Comparison between botulinum toxin and steroid septal injection in the treatment of allergic rhinitis
Institution where the work was done: Taipei City Hospital, Taipei, Taiwan.
Funding information: Taipei City Hospital
Abstract
Objective
To compare the effects of botulinum toxin and steroid septal injections in treating allergic rhinitis (AR) by evaluating improvements in the rhinitis control assessment test (RCAT), visual analog scale (VAS), nasal obstruction symptom evaluation (NOSE) scores, and active anterior rhinomanometry (RMM) measurements.
Methods
This prospective, single-blinded cohort study was conducted at the Department of Otolaryngology, Taipei City Hospital between January 2017 and December 2018. Ninety-five patients were randomized to receive botulinum toxin, dexamethasone, or normal saline (group A, group B, and placebo, respectively). The main outcome measures were pretreatment subjective nasal symptoms (RCAT, VAS, and NOSE) and active anterior RMM measurements. All measurements were repeated during posttreatment 1, 2, and 3 months.
Results
No significant difference was observed in pretreatment questionnaire scores and RMM values between the study and placebo groups. The mean posttreatment RCAT, VAS, and NOSE scores after 1 and 2 months significantly improved in the treatment groups compared to placebo. The VAS and NOSE at posttreatment 2 months and RCAT, VAS, and NOSE at posttreatment 3 months were significantly different comparing group A to group B. All RMM parameters showed better values in group A than in group B at 1, 2, and 3 months posttreatment, with significant differences in four parameters in posttreatment 3 months.
Conclusions
Botulinum toxin septal injection is a safe treatment option for AR and improves subjective nasal symptoms for 3 months. Botulinum toxin A injection tended to be more effective than steroid septal injection in terms of duration and degree.
Level of Evidence: 2b, individual cohort study.
1 INTRODUCTION
The incidence of allergic rhinitis (AR) in Taiwan has been increasing yearly, and has become a major health concern. Studies have shown that the prevalence of AR in Taiwan is approximately 26.3%.1 The patency of the nasal airway is regulated by the autonomic nervous system. Sympathetic activity decreases nasal airway resistance by constricting the nasal capacitance vessels and venous sinusoids, and parasympathetic activity produces nasal mucus from the submucosal seromucinous gland.2 Nasal symptoms in AR are caused by the activation of mast cells by immunoglobulin E (IgE) and the release of inflammatory substances after exposure to allergens, which alters the balance of the autonomic nervous system, resulting in nasal congestion, itchy nose, runny nose, and sneezing. At present, oral antihistamines, antihistamine nasal sprays, oral steroids, steroid nasal sprays, antileukotrienes, and oral decongestants are used for the treatment of AR.3 Besides, botulinum toxin (BTX) and steroid septal injections are alternative treatment options that can improve the symptoms of AR.
BTX is a neurotoxic protein produced by various strains of the spore-forming, obligate anaerobic bacterium, Clostridium botulinum. This exotoxin is divided into types A–G, depending on its immunological specificity.4 The toxic dose of BTX in the human body is about 2500–3000 units.5 Nevertheless, many strategies have been adopted to ensure a safe dose of BTX is used in AR or intrinsic rhinitis. The most recent study recommended a minimal effective dose of 30 units of BTX at the bilateral inferior turbinates.6 This neurotoxin inhibits the release of acetylcholine from preganglionic neurons of the sphenopalatine ganglion and inactivates peripheral cholinergic nerve terminals by blocking the release of acetylcholine.7-9 In addition, it can block vasoactive intestinal polypeptide (a potent vasodilator)10 and cause apoptosis of nasal gland tissue,11 possibly yielding an additional effect on relieving nasal symptoms, such as nasal congestion and nasal discharge. BTX has also been used for the treatment of otolaryngological conditions, such as Frey syndrome, spastic dysphonia, voice tremor, oromandibular dystonia, cervical dystonia, strabismus, and other facial movement disorders.12
This study focused on comparing the effects of BTX-A and steroid (dexamethasone) septal injections in the treatment of AR by performing subjective evaluations of the rhinitis control assessment test (RCAT), visual analog scale (VAS), and nasal obstruction symptom evaluation (NOSE) scores as well as active anterior rhinomanometry (RMM) of the nasal airway at 1, 2, and 3 months after treatment.
2 MATERIALS AND METHODS
2.1 Patients
This prospective, randomized controlled, single-blinded cohort study was conducted in the Department of Otolaryngology at Taipei City Hospital between January 2017 and December 2018 and included 95 patients who were randomized to receive either BTX-A (group A; n = 34), dexamethasone (group B; n = 31), or normal saline (placebo group; n = 30). The diagnostic criteria of AR were based on the clinical guidelines of the American Academy of Otolaryngology-Head and Neck Surgery.13 According to the medical history and physical examination results, AR was diagnosed in patients who had more than one symptom of nasal congestion, runny nose, nasal itching, or sneezing, and more than one sign of clear rhinorrhea, turbinate swelling, pale discoloration of the nasal mucosa, and red and watery eyes, with an IgE index >100 IU/ml. Patients with infectious sinus diseases, nasal polyps or polyposis, prior rhinologic surgery, nasal valve collapse, pregnancy, steroid use, previous head and neck cancer, or any significant systemic disease (asthma, chronic obstructive pulmonary disease, diabetes, or others) were excluded from the study. The patients were randomly assigned to receive either BTX-A, dexamethasone, or normal saline septal injection, which was performed by the same senior otorhinolaryngologist.
2.2 Septal injection
The study and placebo groups received local intranasal anesthesia with 10% lidocaine spray 10 min before the treatment at the clinic. In study group A, 25 U (0.625 ml) BTX-A (Botox, Allergan Inc., Irvine, CA; 2.5 ml = 100 U) was submucosally injected into the nasal septum on each side using a 30-gauge needle. In study group B and the placebo group, 1 ml of dexamethasone (5 mg/ml) and 1 ml of normal saline were submucosally injected into the nasal septum on each side, respectively. Patients were observed for 10 min for any bleeding or discomfort before leaving the clinic. The entire process of nasal septal injection and questionnaire completion lasted for approximately 30 min.
2.3 Subjective evaluation of nasal obstruction symptoms
The RCAT, VAS, and NOSE scores were used to assess AR symptoms. The RCAT is a subjective assessment of the severity of AR containing six questions on (1) nasal congestion, (2) sneezing, and (3) watery eyes, (4) the extent to which nasal or other allergy symptoms interfere with sleep, (5) how often did you avoid any activities because of your nasal or other allergy symptoms, and (6) how well were your nasal or other allergy symptoms controlled during the past week. All six items were scored using a 5-point Likert scale (1 = extremely often; 2 = often; 3 = sometimes; 4 = rarely; and 5 = never). The VAS comprised a 10-cm line with the extremes “no symptoms of allergic rhinitis” (0 cm) and “severe symptoms of allergic rhinitis” (10 cm). The NOSE, a standardized quality-of-life questionnaire, contains five items: (1) nasal congestion or stuffiness, (2) nasal blockage or obstruction, (3) trouble breathing through the nose, (4) trouble sleeping, and (5) unable to receive sufficient air through the nose during exercise.8, 9 All five items were scored using a 5-point Likert scale (0 = not a problem; 1 = a very mild problem; 2 = a moderate problem; 3 = a fairly bad problem; and 4 = a severe problem).
2.4 Active anterior RMM
Active anterior RMM (Rhinomanometer NR6; GM Instruments Ltd, Scotland, UK) is a dynamic and objective assessment of nasal airflow, pressure, and resistance that is performed by measuring the pressure difference between the nasal entrance and the choanae.14 For RMM, the open nostril is used for measuring nasal airflow, while the other is closed with a pressure probe.15 In our study, six dependent variables were assessed: (i) flow on the narrow side (NF), (ii) flow on the wide side (WF), (iii) total flow (TF), (iv) nasal resistance of the narrow side (NNR), (v) nasal resistance of the wide side (WNR), and (vi) total nasal airway resistance (TNR). The variables were recorded at a pressure of 150 Pa in each nasal cavity. Following RMM, the total nasal airflow resistance was calculated on the basis of Ohm's law to determine the nasal resistance in both nasal cavities. A total nasal airflow resistance of 0.3 Pa/ml/s was defined as the upper limit of normal nasal airflow resistance.16
All measurements (RCAT, VAS, NOSE, and RMM) were performed in both the study and placebo groups before treatment and during the three posttreatment visits (at 1, 2, and 3 months).
2.5 Ethical considerations
All patients provided informed written consent to participate prior to enrollment in the study. The study was approved by the Institutional Review Board of Taipei City Hospital, Taipei, Taiwan (TCHIRB-10410116). The RCAT, VAS, and NOSE questionnaires and RMM did not pose any hazard to the patients.
2.6 Statistical analysis
The data were analyzed using SAS version 9.4 software (SAS Institute Inc., Cary, NC) on a personal computer. The Kruskal–Wallis test was used to test the differences in RMM measurements and RCAT, VAS, and NOSE scores in the study and placebo groups. If Kruskal–Wallis test showed significance (p <.05), Dunn's test was performed for post-hoc analysis (Table 1). The Wilcoxon signed-rank test was used to test the pretreatment and posttreatment results in the study and placebo groups (Tables 2 and 3). The Pearson correlation for nonparametric data was used to determine the degree of association between two numerical variables, that is, the RMM data and the subjective sensation of nasal patency (RCAT, VAS, and NOSE scores; Table 4). A p-value <.05 was considered statistically significant.
| Subjective and objective measurement | Placebo | Botulinum toxin type A | Dexamethasone | p-valuea | Post-hoc test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 30) | (n = 34) | (n = 31) | |||||||||
| Mean ± SD | (Min.–max.) | Mean ± SD | (Min.–max.) | Mean ± SD | (Min.–max.) | P versus B | P versus D | B versus D | |||
| Sex (n, %) | Female | 14 | 46.67% | 23 | 67.65% | 17 | 54.84% | ||||
| Male | 16 | 53.33% | 11 | 32.35% | 14 | 45.16% | |||||
| Age | 38.50 ± 13.17 | (21–66) | 37.29 ± 16.01 | (20–70) | 38.52 ± 12.55 | (21–66) | .64 | ||||
| Subjective | |||||||||||
| Pretreatment | RCAT | 14.17 ± 3.06 | (8–23) | 14.94 ± 4.48 | (7–27) | 15.32 ± 3.05 | (9–24) | .42 | — | — | — |
| VAS | 7.57 ± 1.30 | (4–10) | 7.09 ± 1.68 | (3–10) | 7.61 ± 1.15 | (5–10) | .35 | — | — | — | |
| NOSE | 12.47 ± 3.88 | (4–20) | 11.59 ± 4.26 | (2–20) | 13.16 ± 3.27 | (4–20) | .18 | — | — | — | |
| Posttreatment 1 month | RCAT | 15.77 ± 3.29 | (7–21) | 22.68 ± 3.47 | (13–27) | 23.94 ± 3.58 | (15–30) | <.0001 | # | # | — |
| VAS | 7.37 ± 1.13 | (5–10) | 2.91 ± 1.78 | (0–8) | 2.94 ± 1.61 | (0–8) | <.0001 | # | # | — | |
| NOSE | 12.60 ± 3.68 | (5–20) | 5.35 ± 3.26 | (0–14) | 4.97 ± 3.54 | (0–15) | <.0001 | # | # | — | |
| Posttreatment 2 months | RCAT | 15.23 ± 2.94 | (10–22) | 22.15 ± 2.96 | (16–28) | 20.48 ± 3.91 | (12–28) | <.0001 | # | # | — |
| VAS | 7.03 ± 1.10 | (5–9) | 3.03 ± 1.60 | (1–8) | 5.29 ± 1.95 | (1–8) | <.0001 | # | # | # | |
| NOSE | 12.57 ± 3.06 | (7–19) | 5.09 ± 3.15 | (0–15) | 8.29 ± 4.31 | (0–18) | <.0001 | # | # | # | |
| Posttreatment 3 months | RCAT | 14.97 ± 2.85 | (9–20) | 23.15 ± 2.97 | (16–28) | 19.13 ± 4.14 | (10–28) | <.0001 | # | # | # |
| VAS | 7.13 ± 1.31 | (4–9) | 3.65 ± 1.59 | (1–8) | 5.97 ± 1.82 | (0–8) | <.0001 | # | — | # | |
| NOSE | 12.43 ± 3.22 | (6–18) | 5.82 ± 3.06 | (1–15) | 10.61 ± 4.66 | (1–20) | <.0001 | # | — | # | |
| Objective | |||||||||||
| Pretreatment | NF | 154.07 ± 65.88 | (54–311) | 163.00 ± 90.69 | (21–376) | 165.10 ± 85.42 | (29–341) | .89 | — | — | — |
| WF | 279.77 ± 128.86 | (107–647) | 351.79 ± 173.30 | (87–720) | 315.61 ± 185.62 | (57–821) | .27 | — | — | — | |
| TF | 433.83 ± 166.24 | (186–958) | 515.06 ± 223.54 | (136–917) | 480.45 ± 239.33 | (92–1040) | .27 | — | — | — | |
| NNR | 1.28 ± 0.70 | (0.502–3.291) | 1.46 ± 1.41 | (0.418–7.019) | 1.33 ± 1.11 | (0.44–5.214) | .50 | — | — | — | |
| WNR | 0.65 ± 0.28 | (0.233–1.416) | 0.57 ± 0.35 | (0.207–1.801) | 0.73 ± 0.60 | (0.188–2.632) | .21 | — | — | — | |
| TNR | 0.42 ± 0.17 | (0.159–0.862) | 0.39 ± 0.29 | (0.151–1.431) | 0.47 ± 0.38 | (0.16–1.633) | .09 | — | — | — | |
| Posttreatment 1 month | NF | 152.23 ± 79.10 | (44–451) | 271.53 ± 145.51 | (73–588) | 200.16 ± 114.65 | (38–652) | <.05 | # | — | — |
| WF | 317.33 ± 173.71 | (90–755) | 464.26 ± 198.14 | (134–830) | 362.48 ± 193.46 | (94–786) | <.05 | # | — | — | |
| TF | 469.57 ± 218.48 | (134–1106) | 735.79 ± 299.75 | (222–1412) | 562.65 ± 273.87 | (132–1425) | <.05 | # | — | # | |
| NNR | 1.23 ± 0.60 | (0.336–3.362) | 0.80 ± 0.54 | (0.255–2.068) | 1.05 ± 0.77 | (0.23–3.999) | <.05 | # | — | — | |
| WNR | 0.60 ± 0.28 | (0.169–1.524) | 0.42 ± 0.23 | (0.179–1.069) | 0.56 ± 0.34 | (0.186–1.604) | <.05 | # | — | — | |
| TNR | 0.39 ± 0.21 | (0.105–1.262) | 0.26 ± 0.15 | (0.106–0.698) | 0.36 ± 0.23 | (0.105–1.145) | <.05 | # | — | # | |
| Posttreatment 2 months | NF | 166.73 ± 80.55 | (54–399) | 274.29 ± 166.37 | (43–770) | 184.94 ± 99.87 | (63–530) | <.05 | # | — | — |
| WF | 295.07 ± 145.65 | (87–649) | 460.65 ± 195.79 | (44–809) | 356.42 ± 158.24 | (143–789) | <.05 | # | — | — | |
| TF | 462.00 ± 210.97 | (141–909) | 734.94 ± 340.15 | (87–1579) | 541.35 ± 241.00 | (218–1319) | <.05 | # | — | # | |
| NNR | 1.16 ± 0.61 | (0.374–2.845) | 0.91 ± 0.81 | (0.197–3.522) | 1.02 ± 0.60 | (0.261–2.38) | <.05 | # | — | — | |
| WNR | 0.65 ± 0.37 | (0.231–1.781) | 0.50 ± 0.62 | (0.191–3.442) | 0.51 ± 0.23 | (0.188–1.048) | <.05 | # | — | — | |
| TNR | 0.41 ± 0.21 | (0.156–1.123) | 0.32 ± 0.33 | (0.101–1.741) | 0.34 ± 0.16 | (0.113–0.689) | <.05 | # | — | — | |
| Posttreatment 3 months | NF | 175.77 ± 92.85 | (70–452) | 261.82 ± 149.13 | (30–677) | 193.32 ± 112.65 | (45–544) | <.05 | # | — | — |
| WF | 340.70 ± 146.05 | (118–771) | 455.97 ± 164.94 | (88–833) | 359.06 ± 229.02 | (88–890) | <.05 | # | — | # | |
| TF | 516.47 ± 226.60 | (205–1223) | 717.79 ± 286.77 | (118–1510) | 552.39 ± 304.10 | (136–1346) | <.05 | # | — | # | |
| NNR | 1.13 ± 0.51 | (0.334–2.331) | 0.92 ± 1.05 | (0.223–5.482) | 1.07 ± 0.71 | (0.277–3.355) | <.05 | # | — | — | |
| WNR | 0.53 ± 0.24 | (0.193–1.378) | 0.42 ± 0.33 | (0.18–1.81) | 0.63 ± 0.41 | (0.171–1.733) | <.05 | # | — | # | |
| TNR | 0.36 ± 0.16 | (0.125–0.772) | 0.28 ± 0.28 | (0.103–1.422) | 0.38 ± 0.25 | (0.109–1.152) | <.05 | # | — | # | |
- Note: Post-hoc test: Correction by Dunn's test. P: Placebo; B: BOTOX; D: Dexamethasone, #: there are statistically difference between these two group, —: no statistically difference.
- Abbreviations: NF, flow on the narrow side; NNR, nasal resistance of the narrow side; NOSE, nasal obstruction symptom evaluation; RCAT, rhinitis control assessment Test; TF, total flow; TNR, total nasal airway resistance; VAS, visual analogue scale; WF, flow on the wide side; WNR, nasal resistance of the wide side.
- a Kruskal–Wallis test.
| BTX-A | Pretreatment | Posttreatment 1 month versus pretreatment | p-valuea | Posttreatment 2 months versus pretreatment | p-valuea | Posttreatment 3 months versus pretreatment | p-valuea | |
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||||
| Subjective | ||||||||
| RCAT | 14.94 ± 4.48 | 22.68 ± 3.47 | <.0001 | 22.15 ± 2.96 | <.0001 | 23.15 ± 2.97 | <.0001 | |
| VAS | 7.09 ± 1.68 | 2.91 ± 1.78 | <.0001 | 3.03 ± 1.60 | <.0001 | 3.65 ± 1.59 | <.0001 | |
| NOSE | 11.59 ± 4.26 | 5.35 ± 3.26 | <.0001 | 5.09 ± 3.15 | <.0001 | 5.82 ± 3.06 | <.0001 | |
| Objective | ||||||||
| NF | 163.00 ± 90.69 | 271.53 ± 145.51 | <.0001 | 274.29 ± 166.37 | <.0001 | 261.82 ± 149.13 | <.0001 | |
| WF | 351.79 ± 173.30 | 464.26 ± 198.14 | <.0001 | 460.65 ± 195.79 | .0001 | 455.97 ± 164.94 | <.0001 | |
| TF | 515.06 ± 223.54 | 735.79 ± 299.75 | <.0001 | 734.94 ± 340.15 | <.0001 | 717.79 ± 286.77 | <.0001 | |
| NNR | 1.46 ± 1.41 | 0.80 ± 0.54 | <.0001 | 0.91 ± 0.81 | .0002 | 0.92 ± 1.05 | .0007 | |
| WNR | 0.57 ± 0.35 | 0.42 ± 0.23 | <.0001 | 0.50 ± 0.62 | .0103 | 0.42 ± 0.33 | <.0001 | |
| TNR | 0.39 ± 0.29 | 0.26 ± 0.15 | <.0001 | 0.32 ± 0.33 | .0052 | 0.28 ± 0.28 | <.0001 | |
- Abbreviations: NF, flow on the narrow side; NNR, nasal resistance of the narrow side; NOSE, nasal obstruction symptom evaluation; RCAT, rhinitis control assessment Test; TF, total flow; TNR, total nasal airway resistance; VAS, visual analogue scale; WF, flow on the wide side; WNR, nasal resistance of the wide side.
- a Wilcoxon signed-rank test.
| Dexamethasone | Pretreatment | Posttreatment 1 month versus pretreatment | p-valuea | Posttreatment 2 months versus pretreatment | p-valuea | Posttreatment 3 months versus pretreatment | p-valuea | |
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||||
| Subjective | ||||||||
| RCAT | 15.32 ± 3.05 | 23.94 ± 3.58 | <.0001 | 20.48 ± 3.91 | <.0001 | 19.13 ± 4.14 | <.0001 | |
| VAS | 7.61 ± 1.15 | 2.94 ± 1.61 | <.0001 | 5.29 ± 1.95 | <.0001 | 5.97 ± 1.82 | <.0001 | |
| NOSE | 13.16 ± 3.27 | 4.97 ± 3.54 | <.0001 | 8.29 ± 4.31 | <.0001 | 10.61 ± 4.66 | .0007 | |
| Objective | ||||||||
| NF | 165.1 ± 85.42 | 200.16 ± 114.65 | <.05 | 184.94 ± 99.87 | .27 | 193.32 ± 112.65 | .08 | |
| WF | 315.61 ± 185.62 | 362.48 ± 193.46 | <.05 | 356.42 ± 158.24 | .05 | 359.06 ± 229.02 | .10 | |
| TF | 480.45 ± 239.33 | 562.65 ± 273.87 | <.01 | 541.35 ± 241.00 | <.05 | 552.39 ± 304.1 | .06 | |
| NNR | 1.33 ± 1.11 | 1.05 ± 0.77 | <.05 | 1.02 ± 0.60 | <.05 | 1.07 ± 0.71 | .14 | |
| WNR | 0.73 ± 0.60 | 0.56 ± 0.34 | <.05 | 0.51 ± 0.23 | .0006 | 0.63 ± 0.41 | <.05 | |
| TNR | 0.47 ± 0.38 | 0.36 ± 0.23 | <.01 | 0.34 ± 0.16 | .0007 | 0.38 ± 0.25 | <.05 | |
- Abbreviations: NF, flow on the narrow side; NNR, nasal resistance of the narrow side; NOSE, nasal obstruction symptom evaluation; RCAT, rhinitis control assessment Test; TF, total flow; TNR, total nasal airway resistance; VAS, visual analogue scale; WF, flow on the wide side; WNR, nasal resistance of the wide side.
- a Wilcoxon signed-rank test.
| Correlation coefficient | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BTX-A (n = 34) | Pretreatment | Posttreatment 1 month | Posttreatment 2 months | Posttreatment 3 months | ||||||||
| p-value | RCAT | VAS | NOSE | RCAT | VAS | NOSE | RCAT | VAS | NOSE | RCAT | VAS | NOSE |
| RCAT | (N/A) | <.05 | <.0001 | (N/A) | <.05 | <.05 | (N/A) | .18 | <.05 | (N/A) | <.01 | <.05 |
| VAS | <.05 | (N/A) | <.01 | <.05 | (N/A) | <.0001 | .1765 | (N/A) | <.0001 | <.01 | (N/A) | <.0001 |
| NOSE | <.0001 | <.01 | (N/A) | <.05 | <.0001 | (N/A) | <.05 | <.0001 | (N/A) | <.05 | <.0001 | (N/A) |
| NF | .28 | .65 | .10 | .44 | .77 | .44 | .30 | .56 | .84 | .91 | .77 | .95 |
| WF | .09 | .34 | .05 | .49 | .50 | .57 | .88 | .19 | .89 | .40 | .73 | .32 |
| TF | .08 | .35 | <.05 | .93 | .76 | 1.00 | .68 | .30 | .86 | .59 | .96 | .59 |
| NNR | .18 | .24 | .10 | .42 | .48 | .16 | .42 | <.05 | .36 | .71 | .31 | .46 |
| WNR | .15 | .45 | .08 | .72 | .71 | 1.00 | .79 | .16 | .65 | .56 | .55 | .93 |
| TNR | <.05 | .19 | <.05 | .81 | .71 | .71 | .64 | .09 | .99 | .49 | .65 | .97 |
| Correlation coefficient | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dexamethasone (n = 31) | Pretreatment | Posttreatment 1 month | Posttreatment 2 months | Posttreatment 3 months | ||||||||
| p-value | RCAT | VAS | NOSE | RCAT | VAS | NOSE | RCAT | VAS | NOSE | RCAT | VAS | NOSE |
| RCAT | (N/A) | <.05 | .13 | (N/A) | <.01 | <.01 | (N/A) | <.0001 | <.0001 | (N/A) | <.0001 | <.0001 |
| VAS | <.05 | (N/A) | <.01 | <.01 | (N/A) | <.0001 | <.0001 | (N/A) | <.0001 | <.0001 | (N/A) | <.0001 |
| NOSE | .13 | <.01 | (N/A) | <.01 | <.0001 | (N/A) | <.0001 | <.0001 | (N/A) | <.0001 | <.0001 | (N/A) |
| NF | .55 | .72 | .85 | <.05 | <.01 | <.05 | .45 | .90 | .66 | .92 | .43 | .66 |
| WF | .90 | .20 | .62 | .14 | <.05 | .17 | .44 | .35 | .34 | .07 | .11 | .19 |
| TF | .91 | .26 | .64 | <.05 | <.01 | <.05 | .85 | .51 | .66 | .19 | .14 | .25 |
| NNR | .62 | .97 | .96 | .16 | .25 | .22 | .37 | .87 | .78 | .60 | .62 | .75 |
| WNR | .75 | .44 | .62 | .16 | .13 | .17 | .72 | .59 | .47 | .31 | .36 | .71 |
| TNR | .64 | .46 | .63 | .13 | .14 | .22 | .81 | .95 | .82 | .31 | .38 | .67 |
- Abbreviations: NF, flow on the narrow side; NNR, nasal resistance of the narrow side; NOSE, nasal obstruction symptom evaluation; RCAT, rhinitis control assessment Test; TF, total flow; TNR, total nasal airway resistance; VAS, visual analogue scale; WF, flow on the wide side; WNR, nasal resistance of the wide side.
- Note: p value <.05: including positive correlation or negative correlation in bold type.
3 RESULTS
A total of 102 patients with AR without systemic diseases were treated using BTX-A, dexamethasone, or normal saline nasal septal injection. Of these 102 patients, 7 (6.9%) did not complete the 3-month posttreatment follow-up. Therefore, data from only 95 patients were enrolled in the study. Study group A receiving BTX-A included 23 women and 11 men; their mean age was 37.29 ± 16.01 (range: 20–70) years. Study group B receiving dexamethasone included 17 women and 14 men; their mean age was 38.52 ± 12.55 (range: 21–66) years. The placebo group included 14 women and 16 men; their mean age was 38.50 ± 13.17 (range: 21–66) years. The groups showed no statistical differences in age and sex.
The study and placebo groups showed no differences in the medical treatment for AR before the current treatment. All patients used oral antihistamine or steroid nasal spray before treatment and stopped any medical treatment 2 weeks before the current treatment. No oral antihistamine or steroid nasal spray was used during the treatment and after the treatment.
3.1 Pretreatment and posttreatment evaluations
No significant difference was observed in the pretreatment RCAT, VAS, and NOSE scores between the study and placebo groups (Table 1). However, the mean posttreatment RCAT, VAS, and NOSE scores after 1, and 2 months had significantly improved in the study groups compared to the placebo group (p <.0001). The posttreatment scores for all three tests were significantly different between group A and the placebo group after 3 months. Only RCAT scores showed significant difference between group B and the placebo group after 3 months. No significant different in the scores for all three tests was observed between groups A and B after 1 month of treatment. VAS and NOSE scores were significantly different between groups A and B at 2 months posttreatment. The scores for all three tests were significantly different at the 3-month visit when groups A and B were compared.
The pretreatment RMM values showed no significant differences between the study and placebo groups (p >.05, Table 1). Nevertheless, all six posttreatment RMM values showed significant differences between group A and the placebo group at all time points (p <.05), but no significant difference between group B and placebo group. All six RMM parameters, showed better values in group A than in group B posttreatment at 1, 2, and 3 months, with significant differences for TF at all time points and WF, WNR, and TNR 3 months posttreatment (p <.05).
The subjective measurements of RCAT, VAS, and NOSE scores in the study groups showed significant improvements at 1, 2, and 3 months after treatment compared to before treatment (p <.05). In study group A (Table 2), a significant improvement was observed in all RMM measurements at 1, 2, and 3 months. In group B, significant improvement was observed in all RMM measurements at 1 month (Table 3), but the improvement was significant for TF, NNR, WNR, and TNR values at 2 months and for only WNR and TNR values at 3 months posttreatment. In group B, the efficacy of dexamethasone decreased over time.
3.2 Subjective and objective correlation
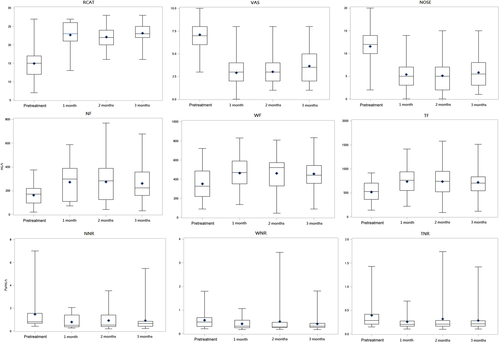
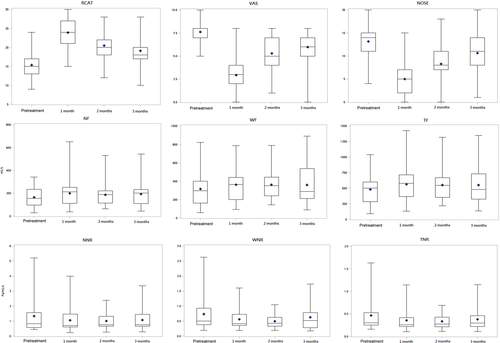
Table 4 shows the correlation results for the subjective measurements of RCAT, VAS, and NOSE and the objective measurement of RMM in study groups A and B. Significant correlations were found between the pretreatment RCAT, VAS, and NOSE scores and those measured at 3 months posttreatment (p <.05) in both the study groups, except for the correlation between posttreatment RCAT and VAS scores at 2 months in study group A (p >.05) and pretreatment RCAT and NOSE scores in study group B. Figures 1 and 2 show the box plot analysis of the values of RCAT/VAS/NOSE and RMM measurements in study groups A and B at pretreatment and posttreatment 1, 2, and 3 months respectively. The RCAT, VAS, and NOSE scores and RMM measurements showed an improving trend after treatment in groups A and B. The posttreatment RCAT, VAS, and NOSE scores did not significantly correlate with total nasal resistance in either study group (Table 4). However, a significant correlation was observed between RCAT, VAS, and NOSE scores and NF and TF values in study group B at 1-month posttreatment. No serious complications occurred in our study population. Only two patients in group B had dry mucosa and crust at the injection site on the nasal septum at 7 days posttreatment (bilateral in one patient and left-sided in the other), which appeared normal in both patients at the 1-month posttreatment follow-up.
4 DISCUSSION
In this study, we compared the effects of BTX and steroid septal injections in the treatment of AR, and our subjective and objective assessments revealed that both treatments produced significant improvements compared to placebo, BTX injection tended to be more effective than steroid septal injection in terms of duration and degree.
Kim et al.17 first used BTX-A to treat intrinsic rhinitis by injecting 2 units of BTX-A into each of the middle and inferior turbinate. Their results showed that the BTX-A group had a significant decrease in runny nose symptoms and the size of nasal tissue, with the effect lasting for 4 weeks. Subsequent studies investigated increasing doses of BTX-A to prolong its duration of action in treating AR. Unal et al.18 used BTX-A to treat AR by injecting a total of 40 or 60 units of BTX-A into the middle and inferior turbinates. In another study, patients received injections of 25 units of BTX-A into each inferior turbinate (total, 50 units).19 Braun et al.20 injected a total of 80 units of BTX-A (Dysport) into four regions of the nasal septal mucosa of patients with idiopathic rhinitis. A review of BTX-A in rhinitis by Zhong et al.,21 found that the total dosage 20–60 units of BTX-A could effectively improve the symptoms of runny nose, nasal congestion, sneezing, and itching for 8–12 weeks. We also observed that mean RCAT, VAS, and NOSE scores significantly improved for 12 weeks (3 months) posttreatment when compared to the corresponding scores in the placebo group as well as the pretreatment values in study groups A and B (Tables 1–3). All posttreatment RMM values at 1, 2, and 3 in the BTX group were better when compared with the values in group B and the placebo group, though the differences were only significant between the BTX group and the placebo group.
Yang et al.19 compared the effects of BTX-A and steroid injections in AR. In their study, patients received injections of either 25 units of BTX-A, 1 ml of triamcinolone (20 mg/ml), or 1 ml of isotonic saline into each inferior turbinate. Their results showed that BTX-A provided better symptom relief in terms of duration and degree than steroid injection. Similarly, our study showed that the posttreatment RCAT, VAS, and NOSE scores at 3 months were better in group A than in group B. Moreover, all six RMM parameters showed better values in group A than in group B at 1, 2, and 3 months after treatment. Therefore, BTX-A injection produced superior results to those of steroid injection when used for the treatment of AR symptoms.
The correlation between the subjective and objective outcomes of nasal patency remains a matter of debate.22, 23 Hsu et al.14 concluded that the subjective and objective measurements of nasal obstruction lacked significant correlation after treatment. In our study, the RCAT, VAS, and NOSE scores also did not significantly correlate with total nasal resistance in both the study groups after treatment.
In most previous studies, BTX-A was injected into either the inferior turbinate or middle turbinate, and the injection site was the anterior part of the nasal turbinate or the posterior lateral part of the nasal turbinate.21 Braun et al.20 first injected BTX-A into the nasal septal mucosa of patients with idiopathic rhinitis, and Mozafarinir et al. compared the effects of injecting BTX-A into the septum of patients who suffered from idiopathic rhinitis or AR with placebo.24 They found that BTX-A injected into the septum relieved symptoms lasting for 8–12 weeks compared to placebo. Based on their clinical experience, they suggested that submucoperichondrial injection of BTX-A into the nasal septum is an easy alternative for the therapist and is well tolerated by the patients.
The rate of adverse effects for septal injection is lower than that for inferior turbinate injection.25 The advantages of this approach are good visual control of injection by observing the typical bleaching effect of the mucosa and a lower risk than that associated with intravascular injection.26 The possible mechanism of decreasing the symptoms of AR by the injection of BTX-A into the nasal septum is that BTX-A inhibits the release of acetylcholine from preganglionic neurons of the sphenopalatine ganglion and peripheral cholinergic nerve endings affecting parasympathetic function of nasal mucosa. Furthermore, there are more serous glands in the nasal septum than in the nasal turbinate,27 which can lead to a decrease in secretion. Besides, the blood flow in the nasal septum was less than nasal turbinate, prolonging the effect of BTX-A.26
In view of the safety of BTX-A and steroid injections, previous studies have found that intranasal injection of steroids with small particles could reportedly cause vascular embolism and blindness, even though the probability of blindness is extremely low (0.003%–0.006%).28-30 However, intranasal injection of BTX-A entails no serious complications, such as dry eyes or systemic immune response.18, 19 In our study, two patients who received steroid septal injections developed dry mucosa and crust at the injection site of the nasal septum, whereas no complications were reported in patients who received BTX-A injections.
4.1 Study limitation
The main limitation of this study is its small sample size. However, considering the dearth of studies comparing the effects of nasal septal injection of BTX and steroids, our findings provide valuable information based on the subjective and objective evaluations of both methods of treatment in AR. Further studies with larger sample sizes and comparing different injection sites are nonetheless warranted.
5 CONCLUSION
Our novel investigation using both subjective tools and objective measurements to determine the efficiency of BTX-A and steroid injection in the nasal septum for the treatment of AR and found BTX-A injection is an easy, safe, and effective method, which improves subjective nasal symptoms for 3 months. Furthermore, compared to steroid septal injection, BTX-A injection is potentially more effective in terms of its duration and degree.
ACKNOWLEDGMENTS
The authors thank the Research Office for Health Data and the Department of Education and Research, Taipei City Hospital, Taiwan, for their contributions to data management and statistical analyses.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.




