Aspergillus otitis externa: A retrospective study of predisposing factors, treatment, and complications
Abstract
Objectives
To study the predisposing factors, treatment, and complications of Aspergillus otitis externa.
Methods
A retrospective analysis of patients diagnosed with Aspergillus otitis externa at the Department of Otorhinolaryngology, Helsinki University Hospital, between January 2010 and December 2018 was performed.
Results
Of the 269 Aspergillus otitis externa (OE) patients, 96 developed otitis media (OM) and 7 developed mastoiditis. Antibiotic and steroid treatment and otological history were risk factors for Aspergillus OE. Systemic diseases and immunocompromising states were more common in mastoiditis patients. Repetitive ear cleaning and topical drugs are primary treatments, but systemic drugs and surgery were needed in resistant and invasive cases. Forty-five novel tympanic membrane (TM) perforations were reported. A strong association between Aspergillus species and final infection types was found; A. niger was the dominant species in OM and in novel TM perforations, whereas A. flavus and A. fumigatus caused mastoiditis. Some of the TM perforations persisted despite treatment. Permanent hearing impairment was associated with OM and mastoiditis.
Conclusion
As Aspergillus OE has the potential to cause acute and chronic complications, fungal OE should be suspected early on if the infection persists after conventional treatment. The identification of Aspergillus species could aid in spotting patients at risk for more severe disease and complications. Intensive local treatment is sufficient in most cases of OE and OM but effective topical antifungals are limited. Patients with Aspergillus OM and mastoiditis should be followed up for hearing impairment and permanent TM perforations after the infection resolves.
Level of evidence
Level 4 (The Oxford 2011 Levels of Evidence).
1 INTRODUCTION
Along with Candida species, Aspergillus species are the most common fungi that cause otitis externa (OE). Predisposing factors for fungal OE are the use of topical antibiotics, steroids, and nonsterile oils, a warm environment, swimming, and mechanical trauma to the external auditory canal.1, 2
Most fungal OE are superficial but they may result in severe complications, such as malignant OE, which is typically associated with diabetes, AIDS, and other immunocompromising conditions. Malignant OE is an invasive infection of tissues around the external auditory canal and may lead to further complications, such as cranial nerve palsies.3-5 Aspergillus species are among the most common fungi in malignant OE.4 Fungal OE may develop into otitis media (OM) and mastoiditis via tympanic membrane (TM) perforation or tympanostomy.5, 6 Few earlier studies have focused on TM perforations and acute OM caused by fungi.7, 8
The most important Aspergillus species in OE are A. niger, A. flavus, and A. fumigatus.2 Typically, Aspergillus forms a biofilm on the surface of skin and mucosa. This may enhance fungal growth and penetration and increase resistance to the host's immune system and antifungal drugs.9 Adequate treatment includes mechanical cleaning of the biofilm and discharge with irrigation and suction, topical antifungals or other antimicrobial agents, and systemic antifungal drugs.1, 3, 6 Surgery and hyperbaric oxygen therapy are additional treatment options for complicated cases and malignant OE.4, 5
This study aimed to characterize the predisposing factors, treatment, and complications of Aspergillus OE with a special focus on TM perforations, acute OM, and mastoiditis.
2 MATERIALS AND METHODS
This was a retrospective analysis of patients diagnosed with Aspergillus OE at the Department of Otorhinolaryngology, Helsinki University Hospital, a tertiary care center, between January 2010 and December 2018. The data were collected from the Helsinki University Hospital databases. We included all patients with clinically diagnosed and microbiologically verified Aspergillus OE. None of the patients were excluded due to limited follow-up data or for any other reason. The follow-up data were collected until the end of 2019.
The following patient data were collected: age, sex, comorbidities, body mass index (BMI), smoking history, alcohol and drug use status, immunocompetence status, and otological history. The following data were collected for the studied Aspergillus OE: infection characteristics, Aspergillus species, susceptibility results, other concomitant pathogens, treatment, number of outpatient visits, follow-up, outcomes, hearing test results, surgical procedures, and complications.
The onset of Aspergillus OE was defined as the date when Aspergillus was microbiologically verified. The duration of infection was calculated from the time of diagnosis until complete recovery, as determined by a physician, or until the end of follow-up, if earlier. The treatments targeted to Aspergillus after microbiological verification were included in the analysis of effective treatments.
Mycological and bacteriological samples were cultivated and morphologically analyzed at the laboratory of the Department of Microbiology, Helsinki University Hospital. Susceptibility testing was performed only if mastoiditis was diagnosed or suspected.
Hearing test results were available for a limited number of patients. Only patients with both pre- and postinfection hearing results were included in the hearing level analysis. Hearing results were reported as pure tone average (PTA) decibels from the frequencies of 500, 1000, 2000, and 4000 Hz.
The clinical and demographic data of the patients were analyzed using descriptive statistics and the IBM® SPSS® Statistics Chi-square test, ANOVA, and multinomial logistic regression. p < .05 was considered to indicate statistical significance.
The research protocol was approved by the Ethics Committee of Helsinki University Hospital. The analyses were anonymous and based on retrospective data. No patient was contacted or prospectively followed up.
3 RESULTS
A total of 269 patients with Aspergillus OE were included in the study. Most patients had a noncomplicated OE with an intact TM and no signs of invasion but some developed OM and mastoiditis, through either an existing (chronic) or an infection-born (novel) TM perforation. In Tables 1–3, the cohort was divided into subgroups based on the final infection type: OE (n = 166, 61.7%), OM (n = 96, 35.7%), and mastoiditis (n = 7, 2.6%).
| OE (N = 166, 61.7%) | OM (N = 96, 35.7%) | Mastoiditis (N = 7, 2.6%) | p-value | |
|---|---|---|---|---|
| Gender, N (%) | .137 | |||
| Female | 76 (45.8) | 56 (58.3) | 3 (42.9) | |
| Male | 90 (54.2) | 40 (41.7) | 4 (57.1) | |
| Age, years | ||||
| Range | 4.2–94.4 | 5.9–93.9 | 16.5–88.2 | |
| Mean | 51.7 | 51.7 | 52.7 | .826 |
| Median | 52.4 | 52.4 | 53.0 | |
| BMI, N (%) | .813 | |||
| <30 | 120 (72.3) | 64 (66.7) | 5 (71.4) | |
| >30 | 36 (21.7) | 24 (25.0) | 2 (28.6) | |
| Not reported | 10 (6.0) | 8 (8.3) | 0 | |
| Intoxicants, N (%) | ||||
| Current smoking | 35 (21.1) | 12 (12.5) | 2 (28.6) | .173 |
| Current alcohol abuse | 11 (6.6) | 5 (5.2) | 1 (14.3) | .616 |
| Comorbidities, N (%) | ||||
| Cardiovascular | 61 (36.7) | 35 (36.5) | 3 (42.9) | .945 |
| Diabetes | 30 (18.1) | 11 (11.5) | 2 (28.6) | .245 |
| Chronic pulmonary disease | 18 (10.8) | 11 (11.5) | 2 (28.6) | .358 |
| Chronic auricular skin disease | 17 (10.2) | 5 (5.2) | 0 | .263 |
| Hypothyreosis | 11 (6.6) | 10 (10.4) | 1 (14.3) | .471 |
| Psychiatric disease | 8 (4.8) | 5 (7.2) | 2 (28.6) | .027 |
| Rheumatic or other autoimmune disease | 5 (3.0) | 6 (6.3) | 1 (14.3) | .211 |
| Chronic rhinosinusitis | 6 (3.6) | 3 (3.1) | 1 (14.3) | .322 |
| Neurological disease | 4 (2.4) | 6 (6.3) | 0 | .251 |
| Active malignancy | 1 (0.6) | 0 | 1 (14.3) | <.001 |
| None | 65 (39.2) | 34 (35.4) | 2 (28.6) | .688 |
| Ongoing immunosuppressive therapy | 3 (1.8) | 1 (1.0) | 1 (14.3) | .043 |
| Otological history, N (%) | ||||
| Multiple OE | 96 (57.8) | 60 (62.5) | 3 (42.9) | .516 |
| Multiple Aspergillus OE | 36 (21.7) | 23 (24.0) | 1 (14.3) | .801 |
| Ear surgery on the infection side | 47 (28.3) | 30 (31.3) | 1 (14.3) | .606 |
| Hearing aid on the infection side | 16 (9.6) | 10 (10.4) | 1 (14.3) | .913 |
- Note: The demographic data and medical history of the whole cohort and the subgroups are based on the final infection type.
| Treatment, N (%) | Total (N = 269) | OE (N = 166, 61.7%) | OM (N = 96, 35.7%) | Mastoiditis (N = 7, 2.6%) |
|---|---|---|---|---|
| Mechanical ear cleaning | 264 (98.1) | 163 (98.2) | 94 (97.9) | 7 (100.0) |
| Local antiseptics | 238 (88.5) | 153 (92.2) | 80 (83.3) | 5 (71.4) |
| Boric acid | 179 (66.5) | 122 (73.5) | 55 (57.3) | 2 (28.6) |
| Methylene blue | 187 (69.5) | 121 (72.9) | 62 (64.6) | 4 (57.1) |
| Clioquinol | 73 (27.1) | 33 (19.9) | 38 (39.6) | 2 (28.6) |
| Local antifungals | 43 (16.0) | 20 (12.0) | 21 (21.9) | 2 (28.6) |
| Itraconazole | 16 (5.9) | 7 (4.2) | 8 (8.3) | 1 (14.3) |
| Miconazole | 31 (11.5) | 15 (9.0) | 14 (14.6) | 2 (28.6) |
| Systemic antifungals | 25 (9.3) | 6 (3.6) | 13 (13.5) | 6 (85.7) |
| Itraconazole p.o. | 23 (8.6) | 6 (3.6) | 13 (13.5) | 4 (57.1) |
| Voriconazole i.v. | 4 (1.5) | 0 | 0 | 4 (57.1) |
| Surgery | 6 (2.2) | 0 | 0 | 6 (85.7) |
| Hyperbaric oxygen | 1 (0.3) | 0 | 0 | 1 (14.3) |
- Note: The treatments administered in the whole cohort and the subgroups based on the final infection type.
| Aspergillus species, N (%) | Total (N = 269) | OE (N = 166, 61.7%) | OM (N = 96, 36.7%) | Mastoiditis (N = 7, 2.6%) | Novel TM perforation (N = 45, 16.7) |
|---|---|---|---|---|---|
| Niger | 149 (55.4) | 78 (47.0) | 70 (72.9) * | 1 (14.3) | 37 (82.2) * |
| Flavus | 62 (23.0) | 51 (30.7) | 8 (8.3) | 3 (42.9) * | 3 (6.7) |
| Fumigatus | 29 (10.8) | 20 (12.0) | 6 (6.3) | 3 (42.9) * | 2 (4.4) |
| Sydowi | 9 (3.3) | 5 (3.0) | 4 (4.2) | 0 | 0 |
| Terreus | 8 (3) | 6 (3.6) | 2 (2.1) | 0 | 1 (2.2) |
| Ochraceus | 1 (0.3) | 0 | 1 (1.0) | 0 | 0 |
| Glaucus | 1 (0.3) | 1 (0.6) | 0 | 0 | 0 |
| Hollandicus | 1 (0.3) | 0 | 1 (1.0) | 0 | 0 |
| Not specified | 9 (3.3) | 5 (3.0) | 4 (4.2) | 0 | 2 (4.4) |
- Note: Aspergillus species in the cohort, in the subgroups based on the final infection type, and in the OM and mastoiditis cases with a novel TM perforation.
- *p < .05.
The average age was 51.7 years, and 135 (50.2%) patients were females. The majority (n = 168, 62.5%) had systemic comorbidities, most frequently cardiovascular diseases and diabetes. A history of repetitive OE (59.1%) was common. Psychiatric diseases, active malignancy, and ongoing immunosuppressive therapy were more common among patients with mastoiditis than others (p < .05). The demographic data and medical history are shown in Table 1.
The patients were diagnosed with OE in a primary or secondary care center or at our clinic, and most of them (n = 216, 80.3%) received local or systemic antibiotics, corticosteroids, or other drugs that were initiated empirically or targeted to other concomitant microbes (most frequently Staphylococcus aureus, Pseudomonas, and Candida parapsilosis). Usually, after treatment failure, the patients were referred to our clinic where microbiological samples were taken at the latest and afterward repeated in case of a weak response to treatment. Overall, a majority (n = 172, 63.9%) of the patients had monomicrobial Aspergillus OE. The mean and median durations of OE symptoms before Aspergillus diagnosis were 56 and 21 days, respectively. The management of Aspergillus OE required up to 542 days (mean 47 days, median 20 days) before recovery. Patients visited the outpatient clinic 1– 25 times. Repetitive visits were carried out to perform mechanical ear cleanings and to follow up recovery. Based on the TM status and signs of invasion, the final infection type was categorized as OE, OM, or mastoiditis. 10 (3.7%) patients were hospitalized to enable intensive local treatment or to perform surgery and administer intravenous medication; 6 of them had mastoiditis. The duration of infection did not vary significantly between the final infection types but patients with A. niger infection had more outpatient visits (mean 4.60 vs. 3.89, p < .05) and more ear debridements (mean 4.19 vs. 3.54, p < .05) than did the other patients.
The primary treatment for Aspergillus OE was debridement, and topical drugs and antimicrobial agents, such as boric acid, methylene blue, clioquinol, miconazole, and off-label itraconazole. Additionally, oral itraconazole was used in 8.6% of the cases. Susceptibility testing that was performed only in 9 cases found no resistance to voriconazole. Mastoiditis was managed with long periods of intravenous voriconazole and/or oral itraconazole in 6 cases, with mastoidectomy in 6 cases, and with hyperbaric oxygen treatment in 1 case. (Table 2) No mortality or persistent Aspergillus infection was reported.
A. niger was the most common species and was isolated from 149 (55.4%) patients, followed by A. flavus from 62 (23.0%) patients and A. fumigatus from 29 (10.8%) patients. A. niger was the major species in the OM (72.9%, p < .05) and novel TM perforation (82.2%, p < .05) groups, whereas A. flavus (42.9%) and A. fumigatus (42.9%) were the dominant species in the mastoiditis group (p < .05) (Table 3). In a multinomial logistic regression analysis for severity of infection with Aspergillus species as factor, and age, gender, hearing aid use, smoking, and psychiatric diseases as covariates, we found Aspergillus species to be significant in the model (p = .002).
Of the 7 patients, 3 with mastoiditis had further complications—1 with facial nerve palsy, 1 with facial and abducens nerve palsy and temporal bone osteomyelitis, and 1 with destruction of the ossicles. All of them had a monomicrobial Aspergillus infection. Both patients with cranial nerve palsies also had novel TM perforations due to A. flavus and A. fumigatus.
A novel, infection-born, TM perforation was diagnosed in 42 OM and 3 mastoiditis patients. In addition, 58 patients had a chronic or iatrogenic TM perforation. The total incidence of novel TM perforations was 16.7%. Nine perforations were closed surgically, 18 closed spontaneously during follow-up, 12 persisted, and 6 patients were lost to follow-up. The management of the novel TM perforations is shown in Figure 1.
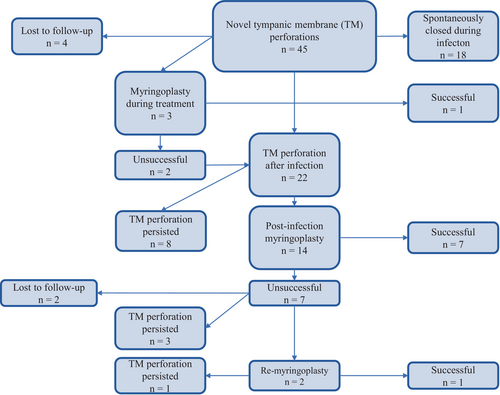
Full hearing test results were available for 47 patients. The average decrease in the air conductivity PTA was 3.1 dB in the OE group, 13.8 dB in the OM group, and 19.0 dB in the mastoiditis group (p = .178). During follow-up, the number of hearing aid users increased from 44 to 58.
4 DISCUSSION
We retrospectively studied a cohort of 269 patients treated for Aspergillus OE: 96 developed OM, and 7 developed mastoiditis. The risk factors for Aspergillus OE were previous antibiotic and steroid treatments and a history of otological disorders. These results are in line with the literature.1, 2, 7, 10 Mastoiditis patients more often had active malignancies and received immunosuppressive therapy (p < .05). Immunocompromised states, including diabetes, HIV/AIDS, malignancies, and organ transplants, have been identified as risk factors for invasive Aspergillus infections.3, 5, 11 Except for diabetes, these etiologies were rare in our cohort. The small number of patients with mastoiditis was a limitation in the analysis of risk factors.
The symptoms of fungal OE are less fulminant with less pain than those of bacterial OE,2 which may partly explain the prolonged symptoms. In our cohort, the diagnosis was delayed if Aspergillus was not suspected and microbiologically tested in the early stage. In some mixed infections, Aspergillus was not considered clinically relevant, and the treatment was targeted at bacteria. In both noncomplicated and invasive fungal OE, the latency before diagnosis and onset of effective treatment was generally long, as has been observed in previous studies.3, 7, 10, 12, 13 This may increase Aspergillus growth, prolong infection, and predispose patients to complications. In addition, delayed diagnosis increases patient burden and overall costs.
Treatment of noninvasive Aspergillus OE requires time and effort.6, 10 In our cohort, the response was often observed only after multiple debridements and treatment trials. Local treatment mainly consisted of nonselective antimicrobial agents, mostly because effective and nontoxic topical antifungal drugs are poorly available.8 Adequate clotrimazole products were not available at all in our country, even though topical clotrimazole can be recommended for treating Aspergillus OE and OM.1, 2, 14, 15 Nevertheless, most noninvasive infections were successfully managed without systemic antifungals, indicating that careful local treatment is sufficient for eradicating Aspergillus. Instead, in invasive infections, long-term systemic antifungals are essential, and surgical treatment must often be combined.3, 4, 12 In our study, severe complications such as mastoiditis were successfully managed according to these principles. In most cases with mastoiditis, voriconazole, which is a recommended drug,3, 16 was used.
A significant percentage of our Aspergillus OE patients developed OM, and a minority further developed mastoiditis. We found strong associations between Aspergillus species and final infection type. A. niger was the most common species in the OE.1, 2, 17, 18 A. niger also caused the most OM (72.9%) and infection-born TM perforations (82.2%) and was overrepresented in these groups (p < .05). After excluding patients with chronic TM perforation, the rate of novel TM perforations in A. niger OE patients was 32.2%. To our knowledge, such a strong potential to cause complications has not been reported before. However, mastoiditis was mainly caused by A. flavus and A. fumigatus (p < .05), which were much less common in the cohort. This was somewhat expected, as A. flavus and A. fumigatus are known to cause invasive infections.3, 5, 11 Compared to other studies on fungal invasive OE, two patients developed cranial nerve palsy but we observed no persistent infections or mortality.4, 5, 12 This could be explained by the low number of immunocompromising comorbidities in our cohort.
TM perforations have been previously reported in fungal OE.1, 7, 8, 10, 18 In our cohort, an infection-born TM perforation was diagnosed in 16.7% of the patients. The rate of spontaneous recovery was 40% and the rate of surgical closure was only 20%. Thus, the rate of persistent novel TM perforations was relatively high compared to that in other studies on fungal OE.8 This suggests that TM perforations caused by A. niger might have a lower healing potential than those caused by other fungal etiologies. This perspective warrants further study. Furthermore, this highlights that even noninvasive Aspergillus OE may cause permanent TM perforations and subsequent disadvantages for the patient.
We found evidence of permanent hearing impairment caused by Aspergillus OE. Hearing loss in patients with noncomplicated OE was minor and not clinically significant. However, the difficulty of hearing loss in OM and mastoiditis correlated with infection severity. Fourteen new patients initiated hearing rehabilitation after Aspergillus OM or mastoiditis, indicating that these infections carry a risk of significant hearing loss. Similar results have been reported in fungal malignant OE.3, 4
5 CONCLUSION
Aspergillus etiology in OE should be suspected early on to prevent delays in the onset of intervention. Patients with a history of otological disorders are exposed to superficial Aspergillus OE and invasive infections may develop in immunocompromised patients with intact ears. Our results suggest that most noninvasive Aspergillus OE can be managed with intensive debridements and topical antifungals, provided that adequate drugs are available. The approach for treating mastoiditis, however, is different, warranting systemic antifungals and surgery. Complications such as OM and infection-borne TM perforations in superficial Aspergillus OE may be more common than previously thought. In particular, A. niger OE can be resilient and carry a risk for TM perforation. The management of TM perforations caused by Aspergillus is challenging, and repetitive surgical attempts may be needed. Hearing tests should be performed in the case of Aspergillus OM and mastoiditis to identify patients with impaired hearing. Our results indicate that even noninvasive Aspergillus OE may cause permanent disadvantages for patients, and these infections should be taken seriously.
ACKNOWLEDGMENTS
The authors greatly thank Nathalie Friberg, MD, PhD, for sharing her expertise in the clinical comprehension of the features of Aspergillus. They thank Maija Lappalainen, MD, PhD, and Päivi Tissari, MD, PhD, for their help in collecting the patient data.
FUNDING INFORMATION
The costs were covered by funding received from Helsingin ja Uudenmaan sairaanhoitopiiri and the Leo, Mary, and Mary-Ann Hackman Foundation.
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest. Outside of this study, R. Saarinen is employed by Bayer Pharmaceuticals.




