the middle ear is underdeveloped. Aural atresia is commonly associated with microtia, a smaller and/or malformed outer ear. Microtia is classified from Grade I (slightly smaller ear with essentially normal features) to Grade IV (anotia).4
Branchial cleft anomalies are some of the most common etiologies for congenital neck masses and are due to incomplete involution of the branchial clefts.5 Depending on the degree of incomplete involution, they can present as fistulas (epithelial lining with two openings), cysts (epithelial lining with no external opening), sinus tracts (epithelial lining with one opening), or cartilaginous remnants. There are four clefts on each side; the most common branchial cleft anomalies arise from the second branchial cleft. In this study, we focused on first branchial cleft anomalies. First branchial cleft anomalies make up 5%–25% of all branchial cleft anomalies and are commonly categorized into Work types I and II.5, 6 A Work type I consists of ectodermal tissue and is a duplication of the EAC; Work type II anomalies consist of ectodermal and mesodermal tissue and are duplications of the EAC and pinna. Classically, these first branchial cleft anomalies are present at the angle of the mandibule or in the submandibular region, and end in or near the EAC, generally at the inferior aspect of the middle ear.5 The course of the tract is often closely associated with the facial nerve and can pass medially, laterally, deep, or superficial to it.5, 7, 8
Cholesteatomas are keratinized epidermal inclusion cysts of the middle ear, mastoid, or ear canal, which are classically present as a round, pearly mass behind the tympanic membrane or as an accumulation of keratin debris with associated bony erosion.9 Cholesteatomas can be classified as congenital or acquired, the latter being more common. Risk factors for acquired cholesteatoma include recurrent acute otitis media, chronic middle ear effusion, and cleft palate. The exact mechanism for acquired cholesteatoma remains to be elucidated, but one hypothesis is that excessive negative middle ear pressure results in the development of retraction pockets in the tympanic membrane, which eventually traps epithelium and debris, resulting in the formation of a cholesteatoma. Although most cholesteatomas arise in the middle ear and mastoid, they can also occur in the ear canal, involving the bony EAC lateral to the tympanic membrane.10
There is limited understanding of how congenital aural atresia, first branchial cleft anomalies, and cholesteatoma interact with each other. The incidence of cholesteatoma has been reported in 2%–8% of children with aural atresia and microtia, with an increased risk for patients with congenital EAC stenosis.11, 12 Dermoid or epidermoid cysts of the head and neck are, embryologically, related to congenital cholesteatoma and branchial cleft anomalies as a failure of involution of epithelialized tracts. As of writing, we only found four studies describing children with all three conditions1, 13-15; all of them were case reports.
We sought to add to the literature by describing the prevalence and clinical significance of first branchial cleft anomalies in children with congenital aural atresia. We also sought to understand how the presence of a first branchial cleft anomaly for children with congenital aural atresia would affect their management.
2 METHODS
We performed a retrospective cohort study of children with aural atresia and first branchial cleft anomalies compared to aural atresia alone. We also present details on the case series of the children with aural atresia and first branchial cleft anomalies.
A retrospective chart review was performed on an existing database of children identified by ICD-10 search (Q16.0, congenital absence of ear (auricle); Q16.1, congenital absence, atresia, and stricture of auditory canal (external); Q16.9, congenital malformation of the ear causing impairment of hearing, unspecified; Q17.2, microtia; and Q17.9, congenital malformation of ear, unspecified) in their medical record and who were seen at UCSF Benioff Children's Hospital from 2012 to 2020 for aural atresia. Charts were manually reviewed to confirm the diagnosis of aural atresia.
For the retrospective cohort study, children were categorized as having aural atresia and first branchial cleft anomalies if the presence of a first branchial cleft anomaly was noted in otolaryngology provider notes; otherwise, they were categorized as having aural atresia alone. Age, sex, atresia laterality, type, comorbidities, microtia grade, presence of first branchial cleft anomaly, and presence of cholesteatoma were collected manually through chart abstraction. Because of the small sample size, rates of cholesteatoma in children with aural atresia, with or without associated first branchial cleft anomaly, were performed using Fisher's exact test.
For the case series, clinical and operative details were extracted from medical records of children with aural atresia and first branchial cleft anomalies. Details are reported below.
This study was approved by the Institutional Review Board at the University of California, San Francisco, CA.
3 RESULTS
125 children with aural atresia were included. We identified five subjects with an associated first branchial cleft anomaly.
Among the 125 children, 31 had partial atresia, and 97 had complete atresia. Three of the subjects had bilateral atresia, with partial on one side and complete on the other. None of the subjects had EAC stenosis.
Of the 31 children with partial atresia, 2 had concurrent first branchial cleft anomalies (cases 2 and 4). None of the 31 children with partial atresia had cholesteatoma, including the 2 with concurrent first branchial cleft anomalies. Of the 97 children with complete atresia, 4 had cholesteatoma: 1 out of the 94 without concurrent first branchial cleft anomalies, and all three of the children with concurrent first branchial cleft anomalies.
Among children with aural atresia and first branchial cleft anomalies (n = 5), compared with children with aural atresia alone (n = 120), there was no significant difference in sex (p = 1.00), microtia grade (Grade 0: p = .08; Grade 1: p = .43; Grade 2: p = .58; Grade 3: p = .66), or laterality of atresia (p = .50). However, children with aural atresia and first branchial cleft anomalies were significantly more likely to have syndromes such as Goldenhar and Treacher Collins (p = .04). See Table 1 for more details.
| Children with aural atresia and first branchial cleft anomalies (n = 5), # (%) | Children with aural atresia alone (n = 120), # (%) | Statistical significance | |
|---|---|---|---|
| Female sex | 2 (40) | 53 (44) | p = 1.00 |
| Craniofacial syndrome | 3 (60) | 20 (17) | p = .04 |
| Comorbidities | 3 (60) | 51 (44) | p = .65 |
| Grade 0 microtia | 1 (20) | 1 (0.8) | p = .08 |
| Grade 1 microtia | 1 (20) | 12 (10) | p = .43 |
| Grade 2 microtia | 0 (0) | 26 (22) | p = .58 |
| Grade 3 microtia | 3 (60) | 81 (68) | p = .66 |
| Unilateral atresia | 4 (80) | 105 (88) | p = .50 |
| Partial atresia | 2 (40) | 29 (24) | p = .60 |
| Complete atresia | 3 (60) | 94 (78) | p = .31 |
| Cholesteatoma | 3 (60) | 1 (0.8) | p < .001 |
Rates of cholesteatoma formation were not associated with craniofacial syndromes (p = .15), Grade 3 microtia (p = 1.00), or laterality of the atresia (p = .43). 3/5 (60%) children with aural atresia and first branchial cleft anomalies presented with cholesteatoma compared with 1/120 (0.8%) children with aural atresia alone, a significant difference (p < .001; Table 1).
Case details are as follows.
3.1 Case 1
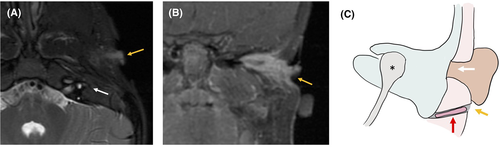
A 10-year-old boy, LD, with Goldenhar syndrome, presented with right microtia and aural atresia with maximal conductive hearing loss, having undergone first-stage microtia repair at an outside institution. He had an infra-auricular blind-sac sinus tract just superior to the angle of the mandible with no history of infection or drainage. A CT scan revealed a severely contracted middle-ear space with soft-tissue and bony atresia in the normal location of the EAC, with a bony tract exiting the inferior aspect of the middle ear toward the externally visible sinus in a path characteristic of a first branchial cleft anomaly. There was a bony expansion characteristic of cholesteatoma, which was confirmed with diffusion-weighted MRI (DW–MRI) (Figure 1). The patient was taken to the operating room, where the temporal bone was approached via a postauricular incision. A typical dimple in the bone was seen at the expected location of the EAC, as is typical in cases of aural atresia. Anteroinferior to this was the expanded bone of the first branchial cleft tract, with cholesteatoma. The tract was confirmed to be continuous with the external pit and was excised in its entirety. The facial nerve was just posterior to this tract and was preserved. As the middle-ear space was extremely contracted, ear canal reconstruction was not possible, and a bone-anchored hearing aid was placed. Subsequent surveillance imaging revealed no evidence of recurrent cholesteatoma after 5 years of follow-up.
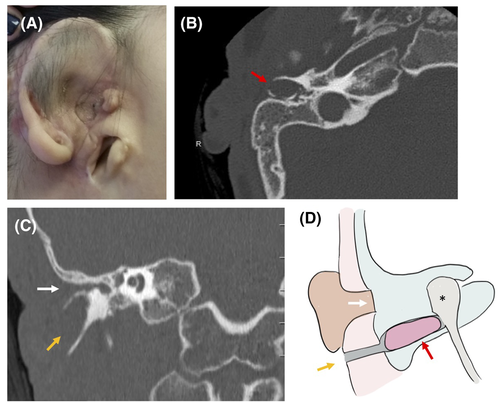
3.2 Case 2
A 10-year-old girl, KV, also with Goldenhar syndrome, presented with left microtia and partial aural atresia with maximal conductive hearing loss, having undergone first-stage microtia repair at an outside institution. Her ear canal was markedly stenotic. A CT scan revealed stenosis of the EAC and small tympanic membrane with fixation of the malleus on the medial bony canal, consistent with partial aural atresia. Also found was an ear canal duplication, posterior to the actual canal, which exited the temporal bone inferiorly in a path characteristic of a first branchial cleft anomaly. DW-MRI showed no cholesteatoma associated with this tract, and no external pit was noted (Figure 2). The patient was taken to the operating room, where the partially atretic native EAC was opened into the EAC duplication to create a widened EAC. Lateral graft tympanoplasty was performed on the native ossicular chain, with a scalp split-thickness skin graft. The postoperative hearing was in the normal range (PTA0.5–2 kHz: 17 dB HL) at 2 year follow up.
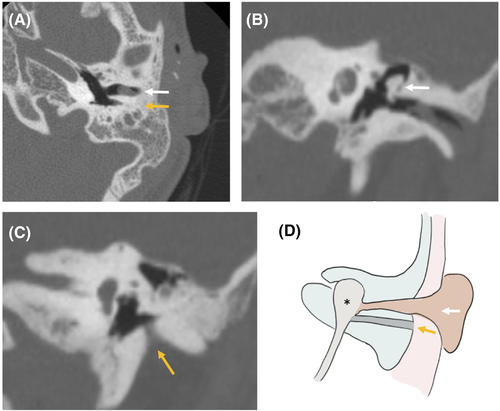
3.3 Case 3
A four-week-old boy, JR, presented with right-sided aural atresia and a normal pinna, with a blind sac at the normal EAC and an additional pit inferior to the tragus. He had right-sided maximal conductive hearing loss, which was managed with a softband BAHA. At 22 months of age, he presented with otalgia and otorrhea and was found to have an abscess inferior to the pinna, consistent with an infected branchial cleft cyst (Figure 3). He underwent incision and drainage with antibiotic management. Two months later, he underwent definitive excision. He was found to have a blind-sac EAC terminating in a bony EAC atresia. Inferior to the normally-placed canal, the external pit continued to a duplicated cartilaginous EAC, from which the prior abscess had originated. A well-encapsulated canal cholesteatoma lateral to the middle ear was noted and excised. This first branchial cleft anomaly terminated in the glenoid capsule and was excised in its entirety, together with the surrounding healed inflammatory tissue. We then completed the bony atresiaplasty and identified the ossicles. A lateral graft tympanoplasty onto the native ossicular mass was performed, and the neo-EAC was lined with a split-thickness skin graft from the scalp. The postoperative hearing was in the normal-borderline range (PTA0.5–2 kHz: 20 dB HL) at 1-year follow-up.
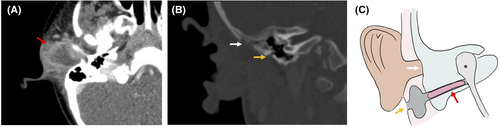
3.4 Case 4
A three-month-old girl, MV, presented with left-sided aural atresia and normal pinna, with a blind sac at the normal EAC and an additional pit inferior to the tragus. She had left-sided maximal conductive hearing loss, which was managed with a softband BAHA. A CT scan was performed that demonstrated a similar configuration to Case 3—absent native EAC but a tract emanating from the inferior middle ear with soft tissue, oriented inferiorly toward the known external ear pit (Figure 4). The family has opted for monitoring rather than excision, and at 21 months of age, the patient has not experienced any drainage or infection at the site.
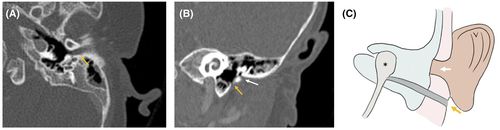
3.5 Case 5
A one-day-old boy, KS, presented with bilateral microtia and atresia, and was found to have Treacher Collins syndrome with severe midface hypoplasia and micrognathia managed with tracheotomy. He had bilateral maximal conductive hearing loss, which was managed with bilateral softband BAHA. At age two, he presented with a left facial/parotid abscess posteroinferior to a left facial pit at the angle of the mandible. This pit was distinct from a blind sac that he had at the site of his native EAC and microtic remnant (Figure 5). He was taken to the operating room for management and definitive excision and found to have a sinus tract extending from the left facial pit, through the parotid and terminating at the infratemporal skull base. The facial nerve was preserved. A pathology report of the excised sinus tract demonstrated keratinized squamous cell epithelium with associated chronic inflammation and fibrosis. No recurrence was noted at a 3-year follow-up.
4 DISCUSSION
In summary, we focused on the five cases of patients with congenital aural atresia who were found to have concurrent first branchial cleft anomalies, indicating failures at the obliteration and recanalization steps at an embryological level. Three of these children had syndromes associated with aural atresia (2 Goldenhar syndromes, 1 Treacher Collins). Three of five presented with infection and cholesteatoma originating from the first branchial cleft anomaly. To our knowledge, this is one of the first reports of cholesteatoma rates in aural atresia due to branchial cleft anomalies. Based on our retrospective chart review and case–control study, we demonstrated that children with congenital aural atresia and first branchial cleft anomalies are significantly more likely to present with cholesteatoma than those with aural atresia alone (p < .001).
The determination of whether the anomalous tract was a first branchial cleft anomaly was based on the following: if the patient had an atretic EAC as well as a second tract, then the second tract was interpreted as a first branchial cleft anomaly; if the EAC was absent, then the tract was determined to be a first branchial cleft anomaly if it followed the characteristic pathway of a first branchial cleft anomaly; that is, (1) the tract was present at the angle of the mandible and/or (2) the tract entered the temporal bone at the inferior aspect of the middle ear (hypotympanum).
We compiled a table summarizing the known cases of patients with congenital aural atresia and branchial cleft anomalies (Table S1). Of the 6 previously reported cases, 1/6 had syndromic aural atresia (Treacher Collins) and 3/6 prior cases had cholesteatoma, which is consistent with our findings. 5/6 were managed surgically, with the remaining case opting for conservative management due to multiple craniofacial abnormalities. Only one of the cases developed a subsequent recurrence or infection.
The increased risk of cholesteatoma formation for patients with aural atresia and first branchial cleft anomalies is likely due to the fact that the aural atresia prevents desquamated keratin debris from leaving the outer ear. In addition, cholesteatoma in aural atresia is more likely to be diagnosed at a later stage. Therefore, it is important that clinicians have a high index of suspicion for cholesteatoma in patients with aural atresia and branchial cleft anomalies as they may present differently. Of note, one patient, LD, was asymptomatic at the time of diagnosis; he was diagnosed based on imaging alone.
Imaging for each patient was decided on a case-by-case basis. The standard of care at our institution is to obtain a CT at or after age 5 if the family is considering surgical repair of aural atresia, as children can complete the scan without anesthesia; they would receive a scan before age 5 if they are symptomatic, usually with drainage, erythema, or pain. We would consider a DW-MRI at age 4–5 years, when the child is able to have one without anesthesia, or sooner if they begin to experience any symptoms. Patients LD and KV received a CT without anesthesia based on this standard of care; patients JR and KS presented with infections and received imaging to optimize management; patient MV received a CT scan based on our experience with JR, as the appearance of the external ear on the physical exam looked similar.
DW-MRI and CT are both imaging modalities that are used to detect cholesteatoma. CT imaging is preferable for bony anatomy, which allows for visualization of the expansion of bone in cholesteatoma. Recent advances in DW-MRI have increased the sensitivity and specificity of DW-MRI in detecting residual or recurrent cholesteatomas down to 3 mm.16 Children with Grade III microtia, partial atresia, or complete atresia alone without branchial cleft anomalies have been shown to have low rates of cholesteatoma; for these children, imaging is typically considered to evaluate for surgical repair of the aural atresia.
This study had limitations. Due to its small sample size, the study may be underpowered for certain analyses. For example, in our study, we did not find an association between cholesteatoma formation and craniofacial syndromes; however, previous studies have found that craniofacial syndromes are a risk factor for cholesteatoma.
5 CONCLUSION
Here, we present the co-occurrence of aural atresia and first branchial cleft anomalies. We also demonstrate an increased risk for cholesteatoma among patients with both congenital aural atresia and first branchial cleft anomalies, which often necessitates surgical intervention. Radiological imaging is helpful in identifying cholesteatoma in these patients.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.




