Molecular basis of chronopharmaceutics
Abstract
Many pathophysiological circumstances vary during 24 h periods. Many physiologic processes undergo biological rhythms, including the sleep–wake rhythm and metabolism. Disruptive effect in the 24 h variations can manifest as the emergence or exacerbation of pathological conditions. So, chronotherapeutics is gaining increasing interest in experimental biology, medicine, pharmacy, and drug delivery. This science and the plethora of information should be used intelligently for optimizing the effectiveness and safety of the drug, relying on the timing of drug intake. These chronopharmacological findings are affected by not only the pharmacodynamics but also pharmacokinetics of drugs. The mammalian circadian pacemaker is located in the suprachiasmatic nucleus. The molecular mechanisms are associated with Clock genes that control the circadian rhythms in physiology, pathology, and behavior. Clock controls several diseases such as metabolic syndrome, cancer, and so on. CLOCK mutation influences the expression of both rhythmic and nonrhythmic genes in wild-type tissues. These genotypic changes lead to phenotypic changes, affecting the drug pharmacokinetic and pharmacodynamic parameters. This review is intended to elaborate system regulating biological rhythms and the applicability in pharmaceutics from viewpoints of the intraindividual and interindividual variabilities of Clock genes. © 2011 Wiley-Liss, Inc. and the American Pharmacists Association J Pharm Sci 100:3560–3576, 2011
INTRODUCTION
The principal purpose of drug delivery is to formulate dosage forms meeting therapeutic demands relating to particular pathological cases. Circadian changes of physiological and pathophysiological functions throughout the daytime have brought a new approach to the evolvement of drug delivery. As a new and developing discipline, chronopharmaceutics has attracted considerable attention from academia and industry. Studies based on the individualization of pharmacotherapy, by monitoring drug concentrations in each patient, have been held to improve conventional pharmacotherapy to achieve the maximum effectiveness and/or safety.1,2 The traditional classification has classified pharmaceutical variations into interindividual and intraindividual variabilities. The basic pharmacogenomic–pharmacogenetic studies only concentrate on the interindividual variability. Researchers have uncovered the molecular mechanism of the interindividual variation from numerous points of view and taking different levels, ranging from the genetic variation to the protein polymorphism depending on point mutations at the translation and posttranslation stages.3-5 This hypothesis has hypothesized the emergence of diseases due to either upregulated or downregulated genetic expression of certain molecular targets. This concept did not take the intraindividual variability throughout the daytime in its attention. The clock and clock-controlled genes in mammals have been discovered since 1997.6
Both the intraindividual as well as the interindividual variabilities should be taken into our consideration, aiming at further improvement of rational pharmacotherapy. The circadian rhythmicity of biochemical, physiological, and behavioral processes may alter the potency and/or the toxicity of numerous medications depending on the administration time.7-13 Chronopharmacotherapy has been described by the medical society for curing many pathological states such as allergic rhinitis, nocturnal asthma, arthritis, congestive heart failure, myocardial infarction, stroke, and peptic ulcer disease. Chronopharmaceutics may be achieved by the dosing schedule of formulated tablets–capsules and the drug delivery system (DDS) in appropriate timing to keep matching between drug concentrations and rhythms in disease activity.7,11 Pharmaceutical industries have extensively studied the underlying molecular mechanisms and have conducted clinical explorations, including myriad patients aiming at devising chronotherapeutic interventions with many pharmaceutical dosage forms and drugs. New technologies have been developed and tested for delivering active constituents precisely in a time-dependent fashion by bedside or ambulatory pumps to treat human diseases. Careful use of this new technology is the responsibility of chronopharmacologists to ensure that devices and clinical findings are well accepted by scientists currently involved in more classical research. However, drugs are still obeying the classical clinical practice and are administered regardless of the time of day. So, these chronopharmacological investigations should be summarized for clinical practice.
Homeostatic functions of steroid hormones and their receptors and many other physiological processes are subjected to the 24 h variation. Disquiet of these physiological rhythms is related to pathological state such as cancer, depression, and diabetes. Clock gene controls the etiology of several diseases such as metabolic syndrome, cancer, and so on. CLOCK mutation controls the genetic expression of both rhythmic and nonrhythmic genes in wild-type (WT) tissues. Chronopharmacotherapy is now depending on rhythm monitoring, rhythm manipulation, and overcome of rhythmic disruption focusing on the molecular clock system. This leads to more improvement and spreading of this science in the clinical practice. Chrono-DDS is the term used for the modern approaches of DDS that cope with the endogenous 24 h rhythm.11,12 Contemporary technologies of pharmacotherapy have focused on pulsatile drug delivery, gene delivery, and antibody delivery, targeting specific molecular components for some diseases. Clock genes should be also important candidates for research and treatment. Therefore, this review is intended to elaborate an overview on the molecular mechanism regulating the biological rhythm based on Clock genes, the hieratical structure, the feasibility of this basic biology to be applied on the clinical practice, and the applicability in pharmacotherapy from viewpoints of the intraindividual and interindividual variability of Clock genes. Rodents such as mice or rats are mostly fed and active during dark period, namely nocturnal animals and their rhythmic pattern are totally different from that of diurnal human. The difference in act–rest cycle between nocturnal rodents and diurnal human should be considered in the interpretation of experimental findings described in this review.
BIOLOGICAL TIME STRUCTURE
The modern approach in the research of biological rhythms clearly reveals that biological processes are not constant over time. It is changeable in a highly organized, well controlled, and synchronized process. It includes the internal adaptation in response to external stimuli, namely zietgeber (from the German, “time givers”) such as food and light. It is as diverse as cyanobacteria and humans. Biological time structure indicates the sum of non-randam and thus predictable time-dependent biological variations, including a spectrum of rhythm with different frequencies such as growth, development, and aging.6,14-18 The endogenous period length (τ) of rhythms may be very short period, in the range of a second, such as in electrocardiographic and encephalographic tracings. Ultradian rhythms are an expression for rhythms of periods in the range of 30 min to 20 h, which are observed in many endocrine glands and sleep stages. Circadian rhythms with about 24 h period are entrained to the 24 h solar cycle and have been most explored now being applied into clinical practice as one of its numerous applications. Longer period lengths of rhythms of a week (circaseptan), a month (circatrigintan), and a year (circannual) are also known. The circatrigintan includes the menstrual cycle. Many rhythms seem to be genetically orthologous and thus endogenously generated in nature. Endogenous rhythms may or may not be controlled in their timing by environmental cues, synchronizers. Chronobiology objectively quantifies and investigates molecular mechanisms of biological time structure, including rhythmic manifestations of life.
CIRCADIAN TIME STRUCTURE
The circadian clock in mammals is expressed within pacemaker neurons of suprachiasmatic nucleus (SCN) that in turn maintain proper phase alignment of peripheral tissue clocks ubiquitously present in nearly all cells. Thus, the brain SCN clock provides “standard time” for all peripheral tissue clocks (Fig. 1).11,12 The circadian clock consists of three components6,14-18: an input pathway adjusting the time by zeitgebers, a central oscillator generating the circadian signal in response to the input pathway, and an output pathway manifesting itself in the final circadian rhythmicity in physiology and behavior. The 24 h variation in light intensities is considered to be the major environmental input involved in circadian entrainment. Light signals stimulate photoreceptor cells in the retina, namely rhodopsin cone opsins, and transmitted by the retinohypothalamic tract to the SCN neurons. The inherited period of the human pacemaker clock is not exactly 24 h but somewhat longer, closer to 25 h. This is clear under free-running conditions such as constant dark or constant light condition. In free-running experiments, subjects are not entrained to the 24 h cycle in nature and they are shielded from all time cues such as a constant dark protocol. When animals free run, experiments can be performed to investigate what sorts of signals, zeitgebers, are most effective in entrainment. The daily light–dark cycle, the strongest synchronizers, and zeitgebers set the inherited pacemaker circadian time-keeping systems to 24 h each day.
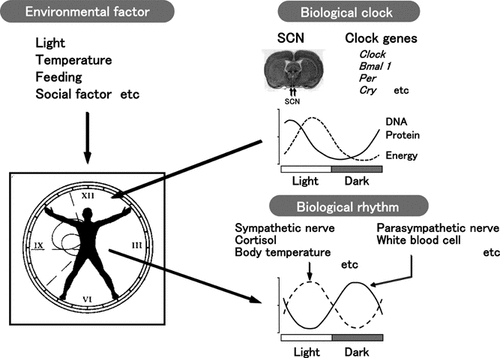
Shematic diagram of the circadian system in mammals. The SCN of the anterior hypothalamus is the site of the circadian pacemaker in mammals.11,12 Light signals, the most important entrainment other than feeding schedule, drugs, and social interaction, are perceived by photoreceptor cells in the retina and transmitted to neurons of the SCN via the retinohypothalamic tract. Clock genes are expressed not only in the SCN but also in other brain regions and various peripheral tissues. Such a cascade of Clock genes may contribute to the organization of biological rhythms in the whole body. The mechanisms employed by circadian output pathways are likely to involve both nervous and hormonal signals.
The circadian rhythms are also affected by many environmental factors other than lighting condition such as feeding schedules, social interactions, and several drugs.11-13 As the endogenous cyclic variation of the central pacemaker is slightly longer than 24 h in human, synchronization occurs by daily phase shift with time advances to the exogenous solar cycle. This synchronization may be the principal of the phototherapy for treating certain psychological disorders. The time-dependent phase shifting effects of light are represented in a phase–response curve (PRC).11-13 Light exposure causes phase shift with time delays in the first half of the night. Meanwhile, it causes phase shift with time advances in the second half of the night. Nonphotic synchronizers such as melatonin are used to study a PRC that is about 12 h out of phase with the PRC to light. Photic and nonphotic effects on intrinsic timekeeping may cause disordered timekeeping in depressive illness.
The circadian clock is encoded by a set of transcriptional activators and repressors that comprise an autoregulatory transcription–translation feedback loop. Period genes (Per1, Per2, and Per3) are centrally expressed in the SCN in a rhythmic manner. Per1 and Per2 only are light inducible.18 Per1 light induction, particularly, is an initial event in light-induced synchronization for resetting and for entraining the circadian biological clock.15 The transcriptional–translational feedback loop machinery of the core clockwork regulates clock-controlled either rhythmic or nonrhythm expression (Fig. 2).6,11,12,16-20 Namely, CLOCK–BMAL1 heterodimers work through an E-box and E-box-like cis-enhancer elements to activate the transcription of Pers, vasopressin, and Dbp mRNA, indicating other output function.16,17,19 This transcriptional upregulation can be inhibited by the PER and CRY proteins by direct protein–protein binding interacting with CLOCK–BMAL1 heterodimer.20 Cyclic variation of Pers mRNA expression is explored not only in the SCN but also in other central and peripheral tissues.21 However, the mechanisms employed by circadian output pathways are not well understood. Cyclic variation in the periphery is governed by that in the SCN through neuronal and hormonal signals, leading to the cyclic variation of the whole body.22,23 This can be proved in the disrupted circadian rhythm in physiological function and the abolished cyclic Pers mRNA expression in SCN-lesioned rats21 and Clock mutant mice.16 Via the hypothalamic–pituitary–adrenal (HPA) axis, SCN controls the circadian rhythm of glucocorticoid levels in plasma. This rhythmic variation of glucocorticoids regulates many physiological responses and developmental processes through the cyclic binding to and modulating the transcriptional activity of their cognate nuclear receptor.24,25 In vitro, single administration of dexamethasone causes brief induction of Per1 and Dbp mRNA levels.25 The role of endogenous glucocorticoid hormones in the circadian entrainment is only for peripheral oscillators but not centrally in the SCN.25 CLOCK mutation, such as mutation in the exon 19, affects the expression of rhythmic and nonrhythmic genes in WT tissues. Intraindividual and interindividual variability of molecular clock should be applied for the clinical practice.26-32 The regulatory mechanism is clarified on the basis of the Clock genes.
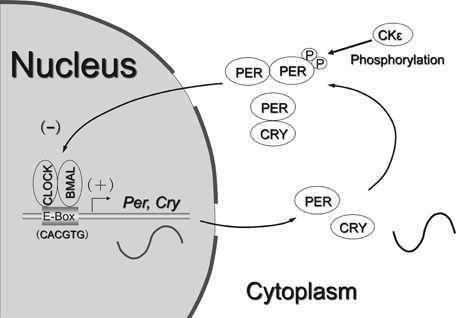
Simplified model of the dual regulation of a core feedback loop.6,11,12,16-20 CLOCK and BMAL1 heterodimers activate clock genes and clock-controlled genes transcription. The PER and CRY proteins shut down CLOCK–BMAL1 upregulation in the nucleus, forming a negative feedback loop. The phosphorylation of PER1 (period) and PER2 by CKIε (casein kinase I epsilon) may regulate their cellular location and stability. Clock-controlled genes products including DBP (D-element-binding protein) and AVP (arginine vasopressin) transduce the core oscillation to downstream output systems.
CHRONOPHARMACOLOGY
Chronopharmacology is the branch of chronobiology dealing with the pharmacological aspects. This science is associated with the time dependency of drug response. Chronopharmacology, including chronotoxicity, consists of chronopharmacodynamics and chronopharmacokinetics and then contributes to chronopharmacotherapy supported by the technique of chrono-DDS.
A surprisingly large number of medical conditions display daily rhythmicity: infant mortality immediately after birth is higher in the late afternoon and early evening than during the rest of the day, adult suicides are more frequent in the late morning and early afternoon, injuries caused by firearms and knives are produced more often around midnight, calls for helicopter ambulance transportation are more common during daylight hours, and unexpected sudden deaths after abdominal surgery are more frequent between midnight and sunrise. The cyclic variation in the drug safety and/or toxicity may be due to the cyclic variation of the molecular target or the chronopharmacokinetic of this drug. The time-dependent effects of drugs on the organism as a whole can be termed as chronergy. It includes both chronoesthesy and chronopharmacokinetics.
Chronesthesy is a term used to describe significant dosing time-dependent differences in the drug pharmacodynamics unrelated to the variation in their pharmacokinetics. Variation (24 h) in receptor number or affinity, differences in the susceptibility or sensitivity of a biological target, second messengers, metabolic pathways, and/or free-to-bound fraction of drugs help explain this phenomenon. Chronesthesy represents prognosticated, rather than randomly distributed, biological time-dependent changes of such molecular target.
Chronopharmacokinetics is a term used to describe biological time-dependent differences in the pharmacokinetics of a drug. Chronopharmacokinetic research has demonstrated that the time of administration is a possible factor of variation in the pharmacokinetics of a drug. Rhythms (24 h) at each process, for example, absorption, distribution, metabolism, and elimination help explain this phenomenon. Thus, 24 h rhythms in gastric acid secretion and pH, motility, renal blood flow, gastrointestinal blood flow, drug–protein binding, and so on may contribute to those in drug pharmacokinetics.11,12 Chronopharmacokinetics, only in some cases, can be one reason responsible for circadian variation in drug effects and/or toxicity.
Chronopharmacology indicates the clinical application of chronobiological approach in pharmacological phenomena.11,12 Chronobiological explanations involve more accurate and/or misleading results than the classical homeostatic point of view. Chronopharmacology constitutes the time-dependent undesirable or harmful effects from chemical, physical, or other agents, namely chronotoxicology. Chronotoxicity may be unintentionally occurred upon biological temporal feature and as a function of biological timing and the chronotherapy endeavoring to cure or prevent disease, with proper regard to temporal features, for example, corticosteroid therapy timed to simulate the adrenocortical cycle in Addison's disease.
CHRONOTHERAPY
Chronotherapy is focusing on the existence of the 24 h variation in pathophysiological conditions.11,12 Under physiological state, the peak of plasma cortisol, aldosterone, androgenic hormones, platelet adhesiveness, blood viscosity, and activity of natural killer cells appears at the initial hours of daytime. Furthermore, hematocrit value is the highest and airway diameter is the widest around the middle and afternoon hours, respectively. On the contrary, the peak of all of insulin, cholesterol, triglycerides, platelet numbers, and uric acid are observed during the late daytime and evening. The rhythms of basal gastric acid secretion, counts of white blood cells, eosinophils, lymphocytes, levels of prolactin, melatonin, adrenal corticotrophic hormone, follicle-stimulating hormone, and luteinizing hormone show also peaks at specific times at night.
Variations (24 h) in the pathological and physiological conditions causes significant rhythmic patterns in the symptoms and particularly noticeable in many therapeutic cases.11,12 The onset of symptoms of migraine headache, sneezing, runny nose, stuffy nose in allergic and infectious rhinitis, and rheumatoid arthritic pain is most frequent around the time of awakening from nighttime in the morning, whereas osteoarthritic pain is the worst at the late of the day. The morbid and mortal events of myocardial infarction, ischemic events, chest pain, and ST-segment depression of angina are greatest in the morning. The highest incidence of thrombotic and hemorrhagic stroke is in the morning. On the contrary, the abdominal pain, the gastric distress onset, and acute exacerbation of peptic ulcer disease are most likely in the early morning and late evening. The epileptic attacks are common around offset in the morning and sleep onset at night. The symptoms of congestive heart failure are worse at night. The elevation of ST-segment in Prinzmetal's angina is most frequent during the middle to later half of the nighttime. The asthma attack is of greatest risk at night.
Awareness of the circadian rhythmicity in the risk of disease plus evidence of 24 h rhythm of pharmacokinetics, effects, and safety of drugs constitute the appropriate pharmacotherapy.11,12,33,34 One strategy to increase the efficiency of pharmacotherapy is the administration of drugs at times at which they are most effective and/or best tolerated. Chronotherapy may be accomplished by the most appropriate timing of formulated tablets and capsules, and the DDS to synchronize drug concentrations to rhythms in disease state. Chronotherapy is relevant to the following cases. The risk and/or intensity of the symptoms of any disease differs predictably over time period such as in case of asthma, arthritis, allergic rhinitis, congestive heart failure, myocardial infarction, stroke, and peptic ulcer disease. The therapeutic-to-toxicity ratio of a drug differs predictably depending on chronobiological factors such as in case of anticancer chemotherapeutics. The pharmacokinetics and pharmacodynamics of a drug differ, depending on the biological clock. The goal of pharmacotherapy of hormonal substitution is to meet the 24 h changes of the hormone levels in normal condition. Also on the horizon are medications used to fix broken biological clocks, perhaps a molecular target in all illness, in the opinion of some clinicians.10-12 Several examples for chronopharmacotherapy are shown (Table 1).10-12
| • The morning daily or alternate-day dosing strategy for methylprednisolone that was introduced during the 1960s constitutes the first chronotherapy to be incorporated into clinical practice. |
| • Evening, once-daily dosing of specially formulated theophylline tablets for treatment of nocturnal asthma. |
| • Before-bedtime administration of verapamil HCL as a unique controlled onset extended-release 24 h dosage form to optimize the treatment of patients with ischemic heart disease and/or essential hypertention. |
| • Evening administration of HMG-CoA-reductase antagonists for the management of hyperlipidemia. |
| • Evening, once-daily dosing of conventional H2-receptor antagonist or morning once-daily administration of protonpump antagonist tablet medications for the management of peptic ulcer disease. |
| • Before-bedtime administration of hypnotics for sleep induction and maintenance. |
| • Morning application of testosterone drug delivery patch systems to achieve a physiologic androgen-replacement therapy. |
| • Programmed-in-time infusion of antitumor medications according to biological rhythms to moderate toxicity and enhance dose intensity in cancer treatment. |
| • Programmed-in-time administration of tocolytic medication relative to the circadian rhythm in uterine contractility to avert preterm labor and birth. |
CHRONOBIOLOGY OF PHYSIOLOGICAL FUNCTION AND DISEASES
Chronobiology is the study of biological rhythms and their molecular mechanisms. Chronopharmaceutics has been developed combining the obsolete goal of pharmaceutics, delivering constant drug concentrations, with contemporary explorations in many fields derived from our progress in chronobiology. Owing to the plethora of information representing the extent of wide spreading, accuracy, and well controlling of biological rhythms, there are great advances in chronobiology and its related sciences that should be used intelligently for developing new advanced drug delivery systems, as well as active ingredients, to optimize their efficacy and safety depending on their need and the biological system rhythmicity. Besides, progress in genomics and nanobiotechnology opens many researchers' mind to new horizons in disease therapy and prevention. The molecular mechanisms underlying 24 h rhythm of physiology and diseases should be explained by Clock genes.
Familial advanced sleep-phase syndrome (FASPS) is a circadian rhythm sleep disorder, which is associated with a life long, stable pattern of early sleep onset around 19:30 h and offset around 04:30 h. It is due to a genetic mutation affecting circadian clock. It is demonstrated in three kindreds.35 Affected individuals experience early evening sleepiness and early morning awakening. Genetic sequencing analysis on a family with FASPS using multiple sets of dense genomic markers to map the mutation and to clarify such mutant genes revealed that there is a mutation in the critical internal at locus to chromosome 2q. One of these was hPer2, the human homolog of mPer2.36 Mutation of hPer2 substitutes serine 662 into a glycine (S662G). This occurs in a region of hPER2 homologous to the casein kinase I epsilon (CKIε)-binding region of mPER1 and mPER2. Serine 662 is in fact part of a consensus CKIε-binding domain for phosphorylation. The S662G substitution renders the mutant protein less readily bound to CKIε than the WT hPER2 in vitro and hypophosphorelated. Thus, a variant in human sleep behavior due to a missense mutation in a clock component, hPER2, changes the circadian period by about 1 h less than normal.
The onset of myocardial infarction occurs frequently in the early morning, and it may be partly associated with 24 h rhythm of fibrinolytic activity of plasminogen activator inhibitor-1 (PAI1).37 Basic helix–loop–helix (bHLH)–PAS domain transcription factors play an important role in controlling the circadian rhythmicity of Pai1 expression and its thrombolytic activity. The positive regulators of the core circadian rhythm components, CLOCK–BMAL1 and CLOCK–BMAL2 heterodimers, regulate hPai1 expression. Cycle-like factor (CLIF), a novel bHLH–PAS protein, is firstly detected in human umbilical vein endothelial cells. CLIF is highly homologous to Drosophila CYCLE, one of the essential transcriptional regulators of circadian rhythm. CLIF is expressed in endothelial cells and neurons in the brain, including the SCN. In endothelial cells, CLIF heterodimerizes with CLOCK and upregulates the Pai1 gene through E-box sites. Furthermore, the negative regulators of the core clockwork system components, PER2 and CRY1, inhibit the Pai-1 promoter activation by the CLOCK–CLIF heterodimer. These findings potentially provide a molecular mechanism for the morning onset of myocardial infarction through the core clock components.
BMAL1, a bHLH/PAS domain transcription factor, forms the main core component of the clock machinery controlling circadian rhythm. It controls adipogenesis and lipid metabolism activity in mature adipocytes.38 In 3T3-L1 cells, the expression level of Bmal1 mRNA increases during adipose differentiation. Knockdown of Bmal1 and 3T3-L1 cells shows minimum amounts of lipid droplets. Bmal1 knockout mice embryonic fibroblast cells fail to be differentiated into adipocytes. Adenovirus-mediated expression of Bmal1 in 3T3-L1 adepocytes resulted in upregulation of several nuclear factors involved in lipogenesis such as peroxisome proliferator-activated receptor (PPAR) in a BMAL1-dependent manner. These factors show 24 h rhythm in adipose tissues of mice. Adenovirus-mediated expression of Bmal1 in Bmal1 knockout mice embryonic fibroblast cells restored the ability of these cells to differentiate. Furthermore, in adipocytes, overexpression of BMAL1 increases lipid synthesis activity. Thus, regulation of adipose differentiation and lipogenesis in mature adipocytes is through one of the core components of the clockwork system, BMAL1.
CHRONOPHARMACODYNAMICS AND MOLECULAR CLOCK
Biological rhythms impact the pathophysiology of diseases, the pharmacokinetics, and pharmacodynamics of drugs.39-43 Chronopharmacology is the science that investigates the relationship between the biological rhythm and the drug. Biological rhythms at different levels ranging from the cellular to subcellular level can give rise to significant time-dependent variations in the pharmacodynamics of drugs. These variations are totally unrelated to their pharmacokinetics. This means chronesthesy. This phenomenon can be explained by 24 h change in receptor number or affinity, second messengers, metabolic pathways, and/or free-to-bound fraction of the drug. Thus, the molecular mechanisms underlying cyclic variation of chronesthesy should be explained by Clock genes.
Angiogenesis is an essential process for tumor growth and metastasis. Recently, the inhibition of angiogenesis is considered as a new molecular target for cancer chemotherapeutics. Under hypoxic conditions in tumor-induced angiogenesis, the expression of vascular endothelial growth factor (VEGF) is upregulated. There is a 24 h rhythmic oscillation of the VEGF expression in tumor cells implanted in mice. In response to hypoxia, the levels of VEGF mRNA are significantly increased.44 By using luciferase reporter gene assay, the negative limbs of the molecular loop, PER2 and CRY1 whose expression also preserved their 24 h rhythmic expression, periodically inhibit the hypoxic induction of VEGF promoter activity. This gave the explanation of such rhythmically oscillated higher levels of VEGF. Methionine aminopeptidase2 (MetAP2) has a crucial role in the endothelial cells growth during the tumor angiogenesis stage. MetAP2, such as VEGF, shows also 24 h rhythm in implanted tumor masses.45 The molecular clock mechanism underlying 24 h rhythm of MetAP2 activity is investigated in tumor-bearing mice. There are eight E-boxes in the 5′ flanking region of MetAP2 promoter region. The circadian rhythmic expression of MetAP2 activity in tumor-bearing mice is regulated by the transcription of clock genes within the clock feedback loops. MetAP2 transcription is activated by the CLOCK–BMAL1 heterodimer and this activation is inhibited by PER2 or CRY1. Furthermore, the resultant antitumor efficacy of antiangiogenic agents and MetAP2 inhibitors are enhanced by administering the drugs at the time of higher VEGF levels and when MetAP2 activity increases, respectively.
Activating transcription factor 4 (ATF4) is highly expressed in cisplatin-resistant cell lines and causes cisplatin resistance. ATF4 is a target of CLOCK through heterodimerization with BMAL1 and binding to the E-box in the promoter region. CLOCK is upregulated in cisplatin-resistant cell lines.46 Clock expression level can be a molecular marker for cisplatin sensitivity and the downregulation of either Clock or ATF4 renders A549 cells to be sensitive to cisplatin. Chemotherapeutic multidrug resistance cell lines show ATF4 overexpression and marked elevation of the intracellular glutathione. Knocking down of ATF4 generally downregulated genes for glutathione metabolism. Taking together, these findings may indicate that the Clock and ATF4 transcription system may, through glutathione-dependent redox system, contribute to multidrug resistance. Also, these results show that the physiological potentials of Clock-controlled redox system might contribute to the oxidative stress-associated disorders, including cancer and systemic chronotherapy. On the contrary, cisplatin causes lesions in the DNA. DNA repair is intimately linked to circadian rhythm. One of the mechanisms of chemotherapeutic resistance for DNA damaging agents such as cisplatin is DNA repairing. This repairing mechanism is under the control of the clockwork system. Incorporation of the chelating agent shows significant time-dependent difference. Understanding of the DNA repairing pathway is essential for the cancer therapy and links the circadian rhythmicity, nucleotide excision repairing, and potentiality of cancer therapy.47
Molecular clocks are time-keeping systems that control the 24 h variations in multiple behavioral, physiological, and biochemical processes. The alteration of the circadian systems is related to the development of many pathological conditions. So, screening approach is performed to introduce modern small molecules capable of pharmacological modulation of the molecular clock to control these pathological situations.48 The evidence for the availability and impact of this approach is still under investigations for both academic and clinical purposes.
CHRONOPHARMACOKINETICS AND MOLECULAR CLOCK
Chronopharmacokinetic research has been extensively studied for many drugs to give the rationale to chronopharmacological observations and to manifest the suitable time of administration as a factor of variation in the pharmacokinetics of a drug. Biological clock controls the drug pharmacokinetics such as circadian rhythmicity in the pharmacokinetic parameters such as absorption, distribution, metabolism, and elimination. Such rhythmicity leads to time-dependent variation in the sum of these parameters and the pharmacokinetics. Variation (24 h) in protein binding, gastric emptying rate, renal blood flow, gastric blood flow, intestinal motility, gastric acidity, intestinal pH and enzymatic activities, and so on play roles in such chronopharmacokinetics.10,11
The Clock genes are ubiquitously expressed and distributed in SCN, other brain regions, and various peripheral tissues. In the liver, a major organ of metabolism and detoxification, many genes show circadian rhythmic expression, which was detected by a microarray analysis.49 The liver is also a biological clock. It is capable of generating its own circadian rhythms.50 So, the 24 h variation of the transcriptional activities in liver tissues may reveal many daily variations in the biochemical and physiological processes in the peripheral tissues and may give vital information for the chronotoxicology as well. The expression of 3906 genes in rat liver was explored as a function of time of day using high-density oligonucleotide microarrays.49 Thirty percent of these genes show lucid expression. Meanwhile, the rest 70% are not expressed or the expression is too low to distinguish from background levels. Less than 1.5-fold of 90% of rhythmic genes is the estimated changes. The temporal expressions of 67 genes show circadian rhythms. These rhythmic genes include many essential genes for fundamental processes such as DNA binding and transcriptional regulation, drug metabolism, ion transportation, signal transduction, and immune response.
Circadian clock controls a significant class of the mammalian transcriptome, including the PAR-domain basic leucine zipper (PAR bZip) transcription factors D-element-binding protein (DBP), HLF, and TEF.51-53 DBP, thyrotroph embryonic factor (TEF), and hepatic leukemia factor (HLF) accumulate in several peripheral tissues such as liver and kidney. Their expression profiles reveal high circadian variation. Triple knockout mice are born at expected Mendelian ratios but die prematurely.54 To identify PAR bZip target genes, the liver and kidney transcriptomes of PAR bZip triple mutant mice were compared with those of WT or heterozygous mutant mice. The triple mutant mice show disrupted gene expression patterns of many enzymes associated with drug metabolism. These genes include aldehyde dehydrogenases, aminolevulinic acid synthase (ALAS1), carboxylesterases, cytochrome P450 enzymes, glutathione-S-transferase (GST), P450-oxidoreductase (POR), sulfotransferases, UDP-glucuronosyltransferases, members of drug transporter families, and constitutive androstane receptor (CAR). Various levels at which PAR bZip transcription factors might interfere the coordination of xenobiotic detoxification system are schematically described.54 Some genes encoding detoxification enzymes, such as CYP2A5, CYP2C50, and CES3, may be PAR bZip direct target genes. The expression of other detoxification enzymes such as CYP2B10 is regulated by CAR, mainly controlled by PAR bZip proteins. Other enzymes in the xenobiotic defense, such as ALAS1 and POR, are under the control of both CAR and PAR bZip proteins, direct and indirect targets. Variation (24 h) in transcriptional regulators will be further explored in future studies.
P-glycoprotein, the product of the multidrug resistance (mdr1a) gene, acts as a xenobiotic efflux transporter endowing the cancer chemotherapeutic resistance and participates in the intestinal barrier hindering the drug intestinal absorption. The expression of mdr1a gene and its efflux function exhibits 24 h rhythm in mice intestine. The 24 h variation in the expression of the mdr1a gene is regulated by the molecular components of the circadian clock.11,12,55 By using luciferase reporter and gel mobility shift analysis, the transcriptional regulation of the mdr1a gene is under the control of clock gene products. Namely, the expression of mdr1a gene is regulated by HLF and E4 promoter-binding protein-4 (E4BP4). They competitively bind to the same DNA-binding site with reciprocal regulatory effect. Molecular and biochemical analyses of HLF- and E4BP4-downregulated colon 26 cells and the intestinal tract of clock mutant mice suggest that these two proteins consist of a reciprocating mechanism in which HLF upregulates the transcription of the mdr1a gene. On the contrary, E4BP4 periodically downregulates mdr1a transcription when E4BP4 is high. So, in mouse small intestine, mdr1a gene expression is controlled by the clock organization. There is a significant 24 h variation in the accumulation of [3H]-digoxin in small intestine of WT mice. The 24 h rhythmic accumulation of [3H]-digoxin is nearly antiphase to that of P-gp. On the contrary, in Clock mutant mice, there is no significant rhythmicity in the intestinal accumulation of [3H]-digoxin, with trace amount of P-gp. Moreover, the [3H]-digoxin levels in small intestine are consistently high during the whole day. The efflux function of mdr1a is decreased in Clock mutant phenotype. H+/peptide cotransporter 1 (PEPT1), the product of solute carrier family 15 member 1 slc15a1 gene, is nutrient transporter widely expressed in the small intestine. It transports dipeptides and tripeptides beside peptidomimetic drugs. The expression profiles of transcription factors of clock-controlled genes, HIF, and DBP under two different kinds of feeding conditions have been studied to investigate the molecular mechanism of the diurnal rhythmcity of PEPT1 expression.56
Cytochrome P450 2E1 (CYP2E1), which has important clinical and toxicological roles exhibits 24 h periodicity in its expression and activity. Hepatic nuclear factor-1alpha (HNF-1alpha) as well as Clock genes with a striking 24 h rhythm in mice liver play an important role in the 24 h variation of CYP2E1 expression (Fig. 3).57,58 The expression of CYP2E1 mRNA is regulated by HNF-1alpha and the clock organization in liver of WT mouse. The expression profile of CYP2E1 mRNA was high in the liver from the late light phase to the early dark phase. Using luciferase reporter analysis, HNF-1alpha upregulated CYP2E1 promoter activity was downregulated by CRY1. This downregulated effect of CRY1 was conserved on the HNF-1alpha-binding site of the CYP2E1 promoter region. In HepG2, approximately 24 h rhythmic oscillation in CYP2E1 mRNA is induced by serum shock. Transfection of HNF-1alpha and CRY1 shRNA disturbed the oscillation of CYP2E1 mRNA in HepG2. In chromatin immunoprecipitation analysis, HNF-1alpha binding to the CYP2E1 promoter was high from the late light phase to the early dark phase. By using the chromatin immunoprecipitation reimmunoprecipitation assay, there are significant time-dependent differences for CRY1 protein interaction with HNF-1alpha transcriptional complexes, including coactivator p300 on the HNF-1alpha-binding site in the CYP2E1 promoter. So, the transcription activator of HNF-1alpha acts periodically and the negative limbs of molecular clocks periodically inhibit CYP2E1 transcription, resulting in the 24 h rhythm of its mRNA expression. There was a significant 24 h rhythm of mortality in mice injected with high dosage of acetaminophen (APAP). Peak mortality was observed in mice injected with APAP in the first half of dark phase, and nadir mortality was observed in mice injected with APAP in the first of light phase. Hepatotoxicity after APAP injection was significantly more severe in mice injected with the drug at 21:00 h than 09:00 h. Immunohistochemical staining using anti-APAP antibody 2 h after APAP injection was detected in centrilobular hepatocytes of mice injected with the drug at 21:00 h but not at 09:00 h. These results suggest that rhythmic expression of CYP2E1 play an important role in the circadian activity associated with APAP toxic metabolite production and the chronotoxicity of APAP.
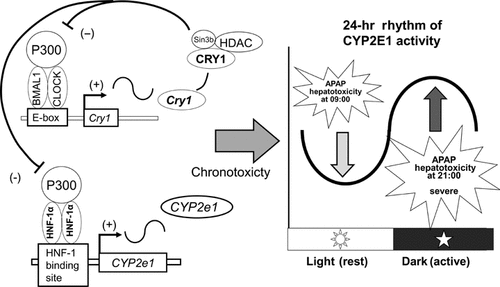
Molecular mechanism of the circadian rhythmicity of Cytochrome P450 2E1 (CYP2E1) and chronotoxicity.57,58 Left panel shows a simplified diagram of the transcriptional regulation of the CYP2E1 circadian rhythmicity by HNF-1alpha and the circadian organization of molecular clocks. The transcriptional activator of HNF-1alpha acts periodically. The molecular clock negative limbs periodically inhibit CYP2E1 transcription, resulting in the 24 h rhythm of its mRNA expression. Right panel shows a significant 24 h rhythm of mortality after acetaminophen (APAP) injection in mice. Peak mortality was observed after APAP injection at 21:00 h and 01:00 h, and nadir mortality was observed after drug injection at 09:00 h. Hepatotoxicity was significantly more severe in mice injected with the drug at 21:00 h than 09:00 h. CYP2E1 circadian rhythmic expression leads to circadian activity, which is associated with APAP toxic metabolite production and chronotoxicity of APAP.
CYP3A4, also a metabolic enzyme, significantly affects pharmacokinetics. The 24 h rhythmic expression of CYP3A4 mRNA was explored in hepatic cells.59 In HepG2 cells, both mRNA levels and metabolic activity of CYP3A4 fluctuate rhythmically with a period length nearly 24 h by serum shock. The 24 h variation in the expression of the cyp3a4 gene seems to be due to that in the metabolic activity. By using luciferase reporter and electrophoretic mobility shift analysis, DBP activates the transcription of the cyp3a3 gene by binding to the box near the upstream of the transcriptional start site. The upregulation of the cyp3a3 gene by DBP is deactivated by the E4BP4. So, DBP and E4BP4 may also consist of a reciprocating mechanism in which DBP activates the transcription of the cyp3a3 gene during the time of day when DBP is high, and E4BP4 downregulates the transcription at other times of day.
Clock genes with their ubiquitous distribution, centrally in SCN and other brain tissues and peripherally in liver, kidney, and intestine, play an important role in the molecular clockworks. The rhythmic oscillation of clock genes in peripheral tissues is controlled under the circadian clock mechanism in the SCN. The mechanism of synchronization and resetting of liver clock function is still obscured. It was hypothesized that neuronal and hormonal signals control such communication. Recently, pseudorabies virus, a transsynaptic tract tracer, has been used to follow neural connections between the SCN and peripheral tissues. It was found that autonomic nervous system connects between SCN and peripheral tissues. More deep investigations may be able to clarify the mechanism underlying neural control of peripheral clock systems, which has great importance for deep understanding of the drug and energy metabolisms.
THE DISRUPTION OF BIOLOGICAL RHYTHMS
The circadian clockwork system is essential for adapting endogenous physiological functions to the 24 h change in environmental conditions. Disruption of the 24 h rhythms, such as the sleep–wake and hormonal cycles is the main etiology of various pathological and psychiatrical disorders. The circadian clock is encoded by a set of transcriptional activators and repressors that comprise an autoregulatory transcription–translation feedback loop. Disruption of molecular clock causes the alteration in circadian rhythm of physiology and behavior. Patients receiving chemotherapeutic agents experience physical and psychological disorders. These disturbances include their adaptation to the 24 h changes.
Many drugs, especially chemotherapeutic agents, cause disruption of the circadian rhythms.13,60,61 This change is linked to the therapeutic effects or may lead to diseases, side effects, and/or altered homeostatic regulation. Interferons (IFNs) are group of proteins, which are widely used as antiviral and antitumor agents. Meanwhile, IFNs have many side effects, including neuropsychiatric disorders such as depression and neurosis. Suicidal attempts are also reported in their neuropsychiatric disorders.62,63 Administration of IFNs in the morning, during the early active phase, in human causes disturbance in the circadian rhythmicity such as alteration in the lymphocyte counts and cortisol concentrations.64 The molecular mechanism of the adverse effects is explained by Clock genes. Interferon-α (IFN-α) disturbed the rhythmicity of Per genes mRNA expression in the SCN in mice.60 It also decreased the mRNA expression of Clock and Bmal1 in the SCN.16,17,19 Also, repetitive administration of IFN-α damped the rhythmicity of locomotor activity and body temperature. IFN-α affected the Clock genes both in the SCN and in the periphery as well. So, it is not so easy to understand whether the IFN-α action on central Clock genes are secondarily related to the IFN-α action on locomotor activity. IFN-α directly acts on the SCN as shown in the induced expression of the multiprotein transcription factor called IFN-stimulated gene factor-3 in the SCN.60 Continuous administration of corticosterone or applying a manipulation of feeding schedule severely disturbed the rhythmicity of locomotor activity without manipulation of the rhythmicity of Clock genes in the SCN.65,66 Thus, the disruption of locomotor activity is not linked with the disruption of Clock gene expression in the SCN. Daily administration of IFN-α at the early active phase (dark period) disturbed the photic induction of the Per1 gene in SCN, leading to malfunctioned resetting entrainment mechanism of SCN. So, behavioral and physiological side effects of IFN-α may be due to the central disruptive effects on the Clock genes in the SCN. IFN-α causes adverse effect on the ocular structure accompanied with retinal or optic neuropathy.67,68 Such effects may also reduce the photic information from the retina to SCN and the stimulation of the light responsive element of the Per gene.
Repeated administration of IFN-α during the early active phase, not the early rest phase, causes the inhibitory effect of mRNA expression of Per gene in the SCN.60 In the same way, repetitive administration of IFN-γ causes similar time-dependent inhibition of Per1 mRNA expression, which can be caused by IFN-α or IFN-β in conjunction with other cytokines.69 The central peak of IFN-γ receptor expression is at the early active phase.70 Being administered in the nadir, so administration of IFN-α during the early rest phase can reduce its side effect. Dosing time dependency of the modulatory effect of IFN-α on circadian clock can be applied to other drugs as shown in the case of IFN-γ. Circadian disruption speeds up liver carcinogenesis in mice.71 So, disturbance of the clock function, as a new perception of side effects, can be overcome by devising a dosing schedule.
MANIPULATION OF BIOLOGICAL RHYTHMS
The endogenous 24 h cyclic rhythmicity in physiology and behavior are affected by genetic factors and exogenous synchronizers of many environmental factors such as lighting condition, several drugs, feeding schedules, and social interactions.13,15,35,65 As the endogenous period length of the central human circadian pacemaker is slightly longer than 24 h, resetting of the endogenous circadian system with the solar cycle follows daily phase shift with time advances. The time-dependent phase shifting effects of light are summarized in PRC.13 Morning light advances the central SCN. Meanwhile, light delays it during the late afternoon and the evening. Midday light causes no phase-shifting effects. Melatonin, 5-hydroxytryptamine, and behavioral arousal are examples of phase-shifting agents that have their own PRC, which is distinct from light one. Exposure between midday and early evening causes phase shift with time advances. Exposure between late night and midday leads to phase shift with time delays. Nonphotic zeitgebers-induced phase shifts are generally similar to dark pulses induced-phase shifts exposed to animals subjected to constant light condition. Effects of photic and nonphotic zeitgebers on intrinsic timekeeping may be important factors of disordered timekeeping in depressive disorders.
The environmental light intensity gives information to the SCN through direct synaptic connections with the retina, leading to adapting the phase of SCN oscillators to the photoperiod.72 Then, clock pacemaker synchronizes rhythms of physiology and behavior. In a circadian rhythmic manner, Per1 and Per2 transcription are rapidly induced by light.15 Per1 mRNA response to light is closely related to behavioral light-induced phase delays. Per1 antisense phosphorothioate oligodeoxynucleotide significantly decreased the locomotor activity during night in mice.73 Therefore, the expression of Per1 may be a rate-limiting step in photic entrainment.
Photic and nonphotic stimuli can synchronize and modulate the SCN clock. Moreover, alteration of the circadian rhythmicity by several drugs has been investigated to cause a phase shift in the rhythm in the central or peripheral nervous system.13 It has been demonstrated that the acute and circadian time-dependent reduction of Per1 and/or Per2 mRNA levels in SCN of the hamster by 5-HT1A/7 receptor agonists is strongly correlated with the phase resetting in response to the drug.74 So, we can predict that nonphotic shifts may involve variation in Per1 and/or Per2 mRNA levels in the SCN. However, with the exception of chronic treatment with antidepressant drugs, which are given over the course of many months, most studies report normal temporal response of the clock to an acute treatment and the response to repetitive administration is generally unknown.
The daily variation of the environmental factors such as feeding condition affect many parameters oscillated in physiological function.66,75 The circadian rhythm of glucocorticoid blood level contribute to control the rhythm of physiological function by the manipulation of feeding condition. Plasma corticosterone levels increases immediately before the timing of feeding. Continuous administration of corticosterone changed the rhythmicity of behavior, physiology, and circadian genes expression. Such effects are not affected by SCN lesions.76 The repetitive manipulation of feeding condition modifies the rhythmic phase of locomotor activity, physiology including corticosterone level, and Clock genes expression in periphery by up to 12 h, whereas it has no effect on the rhythmic phase of Clock genes expression in the SCN.65,66 Food-shifted circadian adrenal rhythmicity can be abolished by ventromedial hypothalamic lesions.77 The site where the feeding-associated circadian oscillation is connected to the HPA axis appears to be the paraventricular nucleus.78 Moreover, skillful management of feeding schedule can be also an intelligent tool that controls the chronopharmacological action and chronopharmacokinetics of drugs.79 The human diet intake pattern dramatically modifies the normal rhythmicity of both the human plasma cortisol levels and the body temperature as well.80 The plasma cortisol rhythmicity can be kept normally only in diurnal feeding pattern. Reversed or altered rhythmicity patterns are under nocturnal or continuous feeding condition. So, a new concept of chronopharmacotherapy is to manipulate the circadian rhythmicity using several drugs or controlled feeding schedule.
CHRONO-DRUG DELIVERY SYSTEM
The therapeutic efficacy and drug toxicity of many conventional medications greatly differ according to the relationship between the time of drug administration and the circadian rhythmicity of physicobiochemical processes. Moreover, many medications cause disturbing circadian rhythms, leading to abnormal homeostatic regulation and side effects. Hence, the disruption of normal biological rhythms is a modern concept of side effects, as mentioned above in case of IFN. Alteration of the normal 24 h rhythms can be decreased to the lowest level by optimizing the dosing time schedule.13 Many reports have demonstrated the rationale of chronotherapy.11,81-83 However, over the past decades, much of research on drug delivery has put the light mainly on constant drug release rate. The DDS designed without considering well proven oscillatory phenomena may lead to unsuccessful remedies and drug delivery limitations.
Chronopharmaceutics presents contemporary challenges to DDS. Owing to rapid progress and diffusion of chronopharmacology, chronobiology, and global market constraints, the traditional pharmaceutical goals, maintaining constant drug release rate or blood level, are becoming no longer used. The availability of appropriate technology, however, may be the major obstacle in the development of DDS that cope with the circadian rhythm. Recent years have witnessed the appearance of what we called “Chrono-DDS” against many diseases. The high research interest concerning chrono-DDS may lead to the emergence of chronopharmaceutics, a new subdiscipline in pharmaceutics. A principal objective of chronopharmaceutics is to introduce the drug in higher concentrations during the time of greatest demand for maximum therapeutic efficiency and in lesser concentrations when the need is less to minimize side effects.
There are many technologies in chronopharmaceutics such as CONTIN®, physicochemical changing of the active pharmaceutical ingredient, TIMERx®, three-dimensional printing, CODAS®, OROS®, DIFFUCAPS®, CEFORM®, chronomodulating infusion pumps, controlled-release erodible polymer, and controlled-release microchip strategies (Table 2).10-12,34,83
| Active Pharmaceutical Ingredient (API) | Proprietary Name Dosages Form | Proprietary Chronopharmaceutical Technology | Disease |
|---|---|---|---|
| USA Theophylline | Uniphyl® extended-release tablets | CONTIN® | Asthma |
| Famotidine | Pepcid® tablets | Physicochemical modification of API | Ulcer |
| Simvastatin | Zocor® tablets | Physicochemical modification of API | Hyperlipideemia |
| Verapamil HCL | Covera-HS® extended-release tablets | OROS® | Hypertension |
| Verapamil HCL | Verelan® PM extended-release capsules | CODAS® | Hypertension |
| Diltiazem HCL Verapamil HCL | Cardizem® LA-extended-release tablets | CEFORM® | Hypertension |
| Propranolol HCL Verapamil HCL | InnoPran® XL-extended-release capsules | DIFFUCAPS® | Hypertension |
| Japan Famotidine | Gaster® tablets | Physicochemical modification of API | Ulcer |
| Simvastatin | Lipovas® tablets | Physicochemical modification of API | Hyperlipidemia |
| Theophylline | Uniphyl® extended-release tablets | CONTIN® | Asthma |
| Tulobuterol | Hokunalin® tape | Transdermal chrono-delivery system | Asthma |
- Theophylline is used for asthma associated with the increased bronchoconstriction in the early morning.
- Famotidine is used for ulcer associated with the increased gastric acid secretion in the evening.
- Simvastatin is used for hypercholesterolemia associated with the increased cholesterol synthesis in evening.
- Verapamil, Diltiazem, and Propranolol are used for hypertension associated with the increased blood pressure in morning.
- Tulobuterol is used for asthma associated with the increased bronchoconstriction in the early morning.
Chrono-DDS in commercial use includes compounds such as theophylline (Uniphyl®), diltiazem (Cardizem® LA), famotidine (Pepcid®), propranolol (InnoPran® XL), simvastatin (Zocor®), and COER-verapamil (Covera-HS®, Verelan® PM). Information has been compiled from specific pharmaceutical company websites, specific product package inserts, United States patents, and US Food and Drug Administration electronic orange book.83 Development in chronopharmaceutics is expected in the near future and it may be made at the border of other emerging terrain such as nanomedicine and system biology. Such modern biological technologies of drug delivery may lead to less toxic and/or more efficient treatment.
Clock gene contributes the etiology of several diseases such as congestive heart failure, asthma, cancer, metabolic syndrome, and so on. Mutation of CLOCK alters the expression profile of many genes in WT mice. Some are rhythmic and others are nonrhythmic genes in WT tissues. Therefore, the role of intraindividual and interindividual variabilities should be applied for the molecular clock, which can be used for the medical practice. However, the alteration of PK/PD is affected by interindividual and intraindividual variabilities of Clock genes as well.
Several reports show that circadian clock disruption increases the rate of cancer growth, and is associated with cancer higher incidence, faster progression, and shorter patient survival.84 The great disruption in circadian activity/sleep cycle is demonstrated for advanced lung cancer patients. Moreover, they suffer severe functional interference, daytime fatigue, greater anxiety and depression, poorer nighttime sleep, and poorer quality of life than comparable patients who maintain good circadian integration. The whole-genomic inspections of mutations in both colon and breast cancers in human have revealed specific retained mutations in Clock gene. Single nucleotide polymorphisms within clock, clock-controlled genes, and melatonin pathways may increase risk of cancer or protection from cancer. The role of Clock gene on cancer disease has been thoroughly explored.85-97
Besides, it is now reported that circadian clock controls cellular proliferation and the expression of cell cycle regulators as well. So, clock disturbed regular rhythmicity may thereby lead to abnormal cell proliferation. It is suggested that the negative regulators of core clock genes (Per2 and Per1) act also as tumor suppressors. Knocking down of these two genes doubles cancer cell numbers. Downregulation of their expression in cancer cells leads to double of cancer growth rate. Meanwhile, upregulation of their expression in cancer cells decreases both cancer growth rate and tumor numbers.84 Recently, clinical trials for the possibility of delivering Clock genes in cancer have been demonstrated.98-106 The miR-192/194 cluster has been determined using a forward genetic screen as a potent inhibitor of the all Per gene family members. So, the exogenous expression of miR-192/194 leads to disturbed 24 h rhythmicity. These results clarify posttranscriptional level control of the circadian clock.107
Modern approaches such as screening for small molecules targeting the circadian clock enhancing its amplitude and stabilizing its phase have been emerged. This strategy contributes to coordinate and consolidate circadian rhythmic organization. This in turn helps prevent and control human cancer. Medications and technologies targeted to molecular clock can be used to help late stage cancer patients feel and survive more normally. Encapsulation of doxorubicin (Adriamycin) in liposome (LipADM) called Adriamycin-encapsulated liposomes. This liposome is modified with the Ala–Pro–Arg–Pro–Gly (APRPG) peptide (APRPG–LipADM). APRPG peptide has high affinity to angiogenic sites.108 Three times of injection of Colon 26 NL-17 tumor-bearing mice with APRPG–LipADM preparation at four different time points induced altered tumor growth suppression depending on the dosing time. More potent tumor growth suppression was observed in the early dark phase than the late dark phase. The findings indicated that tumor growth suppression is correlated to some extent with the blood level of VEGF.
Transferrin receptor 1 (TfR1) regulates the uptake of iron-bound transferring. The abundance of TfR1 cell surface levels correlates with the rate of cellular proliferation.109 It introduces a new selective target for cancer therapy owing to the upregulated genetic expression in cancer than in normal cells. The expression levels of TfR1 in colon cancer cells of mice were under the control of molecular clock. A 24 h rhythm was demonstrated for both TfR1 mRNA and protein levels in colon cancer-bearing mice (Fig. 4, left panel). By using the luciferase reporter analysis and chromatin immunoprecipitation analysis, the clock-controlled gene c-MYC rhythmically activated TfR1 gene transcription. The antitumor efficacy of transferrin-conjugated liposome-delivered oxaliplatin and the platinum incorporation into tumor DNA were enhanced by drug administration at times correlated with the higher TfR1 expression (Fig. 4, right panel). The circadian rhythmicity of TfR1 expression may form an important aspect of strategies for TfR1-targeted cancer therapy.
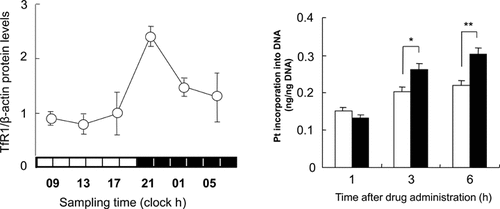
Variation (24 h) in the expression of TfR1 in Colon 26 tumor masses and the Pt incorporation into tumor DNA.109 Left panel shows temporal expression profile of TfR1 protein in implanted Colon 26 tumor cells. Cytoplasmic proteins were measured using each of the antibodies. The data were normalized using β-actin as a control. Mean ±SEM (n = 3) (*p < 0.05, analysis of variance). Right panel shows the influence of dosing time-dependent differences on the intratumoral delivery of L-OHP using Tf-NGPE liposomes in tumor-bearing mice. Colon 26 tumor-bearing mice were injected intravenously with a single dose of Tf-NGPE L-OHP (L-OHP: 7.5 mg/kg) or vehicle (9% sucrose) at 9:00 h (open column) or 21:00 h (closed column). The Pt incorporation into tumor DNA were measured by ICP-MS at the indicated times after an injection of Tf-NGPE L-OHP. Mean ±SEM (n = 3), *p < 0.05, **p < 0.01, comparison between the two groups.
CONCLUSIONS
It has been reported that the drug bioavailability, efficiency, and toxicity change during a 24 h period. However, recently, since 1997, the molecular mechanisms involved in these processes have started to emerge. This review aimed to highlight recent investigations showing the direct role of the molecular components of the circadian clockwork machinery in chronopharmaceutics. Many animal and human studies have deliberated time-dependent variations in the kinetics, effects, and toxicity of drugs that can be explained by cyclic rhythmicity of psycophysiological and biochemical processes under the control of circadian clock. Scheduling can be as important as dosing, or even more so, especially if circadian stage is critical and determines the probability of life versus death in response to the same stimuli, although some practitioners assumed that time to be negligible. The chronopharmacological investigations should be summarized and arranged in an easy format for therapeutic application. Clockwork genes with their well strict regulation ultimately control all physicopathological processes inside the body, representing the intraindividual variability within the 24 h of the day. Clock gene has been proved to directly control several diseases such as cancer, asthma, metabolic syndrome, and so on. Collation of the genetic expression profiles both in tissues from WT and Clock mutant mice showed that mutation in CLOCK alters the expression of many genes that their profiles are both rhythmically and nonrhythmically expressed throughout the day time. So, the 24 h expression profile of clock-controlled genes can be either rhythmic or nonrhythmic. The intraindividual and interindividual variability of molecular clocks should be taken into consideration for the sake of identifying rhythmic markers such as Clock genes for selecting dosing time of the best level of therapeutic efficacy and/or the lowest level of toxicity, rhythm monitoring, overcoming rhythm disruption, and rhythm manipulation (Fig. 5).11 This will lead to improved diffusion of the era of chronopharmacotherapy in the clinical practice. Chronopharmacotherapy may be used in different ways: to induce new rhythmicity of either the process or the gene by manipulating the environment of living organs, by using rhythmic administration of altered feeding schedules, or by using several drugs. This will result in appearance of the new dimension to the chronopharmacotherapy. Alteration of Clock genes rhythmic expression should be taken in our modern attention and consider it an undesirable effect, when it causes disrupted control of the normal circadian system. This serious problem affects the essential functioning of organisms. One suggestion to increase the therapeutic efficiency of pharmacotherapy is drugs administration at times when they are best tolerated. Chrono-DDS, focused on gene delivery and antibody delivery for specific molecular target of some diseases, may introduce new application clinical strategies for several diseases as well as provide perceptions into chronotherapy as a way to optimize contemporary treatments. Further clarifications of the relationship between the pharmacokinetics or pharmacodynamics and clockwork system could augment the development of new medical plans for several diseases intimately linked to clock gene delivery.
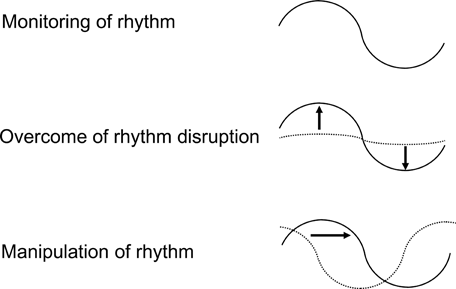
Schematic diagram of chronotherapeutic strategies based on the rhythm monitoring, rhythm manipulation, and rhythmic disruption overcoming from the viewpoints of molecular clock.11 Identification and monitoring the rhythmic marker will improve the dosing time for drug administration and may increase its therapeutic effects and/or reduce their side effects. Many drugs alter the 24 h rhythms of biochemical, physiological, and behavioral processes. Modification of rhythmicity is sometimes associated with therapeutic effects or it may lead to illness and altered homeostatic regulation. Attention should be paid to the alteration of biological rhythm and consider it as an adverse effect when it leads to a serious problem affecting basic functioning of living organisms. Furthermore, production of new rhythmicity by manipulating the conditions of living organs using altered feeding schedules or several drugs appears to lead to the new concept of chronopharmacotherapy.




