Prognostic analysis and establishment of a nomogram in patients with myoepithelial carcinoma of the salivary gland: A population-based study
Ying Liu and Yong Mi contributed this work equally.
Abstract
Background
Currently, the clinicopathological characteristics and prognosis of myoepithelial carcinoma of salivary gland (MC-SG) have not been defined well. The present study aimed to describe the clinicopathological characteristics and prognosis of MC-SG patients.
Methods
The Surveillance, Epidemiology, and End Results database was searched for all patients diagnosed with MC-SG between 1991 and 2016. The Kaplan–Meier method and log-rank tests were used to evaluate the survival. Univariate and multivariate Cox regression analysis were used to identify prognostic biomarkers for overall survival (OS) and disease-specific survival (DSS). Furthermore, a prognostic nomogram was established, and its predictive accuracy and discriminative ability were determined using the concordance index (C-index).
Results
In total, 245 patients diagnosed with MC-SG were identified. The median OS was 152.0 months, with 3-, 5-, and 10-year survival rates of 79.8%, 69.2%, and 50.3%. The 3-, 5-, and 10-year DSS rates were 82.5%, 77.1%, and 61.9%, respectively. Regarding the treatment regimen, most patients (92.2%) underwent surgery, and 103 patients (42.4%) received postoperative radiotherapy. Surgery could significantly prolong OS and DSS (p < .05), but postoperative radiotherapy did not significantly prolong OS and DSS when compared with individuals receiving surgery alone (p > .05). Multivariate Cox analysis revealed that T category (T4), lymph node metastasis (N2), distant metastasis (M1), and poor differentiation were independent unfavorable prognostic factors for OS and DSS. Older age (>62 years) was also independently associated with OS. In addition, the C-index for the established OS- and DSS-specific nomogram was 0.80 (95% CI: 0.72–0.88) and 0.82 (95% CI: 0.73–0.90).
Conclusion
Age, tumor invasion, metastases, and pathological grade were independently associated with prognosis of MC-SG patients, and the prognostic nomogram of this rare disease was established.
1 INTRODUCTION
Myoepithelial carcinoma is a rare malignancy that usually occurs in the epithelium of the major salivary glands, which can also be observed in skin and soft tissues.1 A previous study reported that myoepithelial carcinoma accounted for only 0.2%–1% of all salivary gland tumors.2, 3 Although myoepithelial carcinoma was originally described in 1975, this disease was only categorized into malignant salivary gland tumors in 1991.4, 5 The tumor exhibits wide morphologic and cytologic diversity, similar to myoepithelioma, but with evidence of malignant change such as an infiltrative growth pattern, and angiolymphatic and perineural invasion.6 Thus, myoepithelial carcinoma often has a propensity for occasional regional lymph node invasion and distant metastases.7, 8 The symptoms of myoepithelial carcinoma usually manifest as an initial painless mass, which on occasion rapidly increases in size. Some investigators reported that myoepithelial carcinoma could start growing rapidly after remaining small for a certain period of time.6 Other symptoms vary depending on the site of formation including hoarseness, nasal obstruction, pain, and headaches. However, the clinicopathological characteristics and prognosis of myoepithelial carcinoma of the salivary gland (MC-SG) remain unclear because of its rarity.3, 7, 9 Previous studies demonstrated that the prognosis of myoepithelial carcinoma likely depended on various factors, therefore may be difficult to predict. Because of the limited number of follow-up studies on patients with MC-SG, the factors affecting survival have also not been well defined.
In this study, we performed a retrospective real-world study using the data of patients with MC-SG from the Surveillance, Epidemiology, and End Results (SEER) database. The study aimed to describe the clinicopathological characteristics of MC-SG, define its prognostic factors, and establish the prognostic nomogram for predicting overall survival (OS) and disease-specific survival (DSS).
2 METHODS AND MATERIALS
2.1 Data source and participants
This retrospective study was conducted by acquiring data from the SEER database. The database “SEER 18 Regs Custom Data with additional treatment fields, Nov 2018 Sub (1975-2016)” was searched for all patients with MC-SG (ICD-O-3 histology code 8982) between 1991 and 2016. SEER*STAT 8.3.6 software was used to isolate the information of each patient. Collected variables consisted of demographic information, clinicopathological factors, treatment, and prognosis such as race, sex, age, laterality, primary site, tumor differentiation grade, AJCC-TNM stage (AJCC 7th edition), SEER historic stage, surgery, and radiotherapy. The primary study endpoint was OS and DSS, which were defined as the time from the date of diagnosis to death from all cause (OS), and death specific to MC-SG (DSS), respectively. The present study was deemed exempt from review by the Institutional Review Board of Jinan Stomatological Hospital. All above data was accessed in compliance with the SEER Research Data user's agreement.
2.2 Statistical analysis
Continuous variables were summarized as mean ± standard deviation, whereas categorical variables were denoted as counts and percentages. Survival was evaluated using the Kaplan–Meier curve; log-rank tests were performed to assess the influence of each factor on OS and DSS. Cox proportional hazards model was used in multivariate analysis to determine potential independent prognostic factors. In addition, the prognostic nomograms for OS and DSS were constructed using the independent prognostic factors identified in multivariate Cox regression analysis. We used the concordance index (C-index) to assess the discrimination of the established nomograms, whereas calibration curves were analyzed to determine the difference between predicted and actual survival. All statistical analyses above were performed using R version 3.6.0 (Vienna, Austria). p value less than .05 was considered significant.
3 RESULTS
3.1 Clinicopathological characteristics
In total, 245 patients diagnosed with MC-SG were identified (Table 1). The average age at diagnosis was 62.5 (range, 18–92) years. Of the 245 patients, 114 (46.5%) were male and 131 (53.5%) were female. The most commonly affected anatomic site was the parotid gland (76.0%) followed by the submandibular gland (13.5%), major salivary gland (NOS) (9.4%), and sublingual gland (1.1%). Well-differentiated tumors were detected in 23.5% of all patients, moderately differentiated tumors in 42.0%, poorly differentiated tumors in 17.6%, and undifferentiated tumors in 16.9%. Of 172 patients with AJCC-TNM stage information, 27 had stage I, 53 stage II, 49 stage III, and 43 stage IV disease. There were 26 of 180 patients that had lymph node metastases at initial diagnosis, whereas 14 of 182 patients developed distant metastases. One patient was excluded from survival analysis due to lack of survival information.
| Characteristics | Number (N) | Percent (%) |
|---|---|---|
| Age (year) | 62.5 ± 16.5 | – |
| Gender | ||
| Female | 131 | 53.5 |
| Male | 114 | 46.5 |
| Ethnicity | ||
| White | 183 | 75.3 |
| Black | 37 | 15.2 |
| Other (American Indian/AK Native, Asian/Pacific Islander) | 23 | 9.5 |
| Unknown | 2 | |
| Pathological differentiation | ||
| Well | 32 | 23.5 |
| Moderately | 57 | 42.0 |
| Poorly | 24 | 17.6 |
| Undifferentiated | 2 | 16.9 |
| Unknown | 109 | |
| SEER historic stage classification | ||
| Localized | 112 | 52.6 |
| Regional | 69 | 32.4 |
| Distant | 32 | 15.0 |
| Unstaged | 32 | |
| Primary site | ||
| Parotid gland | 186 | 76.0 |
| Submandibular gland | 33 | 13.5 |
| Major salivary gland, NOS | 23 | 9.4 |
| Sublingual gland | 3 | 1.1 |
| Laterality | ||
| Left | 111 | 49.5 |
| Right | 113 | 50.5 |
| Unknown | 21 | |
| Tumor size | ||
| T1 | 28 | 16.2 |
| T2 | 58 | 33.5 |
| T3 | 57 | 32.9 |
| T4 | 40 | 23.3 |
| Unknown | 72 | |
| Lymph node metastases | ||
| N0 | 154 | 85.6 |
| N1 | 14 | 12.8 |
| N2 | 12 | 6.6 |
| Unknown | 65 | |
| Distant metastases | ||
| M0 | 168 | 92.3 |
| M1 | 14 | 7.7 |
| Unknown | 63 | |
| AJCC-TNM stage | ||
| I | 27 | 15.7 |
| II | 53 | 30.8 |
| III | 49 | 28.5 |
| IV | 43 | 25.0 |
| Unknown | 73 | |
| Surgery | ||
| Yes | 226 | 92.2 |
| No | 19 | 7.8 |
| Radiotherapya | ||
| Postoperative radiotherapy | 103 | 42.4 |
| Preoperative radiotherapy | 5 | 2.0 |
| Radiotherapy alone | 1 | 2.5 |
| No | 130 | 53.1 |
- Abbreviation: NOS, not stated, the type of major salivary gland was not stated in SEER database.
- a Six cases without information on radiotherapy sequence with surgery.
3.2 Survival analysis
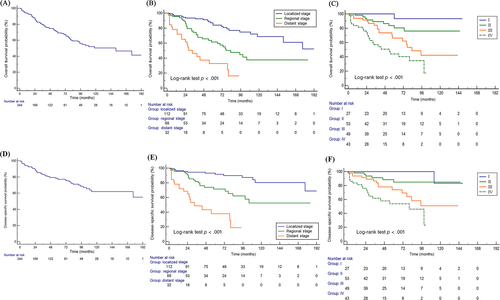
The median follow-up time was 47 (range, 1–197) months, and 80 out of 245 patients died during the follow-up. The median OS was 152.0 months (95% CI: 95.0–177.0, Figure 1A). The OS rates at 3, 5, and 10 years were 79.8%, 69.2%, 50.3%, respectively. The DSS rates at 3, 5, and 10 years were 82.5%, 77.1%, 61.9%, respectively. The OS and DSS curves in entire cohort stratified by tumor stage (AJCC-TNM stage and SEER historic stage) are showed in Figure 1. Overall, the OS and DSS of MC-SG patients became much shorter with an increase in tumor stage. Patients with advanced stage had significantly shorter OS and DSS than those with early stage (p < .001 for all, Figure 1B–D). The stratification analysis revealed that the prognoses worsened with an increase in tumor stage and presence of metastases (Figure 2A–C). Patients who were diagnosed with well-differentiated tumor had significantly longer OS (p < .01, Figure 2D). Furthermore, older patients (>62 years) had significantly shorter survival than younger patients (≤62 years) (p < .01). Apart from the above-mentioned factors, sex, race, primary site, and laterality were not related with OS (p > .05 for all). As for DSS, the stratification analysis also showed that DSS was significantly associated with T/N/M category, pathological differentiation (p < .01 for all, Figure S1).
3.3 Treatment
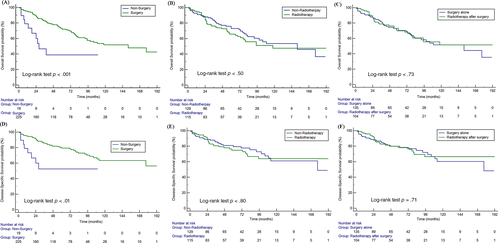
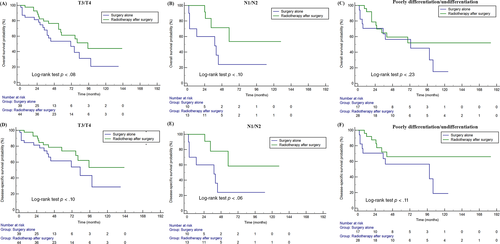
Regarding the treatment regimen, most patients (92.2%) underwent surgery, and 46.9% (115/245) patients also received radiotherapy. Surgery significantly prolonged OS and DSS of MC-SG patients (median OS: 152 months vs. 28 months, p < .01) (Figures 3A,D). Radiotherapy had no significant influence on OS and DSS among these patients (p = .50, p = .80) (Figure 3B,E). In addition, there were 103 patients (42.4%) who received postoperative radiotherapy (Table S1). Postoperative radiotherapy did not significantly prolong OS and DSS when compared with individuals receiving surgery alone in the entire cohort (p = .73, p = .71, respectively) (Figures 3C,F). Further, the survival analysis stratified by high risk factor showed that postoperative radiotherapy could prolong OS and DSS of patients with T3/T4 (p = .08 for OS, p = .10 for DSS) and lymph node metastases (p = .10 for OS, p = .06 for DSS), but without statistical significance (p > .05 for all) (Figure 4).
3.4 Cox regression analyses
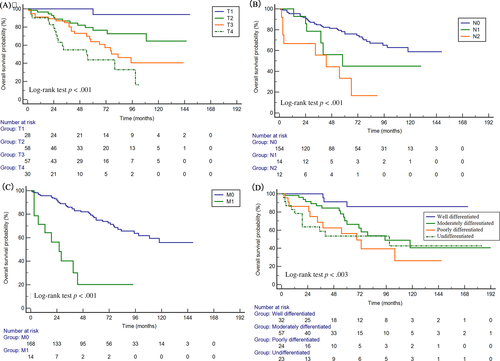
Univariate and multivariate Cox regression analyses were performed to identify the factors potentially influencing OS and DSS (Tables 2 and 3). Univariate Cox analysis showed that pathological grade, tumor stage (SEER historic stage, AJCC-TNM stage), and surgery were significantly related with OS and DSS (p < .05 for all, Table 2). Meanwhile, age was significantly associated with OS in univariate Cox analysis. In multivariate Cox regression analysis, older age (>62 years), T category (T4), lymph node metastasis (N2), distant metastasis (M1), and poor differentiation were independent unfavorable prognostic factors (Table 3). As for DSS, the multivariate Cox analysis demonstrated that T category (T4), lymph node metastasis (N2), distant metastasis (M1), and poor differentiation/undifferentiation were independent indicators for worse survival (Table 3).
| Characteristics | Category | OS | DSS | ||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Agea | >62/≤62 | 2.86 (1.73–4.76) | <.01 | 1.33 (0.69–2.58) | .39 |
| Gender | Male/Female | 1.14 (0.74–1.77) | .56 | 1.21 (0.72–2.03) | .48 |
| Race | White | Reference | Reference | ||
| Black | 0.72 (0.38–1.37) | .32 | 0.64 (0.29–1.41) | .64 | |
| Other | 0.32 (0.10–1.01) | .06 | 0.29 (0.07–1.20) | .09 | |
| Pathological differentiation | Well | Reference | Reference | ||
| Moderately | 3.78 (1.11–12.8) | <.01 | 3.21 (0.70–14.7) | .13 | |
| Poorly | 7.04 (1.97–25.0) | <.01 | 8.34 (1.80–38.6) | <.01 | |
| Undifferentiated | 6.57 (1.84–23.4) | <.01 | 7.76 (1.67–35.9) | <.01 | |
| Primary site | Parotid gland | Reference | Reference | ||
| Submandibular gland | 1.14 (0.61–2.11) | .68 | 1.44 (0.72–2.89) | .91 | |
| Major salivary gland, NOS | 1.61 (0.77–3.37) | .21 | 2.07 (0.92–4.64) | .34 | |
| Sublingual gland | 3.95 (0.54–28.8) | .18 | 5.06 (0.68–37.7) | .11 | |
| Laterality | Right/left | 1.31 (0.93–1.84) | .12 | 1.06 (0.60–1.86) | .83 |
| SEER historic stage classification | Localized | Reference | Reference | ||
| Regional | 2.72 (1.59–4.63) | <.01 | 3.16 (1.57–6.33) | <.01 | |
| Distant | 7.00 (3.81–12.9) | <.01 | 11.0 (5.28–23.0) | <.01 | |
| AJCC-TNM stage | I | Reference | Reference | ||
| II | 5.45 (0.70–42.6) | <.01 | 3.64 (0.44–30.2) | .23 | |
| III | 13.3 (1.80–99.0) | .01 | 10.2 (1.35–78.2) | .02 | |
| IV | 26.9 (3.66–197) | <.01 | 21.1 (2.81–158) | <.01 | |
| Tumor stage | T1 | Reference | Reference | ||
| T2 | 7.66 (1.01–57.9) | <.01 | 5.26 (0.67–41.6) | .11 | |
| T3 | 15.0 (2.04–110) | <.01 | 10.7 (1.42–81.2) | .02 | |
| T4 | 26.4 (3.52–197) | <.01 | 20.8 (2.71–160) | <.01 | |
| Lymph node metastases | N0 | Reference | Reference | ||
| N1 | 1.92 (0.82–4.52) | .14 | 2.16 (0.83–5.62) | .11 | |
| N2 | 4.81 (2.24–10.3) | <.01 | 5.67 (2.46–13.1) | <.01 | |
| Distant metastases | M1/M0 | 5.22 (2.61–10.4) | <.01 | 6.14 (2.90–13.0) | <.01 |
| Surgery | Yes/no | 0.34 (0.17–0.66) | <.01 | 0.32 (0.15–0.68) | <.01 |
| Radiotherapy | Yes/no | 1.21 (0.84–1.74) | .32 | 1.07 (0.64–1.80) | .80 |
- a The average age at diagnosis of 245 included patients was 62.5 years, 62-year-old was set as a cutoff point.
| Characteristics | Category | OS | DSS | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age | >62/≤62 | 2.65 (1.06–6.61) | 0.03 | – | – |
| Pathological differentiation | Well | Reference | Reference | ||
| Moderately | 8.53 (1.76–41.2) | 0.34 | 1.96 (0.29–14.2) | .51 | |
| Poorly | 2.35 (0.41–11.4) | <0.01 | 6.53 (1.04–41.2) | <.01 | |
| Undifferentiated | 6.85 (1.41–33.4) | <0.01 | 7.73 (1.21–49.2) | <.01 | |
| Tumor size | T1 | Reference | Reference | ||
| T2 | 6.16 (0.77–49.0) | 0.09 | 3.75 (0.43–32.5) | .23 | |
| T3 | 5.59 (0.66–47.5) | 0.11 | 3.60 (0.39–33.2) | .26 | |
| T4 | 9.80 (1.09–88.2) | 0.04 | 11.7 (1.21–113) | .03 | |
| Lymph node metastases | N0 | Reference | Reference | ||
| N1 | 0.85 (0.14–5.09) | 0.86 | 1.55 (0.26–9.45) | .63 | |
| N2 | 9.50 (2.25–40.1) | <0.01 | 10.2 (2.39–43.8) | <.01 | |
| Distant metastases | M1/M0 | 11.1 (3.12–39.4) | 0.01 | 12.0 (3.13–46.2) | <.01 |
| Surgery | Yes/No | 0.24 (0.04–1.31) | 0.10 | 0.64 (0.06–6.25) | .70 |
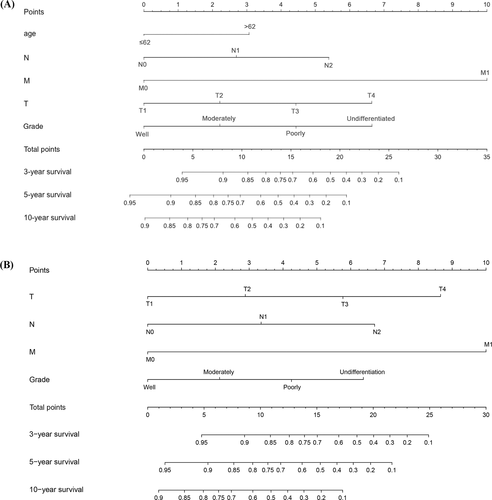
3.5 Prognostic nomogram
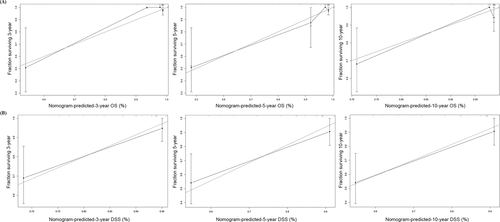
The prognostic nomogram was constructed to predict survival of MC-SG patients using independent prognostic factors. As shown in Figure 5, distant metastases contributed the most to the prediction of OS and DSS followed by T category. The C-indexes for OS and DSS prediction were 0.80 (95% CI: 0.72–0.88), and 0.82 (95% CI: 0.73–0.90), respectively. Calibration plots for the established nomograms showed that the predicted 3-, 5-, and 10-year OS and DSS probabilities were almost identical to the actual observations (Figure 6).
4 DISCUSSION
Myoepithelial carcinoma often occurs in the salivary gland; however, this disease is still rare, and the clinicopathological characteristics and prognosis of patients with MC-SG remains unclear. In this study, we extracted the data of 245 patients with MC-SG from the SEER database and described the clinicopathological characteristics and prognosis of this disease. Further, we determined the prognostic factors and established a nomogram that can be used by clinicians to more precisely predict the survival probability of MC-SG patients.
Because of its rarity, knowledge on MC-SG mainly originates from prior case reports or cases series. Different reports have shown variability in sex distribution, but they reported a generally similar age range. A pooled analysis of 70 patients with myoepithelial carcinoma reported that patients were often in the sixth decade of life at diagnosis, with an age range of 14–86 years.3, 10 Nagao et al. recruited 10 patients and reported an age range of 48–81 years with no pediatric cases and a female to male ratio of 2:1.11 Yu et al. found a male to female ratio of 1.7:1 with an age range of 16–73 years in 27 patients.9 Similar to the results shown by Nagao et al., our data showed 53.3% of patients were women, with a female predominance (1.15:1). In addition, our data showed that the average age was 62.5 (range, 18–92) years with no pediatric cases. This suggests that MC-SG often affects adults, especially elderly persons. In agreement with previous studies, the parotid gland was the most common site of MC-SG in the present cohort.
Nonspecific symptoms of myoepithelial carcinoma often caused a delay in definitive diagnosis; a previous study reported that the average interval from symptoms to the initial diagnosis varied from 3 months to 3 years.12 Myoepithelial carcinoma also exhibits a propensity for metastasis, with an ability for extensive local growth, infiltration, and destruction.9 Consequently, a majority of patients with MC-SG were diagnosed with locally or regionally advanced disease. In the present cohort, 19.4% and 7.7% of patients with MC-SG had lymph node metastases and distant metastases, respectively. With regard to distant metastases, lung metastases were most commonly observed in the previous studies followed by the bone and liver.7 In Kane et al.'s study, distant metastasis was noted in three of seven cases.13 Of the seven cases with information on distant metastases in the present study, four had lung metastases, two bone and liver metastases, and one brain metastases.
MC-SG is supposed to be categorized as the high-grade malignancy with favorable outcomes. Yu et al.’s series including 27 MC-SG patients reported that six patients survived for more than 3 years and four for more than 7 years.9 In the present study, the median OS was 152.0 months, with 5- and 10-year survival rates of 69.2% and 50.3%, respectively. Furthermore, 41.3% of patients with MC-SG could survive longer than 15 years. Multivariate Cox analysis revealed that age and T/N/M categories were independent prognostic factors. Based on these variables, the novel nomograms for predicting OS and DSS were constructed. Clinicians will be able to more precisely predict the survival probability of this rare cancer.
Regarding the treatment regimen, surgery is the first treatment of choice for patients with MC-SG. Our data showed that 92.2% patients, including 35 of 49 patients with stage IV, received surgery. In the present study, surgery could prolong patient survival, but it was not an independent prognostic factor. One possible explanation for this discrepancy is the high recurrence rate after surgical resection. Thus, early surgery with close follow-up are essential for achieving favorable outcomes and decreasing the recurrence risk. Previous study also reported elective neck dissection is generally unnecessary for these patients.9 However, we could not evaluate the value of elective neck dissection among these patients due to the inadequate information. Radiotherapy is also an important treatment regimen.14 Postoperative radiotherapy was used to decrease the risk of recurrence; however, radiotherapy could not significantly reduce the recurrence rate of myoepithelial carcinoma. In Yu et al.’s study, 8 of the 12 patients who underwent postoperative radiotherapy had recurrence.9 In the present study, the data did not show that radiotherapy after surgery could significantly improve survival including OS and DSS. To adjust for confounding factors affecting the conclusion for postoperative radiotherapy, we conducted survival analysis stratifying by tumor invasion, metastases, pathological grade. The results show postoperative radiotherapy could prolong OS and DSS for patients with high risk factor (T3/T4, lymph node metastases), but without significantly statistical difference due to small sample size. Thus, it remains unclear about the role of radiotherapy, which required to be investigated in a large cohort. Regarding chemotherapy, there is limited evidence on the effectiveness of chemotherapy in the treatment of myoepithelial carcinoma, and no standard treatment guidelines have been recommended thus far. Because of the limited information on chemotherapy regimens used in the SEER database, the efficacy of chemotherapy in patients with MC-SG could be not observed. In addition, previous study reported that myoepithelial carcinoma often harbors PLAG fusion with a higher number of copy number alterations, suggesting the potential of therapy-targeting downstream effectors of PLAG.15 The low mutation load of myoepithelial carcinoma is anticipated to be associated with lower clinical benefit from immunotherapy.15
There are several limitations in the present study similar to other SEER database-based studies.16 First, several variables were missing such as gene alterations and chemotherapy information. Second, owing to morphologic heterogeneity, both the cytoarchitectural patterns and the immunohistochemical profile were necessary for the accurate diagnosis of MC-SG.3, 13 The SEER database does not provide detailed information on pathological diagnosis, which raises the possibility of a misdiagnosis. Third, extranodal extension has been introduced in AJCC 8th TNM stage system, which is highly related to treatment outcomes. However, only the AJCC 7th TNM stage system was used in the SEER database, and no pathological details on extranodal extension could be extracted. This flaw may affect the accuracy of these results. In addition, the information on recurrence rates and responses to treatment were not included in the SEER database. Therefore, this limited the accuracy of survival analysis and the establishment of a prognostic nomogram. We constructed the prognostic nomogram for predicting OS and DSS in patients with MC-SG, but the repeatability and reliability of the established nomograms could not be validated because of the lack of an independent cohort.
In summary, to the best of our knowledge, the present study is the largest cohort of patients with MC-SG. We describe the clinicopathological characteristics and prognosis of MC-SG using the data of 245 patients from the SEER database between 1991 and 2016. We also constructed a novel prognostic nomogram for predicting the OS and DSS of patients with MC-SG, and the nomogram could effectively predict the survival of patients with MC-SG. These results are essential for disease management of patients with MC-SG, as well as the future prospective studies of this rare disease.
CONFLICT OF INTEREST
The author declared no conflict of interest.




