Establishment of In Vitro P-Glycoprotein inhibition assay and its exclusion criteria to assess the risk of drug–drug interaction at the drug discovery stage
Abstract
The decision tree to determine whether the P-glycoprotein (P-gp)/multidrug resistance protein 1 (MDR1)-mediated drug–drug interaction (DDI) study is recommended has been proposed by the International Transporter Consortium. We, therefore, designed an in vitro P-gp inhibition assay and determined the appropriate risk criteria for P-gp-mediated DDI at the drug discovery stage. Effects of P-gp inhibitors on digoxin transport across a monolayer of MDR1-expressing cells were examined. The IC50 (half-maximal inhibitory concentration) values generated from the efflux ratio (ER) were smaller than those generated from basolateral-to-apical directional apparent permeability. The difference in IC50 values was kinetically described in a compartment model analysis. This analysis indicated that ER is a highly sensitive parameter that can be used for the degree of P-gp inhibition. Considering IC50 values and the increase in digoxin exposure in clinical DDI studies, the risk criteria of [I2]/IC50 = 30 ([I2], theoretically maximal gastrointestinal concentration) was the optimal cutoff value to predict a clinically relevant DDI. We also investigated whether the IC50 value itself is applicable to assess the DDI risk. In conclusion, compounds with IC50 values less than 2 μM exhibit high risk for P-gp-mediated DDIs. However, compounds with IC50 values greater than or equal to 2 μM are inconclusive because clinical doses should be considered for the precise DDI risk assessment. © 2011 Wiley-Liss, Inc. and the American Pharmacists Association J Pharm Sci 100:4013–4023, 2011
Abbreviations used:
P-gp, P-glycoprotein; DDI, drug–drug interaction; ER, efflux ratio; Papp,BtoA, basolateral-to-apical directional apparent permeability; ITC, International Transporter Consortium; ABC, ATP-binding cassette; BBB, blood–brain barrier; UFLC, ultra-fast liquid chromatography; LC–MS/MS, liquid chromatography with triple quad mass spectrometric detection.
INTRODUCTION
The clinical significance of drug–drug interactions (DDIs) caused by P-glycoprotein [P-gp, ATP-binding cassette B1 (ABCB1), multidrug resistance protein 1 (MDR1)], breast cancer resistance protein (ABCG2), organic cation transporters, organic anion transporters, and organic anion transporting polypeptides has been introduced by the International Transporter Consortium (ITC).1 P-gp is clinically the most important ABC transporter involved in intestinal absorption, biliary and urinary excretion, and brain distribution of drugs.2, 3 Thus, a P-gp-related DDI may cause significant pharmacokinetic changes in the systemic drug exposure or tissue-specific distribution.4, 5 A decision tree to determine whether a clinical P-gp-mediated DDI study is recommended has been detailed in the White paper.1
P-gp and cytochrome P450 3A (CYP3A) generally function in a complementary fashion to reduce systemic exposure, and considerably overlap in substrate specificity.6 Selection of an appropriate P-gp probe substrate, which is not predominantly affected by metabolism and other transporters, is essential to increase the understanding of the P-gp-mediated DDI. Digoxin is a specific P-gp probe substrate used to assess the potential of P-gp inhibition. Owing to the digitalis-mediated toxicity of digoxin, a 25% increase in digoxin exposure in human plasma in the presence of P-gp inhibitors has been recognized as clinically relevant.7 Talinolol is another recommended substrate, which is not greatly metabolized, but secreted in human intestine.8 In this investigation, we focused on digoxin as a P-gp probe substrate.
An in vitro assessment of P-gp inhibition in choosing drug candidates that are not likely to cause P-gp-mediated DDI is important at the drug discovery stage. Scientists from the pharmaceutical industry and academia have established in vitro systems for P-gp inhibition assays using the transcellular transport system [i.e., Caco-2, LLC-PK1, and Madin-Darby canine kidney (MDCK) cells] to predict the P-gp-mediated DDI. However, the parameters used to calculate half maximal inhibitory concentration (IC50) values vary between laboratories. For example, the P-gp-mediated transport rate of digoxin was calculated from the basolateral-to-apical directional apparent permeability (Papp,BtoA).9, 10 In vitro–in vivo correlation analysis focusing on drug transport across the blood–brain barrier (BBB) revealed that the efflux ratio (ER, Papp,BtoA/Papp,AtoB,) across a monolayer of P-gp-expressing cells is a good predictor of in vivo function at the BBB.11, 12 In fact, the ITC recommended that ER should be used as an indicator to determine whether a drug candidate is a P-gp inhibitor. To date, no data have been reported regarding whether there is a difference between the IC50 values generated from ER and Papp,BtoA in the transcellular transport system. In addition, no consideration for the difference between the IC50 values has been done previously. Hence, we compared the IC50 values generated from ER and Papp,BtoA, and discussed an appropriate parameter to predict in vitro P-gp inhibition based on a theoretical approach by using a compartment model analysis.
The US Food and Drug Administration draft guidance document and previous reports have suggested that drugs exhibiting an [I1]/IC50 value greater than or equal to 0.1 or an [I2]/IC50 value greater than or equal to 10 should be evaluated for their clinical DDI potential.1,13 [I1] represents the mean unbound steady-state maximum concentration (Cmax) following administration of the highest clinical dose. However, drug concentrations in the intestine could be higher than those in the systemic circulation after oral administration. Bjornsson et al.14 proposed that the theoretically maximal gastrointestinal concentration ([I2]), defined as the clinical dose divided by 250 mL (volume of a glass of water), should be used as an alternative index to predict the gut-oriented DDI. Although cutoff values to predict a clinically relevant DDI have been proposed,7,15, 16 there is no general approach on DDI risk assessment. The aim of this work was to develop an appropriate in vitro evaluation system for P-gp inhibition and its exclusion criteria to assess the risk of P-gp-mediated DDI with lower false negative and false positive rates at the drug discovery stage.
MATERIALS AND METHODS
Materials
Digoxin, verapamil, and lucifer yellow were purchased from Sigma–Aldrich (St. Louis, Missouri). Elacridar and cyclosporin A were from Toronto Research Chemicals, Inc. (North York, Ontario, Canada). Itraconazole was from MP Biomedicals, LLC (Tokyo, Japan). Ketoconazole and quinidine were from Wako Pure Chemicals Industries (Osaka, Japan). All other chemicals were of the highest reagent grade available from commercial sources.
HTS Transwell® 96-well permeable support plates with a polyethylene terephthalate membrane, 0.4-μm pore size and 0.143-cm2 surface area, were purchased from Corning Life Sciences (Lowell, Massachusetts).
Cell Culture
LLC-PK1 cells expressing human MDR1 were cultured with slight modification as previously described.17 Cells were collected by adding 0.05% trypsin/ethylenediaminetetraacetic acid solution; next, cells were suspended in cell culture media (Dulbecco's modified eagle medium with 20% fetal bovine serum, 1% penicillin, streptomycin, and 150 ng/mL colchicine) and seeded onto 96-well insert plates (1.125×105 cells/well). These plates were incubated in an atmosphere of 5% CO2–95% air at 37°C. Fresh medium was replaced on the third or fourth day and the transcellular transport study was conducted on the seventh day after seeding.
In Vitro P-gp Inhibition Assay for Digoxin Transport
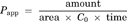 (1)
(1)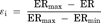 (2)
(2)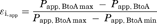 (3)
(3)Quinidine was used as a marker of IC50 variability to ensure the reliability of the generated data. The mean (SD) of the IC50 values based on ER and Papp,BtoA were 1.52 (0.35) and 8.53 (2.41) μM, respectively, indicating that the results of inhibition studies can be independently replicated.
Quantification of Samples from In Vitro P-gp Inhibition Assay
Samples from the in vitro P-gp inhibition assay for digoxin transport were mixed with an equal volume of acetonitrile, including 100 nM of digoxin-d3 (internal standard), and centrifuged at 4°C and 5084 × g for 5 min (Sorvall Legend RT; Thermo Fisher Scientific, Yokohama, Japan). The supernatant was diluted with an equal volume of 0.1% formic acid solution. The sample was mixed, centrifuged at 4°C and 5084 × g for 5 min, and the supernatant was analyzed using MS/MS analysis (API4000, AB SCIEX) equipped with an UFLC apparatus (Shimadzu). The analytical column used was an Atlantis T3, C18 column (20 mm × 2.1 mm, 3 μm) (Waters, Milford, Massachusetts). The total run time, flow rate, and column temperature were 1 min, 1 mL/min, and 50°C, respectively. Mobile phases A and B consisted of 0.2% formic acid in distilled water and 0.2% formic acid in methanol, respectively. The initial concentration of mobile phase B was 5% and this condition was maintained for 0.02 min followed by linear increase of B to 95% over the next 0.03 min, and held for 0.45 min. The condition was then returned to initial concentrations and held for 0.5 min for reequilibration. The lowest limit of quantification was 4.6 nM and the standard curve was linear (r2 > 0.99) over the digoxin concentration range of 4.6–10,000 nM. Detailed MS conditions are shown in Table 1. Accuracy of standards and intraday and interday variability was within ±15%.
| Mass Spectrometric Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | Ionization Mode | Detection Mode | m/z | Source Temperature (°C) | CE (eV) | DP (V) | EP (V) | CXP (V) |
| Digoxin | Electrospray Ionization | Multiple reaction | 825.5/779.4 | 450 | −32 | −90 | −10 | −15 |
| Digoxin-d3 (I.S.) | (Negative-ion Mode) | monitoring (MRM) | 830.5/784.4 | 450 | −32 | −90 | −10 | −15 |
| Elacridar | Electrospray ionization | 564.2/252.2 | 550 | 55 | 100 | 10 | 15 | |
| Itraconazole | (Positive-ion mode) | 705.4/450.4 | 550 | 40 | 100 | 10 | 15 | |
| Cyclosporin A | 1202.8/100.3 | 550 | 80 | 108 | 10 | 8 | ||
| Ketoconazole | 531.3/489.3 | 550 | 30 | 100 | 10 | 15 | ||
| Verapamil | 456.2/165.1 | 550 | 39 | 68 | 10 | 10 | ||
| Quinidine | 326.3/308.1 | 550 | 33 | 61 | 10 | 26 | ||
| Alprenolol (I.S.) | 250.3/116.3 | 550 | 71 | 25 | 10 | 10 | ||
- I.S.: internal standard; CE, collision energy; DP, declustering potential; EP, entrance potential; CXP, collision cell exit potential.
Determination of Plasma Protein Binding
Unbound fractions in human plasma for itraconazole, cyclosporin A, ketoconazole, verapamil, quinidine, and talinolol were determined using the 96-well equilibrium dialysis apparatus (HTDialysis, LLC, Gales Ferry, Connecticut). Pooled human plasma was purchased from KAC (Kyoto, Japan). Tested compounds were added into the control plasma, (final concentration = 1 μM) and 150-μL aliquots were added into the dialysis well, which was separated by the dialysis membrane (singlestrip HTD96a/b; HTDialysis, LLC). The receiver chamber was loaded with 150 μL of 50 mM phosphate buffered saline (pH 7.4). The apparatus was placed on a shaker (87 rpm) (NR-1; TAITEC, Tokyo, Japan) for 20 h at the room temperature. Previous in-house experiments revealed that equilibrium would be adequately achieved within an incubation period of 20 h (data not shown). After incubation, aliquots (50 μL) from donor and receiver sides were mixed with 50-μL aliquots of either the dialyzed control buffer or human plasma to yield an identical matrix so that all samples could be quantified using the same matrix component. Additionally, 150 μL of acetonitrile containing 100 ng/mL alprenolol as an internal standard was added to every sample, vigorously mixed, and centrifuged at 4°C (5084 x g) for 5 min. The supernatant was diluted with 0.2% (v/v) formic acid in 10 mM ammonium formate (pH 3) and the concentration of each aliquot was determined using MS/MS analysis equipped with an UFLC apparatus. The unbound fraction in plasma was calculated by the ratio of concentrations in receiver and donor sides of the dialysate. The analytical column used was a Shim-pack XR-ODS, C18 column (20 mm × 2.0 mm, 5 μm) from Shimadzu. Total run time, flow rate, and column temperature were 2.6 min, 0.5 mL/min, and 50°C, respectively. Mobile phases A and B consisted of 0.2% (v/v) formic acid in 10 mM ammonium formate and acetonitrile, respectively. The initial concentration of mobile phase B was 10%, and this condition was maintained for 0.2 min followed by a linear increase of B to 95% over the next 0.2 min, and held for 1 min. The condition was then returned to the initial concentration and held for 1.2 min for reequilibration. Detailed MS conditions are shown in Table 1. Accuracy of standards and interassay variability were within ±15%.
Risk Assessment of P-gp-Mediated Digoxin DDI
Literature research on DDI studies with digoxin was conducted to assess the effects of potential P-gp inhibitors on digoxin exposure. In this report, a 25% increase of digoxin exposure [area under the curve (AUC) and Cmax] in the presence of P-gp inhibitors was categorized as a clinically significant change because the digitalis-induced toxicity was observed.16 The mean unbound steady-state Cmax following administration of the highest clinical dose is defined as [I1], and the theoretically maximal gastrointestinal concentration (dose/250 mL) is defined as [I2] according to a previous report.14 The unbound fractions in human plasma were ascertained from the literature research18, 19 and in-house experimental data.
The result of risk assessment of P-gp-mediated digoxin DDI can be categorized into four groups. True positive and true negative mean that in vitro P-gp inhibition data are in accordance with the clinical digoxin DDI. False negative means in vitro data are not in agreement with a clinical digoxin DDI, where the increase of AUC is greater than or equal to 1.25-fold; on the contrary, false positive means in vitro data are not in agreement with a clinical digoxin DDI, where the increase of AUC is less than 1.25-fold.
RESULTS AND DISCUSSION
In Vitro P-gp Inhibition Assay for Digoxin Transport
To estimate the inhibitory potency of typical P-gp inhibitors (elacridar, itraconazole, cyclosporin A, ketoconazole, verapamil, and quinidine) on digoxin transport (10 μM), in vitro P-gp inhibition assays using LLC-PK1 cells expressing human MDR1 were performed. The sigmoid inhibition curve indicates that ER and Papp,BtoA of digoxin decreased in a concentration dependent manner (Fig. 1). The inhibitory potency of elacridar on digoxin transport was over 10 times greater than other P-gp inhibitors. These results are comparable to those of a previous report in which the inhibitory effect of compounds was determined according to Papp,BtoA of [3H]digoxin (30 nM) using MDCKII–MDR1 cells.9
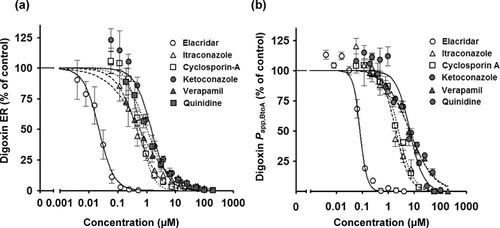
Concentration-dependent inhibition of ER and Papp,BtoA of digoxin (10 μM) by elacridar, itraconazole, cyclosporin A, ketoconazole, verapamil, and quinidine using LLC-PK1 cells expressing human MDR1. Vertical axes are shown as a percentage of (a) digoxin ER and (b) Papp,BtoA in the presence of P-gp inhibitor according to Eqs. 2 and 3, respectively. The transport study was conducted at 37°C for 2 h. Each point represents the mean (SD) of three or four independent measurements.
Comparison of IC50 Values Generated from ER and Papp,BtoA
The IC50 values of 48 compounds were ascertained to assess the potential of P-gp inhibitory activity (Table 2). The IC50 values of compounds that did not achieve more than 50% inhibition at the highest concentration were greater than 60 μM. The IC50 values were calculated using inhibition of ER and Papp,BtoA by using Eqs. 2 and 3, respectively. Elacridar showed the strongest P-gp inhibitory effect with IC50 values of 0.022 [IC50(ER)] and 0.074 [IC50(Papp,BtoA)] μM, followed by carvedilol, itraconazole, verapamil, and cyclosporin A. The IC50 values generated from Papp,BtoA were larger than those from ER for all compounds tested, and there was a positive correlation (r2 = 0.61) between IC50(ER) and IC50(Papp,BtoA) (Fig. 2).
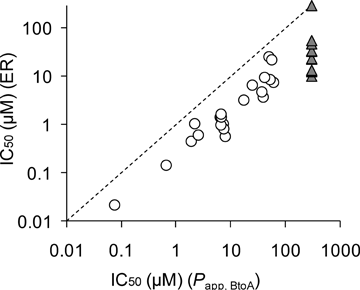
Correlation between IC50 values of 48 compounds generated from ER and Papp,BtoA. Vertical and horizontal axes are IC50 values generated from the inhibition of ER [IC50(ER)] and Papp,BtoA [IC50(Papp,BtoA)]. The dotted line indicates that IC50(ER) is equal to IC50(Papp,BtoA). Closed triangles indicate that IC50(ER) and/or IC50(Papp,BtoA) were not calculated because IC50 values were greater than 60 μM, which is the highest concentration of inhibitors.
| IC50 (μM) | IC50 (μM) | ||||
|---|---|---|---|---|---|
| Compounds | ER | Papp,BtoA | Compounds | ER | Papp,BtoA |
| Elacridar | 0.022 | 0.074 | Nifedipine | 23 | >60 |
| Carvedilol | 0.15 | 0.65 | Chlorpromazine | 24 | >60 |
| Itraconazole | 0.45 | 1.9 | Memantine | 26 | 49 |
| Verapamil | 0.57 | 7.9 | Vinblastine | 34 | >60 |
| Cyclosporin A | 0.62 | 2.5 | Clarithromycin | 34 | >60 |
| Astemizole | 0.83 | 7.4 | Nitrendipine | 35 | >60 |
| Loperamide | 1.0 | 6.5 | Flecainide | 48 | >60 |
| Quinidine | 1.0 | 7.2 | Etoricoxib | 57 | >60 |
| Nicardipine | 1.1 | 2.2 | Atorvastatin | >60 | >60 |
| Ketoconazole | 1.5 | 6.4 | Diazepam | >60 | >60 |
| Lapatinib | 1.5 | 6.6 | Dexamethasone | >60 | >60 |
| Mibefradil | 1.7 | 6.6 | Erythromycin | >60 | >60 |
| Quinine | 3.3 | 17 | Galanthamine | >60 | >60 |
| Bepridil | 3.8 | 39 | Ibuprofen | >60 | >60 |
| Simvastatin | 4.9 | 37 | Propantheline | >60 | >60 |
| Spironolactone | 6.7 | 25 | Ranitidine | >60 | >60 |
| Felodipine | 7.7 | 63 | Fexofenadine | >60 | >60 |
| Propanolol | 8.8 | 52 | Eplerenone | >60 | >60 |
| Dipyridamole | 9.7 | 41 | Aliskiren | >60 | >60 |
| Amiodarone | 10 | >60 | Ramelteon | >60 | >60 |
| Isradipine | 13 | >60 | Talinolol | >60 | >60 |
| Midazolam | 13 | >60 | Captopril | >60 | >60 |
| Diltiazem | 14 | >60 | Scopolamine | >60 | >60 |
| Sertraline | 23 | 56 | Rofecoxib | >60 | >60 |
- IC50 values of compounds that did not show enough inhibition (>50%) at the highest concentration (60 μM) were described as >60 μM. Mean (n = 4).
Theoretsical Consideration of the Difference Between IC50 Values Generated from ER and Papp,BtoA
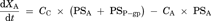 (4)
(4)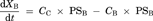 (5)
(5)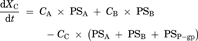 (6)
(6)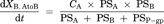 (7)
(7)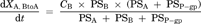 (8)
(8)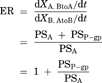 (9)
(9)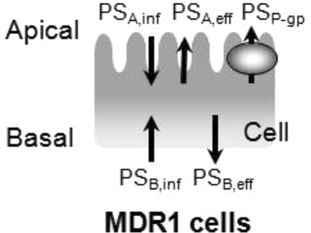
Compartment model to delineate the flux in MDR1-expressing cells. P-gp is expressed on the apical membrane of LLC-PK1 cells and it transports substrates from the intracellular area into the apical chamber of the transwell. PSP-gp represents the permeability–surface area product for P-gp-mediated efflux across the apical membrane. PSA,inf and PSA,eff represent the passive permeability–surface area products of the apical membrane. PSB,inf and PSB,eff represent the passive permeability–surface area products of the basolateral membrane. It is assumed that the influx and efflux passive permeability–surface area are unity (PSA,inf = PSA,eff = PSA, PSB,inf = PSB,eff = PSB).
 (10)
(10)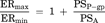 (11)
(11)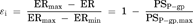 (12)
(12) (13)
(13)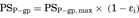 (14)
(14) (15)
(15)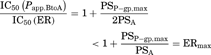 (16)
(16)Consequently, the difference of IC50 values generated from two parameters was kinetically described using the compartment model analysis. The difference between the IC50 values generated from ER and Papp,BtoA may be attributed to the fact that the inhibitory rate based on Papp,BtoA (εi,app) cannot neglect the influence of passive permeability. Additionally, our experimental data and model analysis indicate that ER is a highly sensitive parameter for estimating the degree of P-gp inhibition as compared to Papp,BtoA. Thus, ER is an appropriate parameter for estimating the IC50 values of P-gp inhibitors and was used for the subsequent risk assessment of clinical P-gp-mediated DDI studies.
Risk Assessment of P-gp-Mediated Digoxin DDI
To evaluate whether the investigational drug leads to clinically relevant DDI with digoxin, risk assessment of P-gp-mediated DDI was performed considering IC50(ER) and the increase of digoxin plasma concentration in clinical DDI studies. Clinical drug interaction studies of digoxin and concomitant drugs are summarized in Table 3. In this report, a 25% increase in digoxin exposure (AUC and Cmax) in the presence of P-gp inhibitors was determined to be clinically significant. Literature research indicated that 15 out of 36 DDI studies with digoxin were clinically relevant. Ratios of [I1]/IC50 and [I2]/IC50 were calculated to determine the cutoff limit to predict a clinically relevant digoxin DDI. The highest values of [I1]/IC50 and [I2]/IC50 (0.79 and 7182) were observed in the clinical digoxin DDI concomitantly administered with quinidine. The AUC ratio of digoxin was also the highest value (2.65) in this study (Table 3).
| Efflux Ratio (ER) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compounds | AUCi/AUC Ratio | Cmaxi/Cmax Ratio | Inhibitor Concentration (μM) : [I1] | Gastrointestinal Concentration (μM) : [I2] | Unbound Fraction in Human Plasma18,19 | [I1]/IC50 | [I2]/IC50 | References |
| Cyclosporin A | – | 1.44 | 2.80 | 1167 | 0.045 | 0.20 | 1882.26 | 13 |
| Quinidine | – | 1.44 | 4.50 | 1022 | 0.16 | 0.70 | 992.23 | 13 |
| Quinidine | 2.65 | – | 5.10 | 7398 | 0.16 | 0.79 | 7182.33 | 16 |
| Quinidine | 1.76 | 1.76 | 3.54 | 3397 | 0.16 | 0.55 | 3297.96 | 16 |
| Amiodarone | 1.68 | 1.84 | 2.20 | 4694 | 0.00020 | <0.01 | 455.68 | 16 |
| Itraconazole | 1.68 | 1.34 | 0.95 | 1134 | 0.0020 | <0.01 | 2520.00 | 13 |
| Clarithromycin | 1.64 | 1.83 | 2.70 | 1337 | 0.50 | 0.04 | 39.49 | 13 |
| Amiodarone | 1.63 | 1.72 | 2.49 | 2347 | 0.00020 | <0.01 | 227.86 | 16 |
| Carvedilol | 1.56 | 1.38 | 0.13 | 62 | 0.050 | 0.04 | 418.94 | 16 |
| Diltiazem | 1.51 | 1.37 | 0.17 | 532 | 0.22 | <0.01 | 38.75 | 13 |
| Verapamil | 1.51 | 1.44 | 1.20 | 652 | 0.093 | 0.20 | 1143.23 | 16 |
| Verapamil | 1.50 | 1.44 | 0.13 | 704 | 0.093 | 0.02 | 1235.09 | 13 |
| Diltiazem | 1.44 | 1.38 | 0.70 | 532 | 0.22 | 0.01 | 38.76 | 16 |
| Captopril | 1.39 | 1.59 | 0.23 | 230 | 0.75 | <0.01 | 3.84 | 16 |
| Mibefradil | 1.31 | 1.41 | 2.42 | 1211 | 0.010 | 0.01 | 718.09 | 16 |
| Diltiazem | 1.24 | 1.24 | 0.70 | 798 | 0.22 | 0.01 | 58.14 | 16 |
| Talinolol | 1.23 | 1.45 | 0.62 | 1100 | 0.49 | <0.01 | 18.34 | 32 |
| Nifedipine | 1.23 | 1.06 | 0.23 | 116 | 0.040 | <0.01 | 4.98 | 16 |
| Nifedipine | 1.21 | 1.01 | 0.11 | 58 | 0.040 | <0.01 | 2.49 | 16 |
| Felodipine | 1.18 | 1.34 | 0.030 | 104 | 0.0040 | <0.01 | 13.55 | 16 |
| Nifedipine | 1.18 | 1.08 | 0.46 | 231 | 0.040 | <0.01 | 9.96 | 16 |
| Eplerenone | 1.16 | 1.05 | 4.51 | 965 | 0.40 | 0.03 | 16.08 | 7 |
| Atorvastatin | 1.15 | 1.20 | 0.049 | 265 | 0.020 | <0.01 | 4.42 | 13 |
| Atorvastatin | 1.15 | 1.20 | 0.33 | 573 | 0.020 | <0.01 | 9.55 | 16 |
| Nitrendipine | 1.15 | 1.57 | 0.030 | 222 | 0.01 | <0.01 | 6.40 | 16 |
| Isradipine | 1.11 | 1.26 | 0.020 | 162 | 0.05 | <0.01 | 12.62 | 16 |
| Sertraline | 1.10 | 1.05 | 0.39 | 2612 | 0.01 | <0.01 | 116.00 | 16 |
| Midazolam | 1.10 | 1.05 | 0.032 | 25 | 0.020 | <0.01 | 1.83 | 33 |
| Dipyridamole | 1.08 | 1.23 | – | 2378 | 0.01 | – | 244.27 | 34 |
| Etoricoxib | 1.06 | 1.33 | – | 1338 | 0.08 | – | 23.67 | 35 |
| Nicardipine | 1.06 | 1.06 | 0.18 | 248 | 0.05 | <0.01 | 232.52 | 16 |
| Atorvastatin | 1.03 | 1.10 | 0.020 | 72 | 0.020 | <0.01 | 1.19 | 16 |
| Captopril | 1.03 | 0.95 | – | 230 | 0.75 | – | 3.83 | 36 |
| Rofecoxib | 1.04 | 1.00 | – | 954 | 0.13 | – | 15.91 | 37 |
| Ramelteon | 0.95 | 0.92 | – | 247 | 0.18 | – | 4.11 | 38 |
| Aliskiren | 0.86 | 0.92 | – | 2175 | – | – | 36.25 | 39 |
- [I1] represents the mean unbound Cmax at the highest clinical doses (μM).
- [I2] represents maximal gastrointestinal concentration (μM).
- –represents no data.
The consideration of [I1] and [I2] is one of the key factors to predict the clinical digoxin DDI. The definition of [I1] used in the White paper1 is the steady-state unbound Cmax following administration of the highest clinical dose. Indeed, the unbound drug is thought to be pharmacologically active and accessible to transporters as a substrate and/or an inhibitor. As shown in Table 3, [I1]/IC50 values were higher than 0.1, which is the recommended cutoff limit by the White paper, for cyclosporin A and quinidine that significantly increase the digoxin concentration in the clinical DDI study. However, in most cases, [I1]/IC50 values were less than 0.1, even though clinically relevant DDIs were observed. The [I1] may not be suitable to predict clinically relevant DDIs at the intestinal absorption. The [I2] is defined as the clinical dose divided by 250 mL, which is the volume of a glass of water. Although the potential of P-gp inhibition may be overestimated for poorly soluble drugs, it is important to take a safety risk assessment when it comes to selection of candidate compounds. To improve the predictability of actual intestinal concentration of P-gp inhibitors, consideration of the intestinal fluid volume and solubility of compounds is necessary. Tachibana et al reported that the estimated intestinal fluid volume was 1.92 L. In addition, the solubility of compounds in the estimated intestinal fluid was evaluated for the prediction of P-gp-mediated DDI.22 Further investigations may be required to validate the estimated volume in either fasted or fed condition.
In Vitro Cutoff Values ([I2]/IC50) to Predict the Clinical Digoxin Interaction Studies
The appropriate in vitro cutoff value to predict P-gp-mediated DDIs was investigated using [I2]/IC50 because [I2] indicates the theoretical maximal gastrointestinal concentration. Results of risk assessment are categorized into the true positive, true negative, false positive, and false negative. A true positive indicates an in vitro prediction corresponding to the observation that digoxin AUC and Cmax increased by 25% in the presence of P-gp inhibitors. On the contrary, a true negative indicates an in vitro prediction corresponding to the observation that digoxin AUC and Cmax did not significantly change. The accuracy of DDI risk assessment is shown in Figure 4, indicating that the risk criteria of [I2]/IC50 = 30 was the optimal cutoff value to predict a clinically relevant DDI [the average of true positive and negative percentage (85% accuracy)]. The reliability of the criteria was supported by a previous report using a literature search.22 The IC50 values for compounds that showed less than 50% inhibition of ER at the highest concentration (60 μM) were assumed to be 60 μM in this investigation. The ITC recommended that investigational drugs with an [I2]/IC50 value greater than or equal to 10 should be evaluated using the clinical P-gp-mediated DDI potential.1 However, using these criteria, the false negative rate increased to 52%, which may result in the overestimation of P-gp inhibition potential for drug candidate. At the drug discovery stage, it is primarily important to raise an alert for compounds with severe potential of DDIs in advance to the following clinical study. To set the criteria for compounds selection, the weighting between false positives and false negatives should be well-balanced. From a safety perspective, a false negative error has greater consequences than a false positive because it may result in unexpected side effect caused by P-gp-mediated DDI in clinic. However, from the view of accuracy of risk assessment, the exclusion criteria should not be too conservative; otherwise promising candidate compounds may be discarded with a false positive data. In Figure 4, only captopril was categorized into false negative at [I2]/IC50 values of 10 and 30. And the false negative result with captopril is not due to P-gp inhibition, but is attributed to the reduction of renal clearance of digoxin in the presence of captopril.23 Accordingly, we propose that the cutoff value of [I2]/IC50 = 30 generated from ER would be the appropriate parameter to evaluate the potential of P-gp-mediated DDI in intestinal absorption.
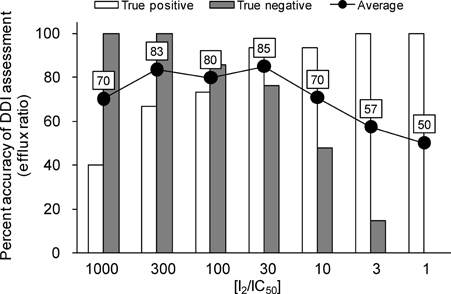
Accuracy of DDI risk assessment using in vitro cutoff values ([I2]/IC50). Vertical axis indicates the accuracy percentage of DDI assessment using the efflux ratio to estimate IC50 values of P-gp inhibitors. Open and closed columns indicate the accuracy percentage of true positives and true negatives, respectively. The average of accuracy percentages of true positives and true negatives are shown by the line in this figure.
Risk Assessment of P-gp-Mediated Digoxin DDI from In Vitro IC50 Values
At the drug discovery stage, it is challenging to set the efficacious doses unless a precise estimation of pharmacodynamics using pharmacokinetic–pharmacodynamic modeling and analysis in humans is possible.24 It is also important to provide the appropriate exclusion criteria of P-gp inhibition for the candidate selection; otherwise a false positive prediction may lead to exclude a number of candidate compounds that may not have the potential DDI in clinic. Therefore, we investigated whether in vitro IC50 value itself is applicable for determining the risk assessment of P-gp-mediated DDIs. A retrospective analysis of clinical digoxin DDI studies revealed that compounds with IC50 values less than 2 μM significantly increased digoxin exposure (9 out of 10 compounds). A false positive result was observed for nicardipine, with an IC50(ER) value of 1.1 μM and [I2]/IC50 value of 232.5. This discrepancy is potentially due to the overestimation of actual intestinal concentration of nicardipine in humans, leading to a false positive in DDI prediction. Indeed, nicardipine is a representative CYP3A substrate in humans.25 Additionally, the intrinsic clearance value of nicardipine determined on the basis of its metabolic stability with pooled human intestinal microsomes was larger than most CYP3A substrates. The fraction of the dose not metabolized by intestinal metabolic enzymes in humans (Fg in human) was 0.64.26 Therefore, [I2] may overestimate the actual intestinal concentration of nicardipine. On the contrary, a false negative result was observed for captopril in DDI prediction (Table 4). The 39% increase of digoxin AUC in the presence of captopril27 was observed because captopril decreases renal clearance of digoxin.23 As a result, a retrospective analysis of clinical digoxin DDI studies suggested that compounds with IC50 values less than 2 μM have high risk for P-gp-mediated DDI.
| IC50 (μM) | ||||
|---|---|---|---|---|
| Digoxin DDI Ratio | IC50 < 2 | 2 ≤ IC50 < 20 | 20 ≤ IC50 < 60 | 60 ≤ IC50 |
| 1.25 ≤ R | 9 | 4 | 1 | 1 |
| R < 1.25 | 1 | 5 | 6 | 9 |
- R: AUC ratio in the presence or absence of P-gp inhibitors.
Extensive research on P-gp-mediated DDIs at the intestinal absorption process with P-gp-specific substrates (i.e., digoxin and talinolol) has been performed in the pharmaceutical industry and academia.10,13,15, 16 At the same time, P-gp-mediated DDIs with CYP3A4/5 and P-gp dual substrates in the intestine have been of great interest. Cyclosporin A and tacrolimus are immunosuppressant drugs and metabolic substrates for CYP3A4 and CYP3A5. They are also actively transported out of cells by P-gp.28 The increase in plasma concentrations of cyclosporin A29 and tacrolimus30 in the presence of ketoconazole, which is a dual inhibitor for CYP3A4/5 and P-gp, were reported. The inhibition of CYP3A4/5 may increase Fg, whereas the inhibition of P-gp may increase the fraction absorbed (Fa) in intestine. In the case of DDI study of paclitaxel (Fa × Fg = 0.045), the increase in systemic exposure of paclitaxel in combination with elacridar is same in magnitude (sevenfold increase) as in combination with cyclosporin A, which is a dual inhibitor for CYP3A4/5 and P-gp.31 Thus, it is important to pay attention to the impact of P-gp inhibition as well as CYP3A4/5 inhibition. Further quantitative investigation will be needed to predict the risk of DDIs involving both CYP3A4/5 and P-gp.
CONCLUSION
We established an in vitro evaluation system of P-gp inhibition with the appropriate risk criteria ([I2]/IC50 value of 30) taking in vitro IC50 values based on ER and the increase of digoxin plasma concentration in clinical DDI studies into account. This investigation also showed that compounds with IC50 values less than 2 μM may increase the risk for P-gp-mediated DDI. On the contrary, compounds with an IC50 value greater than or equal to 2 μM may be inconclusive because clinical doses should also be considered for the precise DDI risk assessment.




