Quantitative targeted absolute proteomics-based ADME research as a new path to drug discovery and development: Methodology, advantages, strategy, and prospects
Abstract
An understanding of the functional roles of proteins, for example, in drug absorption, distribution, metabolism, elimination, toxicity, and efficacy (ADMET/efficacy), is important for drug discovery and development. Equally, detailed information about protein expression is required. Recently, a new protein quantification method, called quantitative targeted absolute proteomics (QTAP), has been developed on the basis of separation and identification of protein digests by liquid chromatography–linked tandem mass spectrometry with multiple reaction monitoring. Target peptides for quantification are selected only from sequence information, so time-consuming procedures such as antibody preparation and protein purification are unnecessary. In this review, we introduce the technical features of QTAP and summarize its advantages with reference to recently reported results. These include the evaluation of species differences of blood–brain barrier protein levels among human, monkey, and mouse. The high selectivity of QTAP and its ability to quantify multiple proteins simultaneously make it possible to determine the absolute expression levels of many proteins in tissues and cells in both physiological and disease states. Knowledge of absolute expression amounts, together with data on intrinsic protein activity, allows us to reconstruct in vivo protein function, and this should be an efficient strategy to predict ADMET/efficacy of drug candidates in humans in various disease states. © 2011 Wiley-Liss, Inc. and the American Pharmacists Association J Pharm Sci 100:3547–3559, 2011
Abbreviations used:
3-OMG, 3-o-methyl-d-glucose; ABC, ATP-binding cassette; ADMET, absorption, distribution, metabolism, elimination, and toxicity; CYP, cytochrome P450; GLUT1, glucose transporter 1; HR-MRM, high-resolution multiple reaction monitoring; LC–MS/MS, liquid chromatography-linked tandem mass spectrometry; m/z, mass-to-charge ratio; MCT1, monocarboxylate transporter 1; MRM, multiple reaction monitoring; PET, positron emission tomography; PPx, pharmacoproteomics; QTAP, quantitative targeted absolute proteomics; QTOF, quadrupole time-of-flight; Triple Q, triple quadrupole; RT-PCR, reverse transcription-polymerase chain reaction.
INTRODCUTION
Transporters, enzymes, receptors, and channels are well known to play key roles in drug absorption, distribution, metabolism, elimination, and toxicity (ADMET) and therefore have an important influence on drug efficacy. Both pharmacogenomics and pharmacogenetics approaches have been used in drug discovery and development to elucidate the functions of these proteins in ADMET research,1,2 as well as to establish the extent of interindividual variability. Recent advances in mass spectrometry (MS) equipment and global proteomics have facilitated the discovery of novel target molecules, surrogate markers, and biomarker proteins. It is now possible to identify thousands of proteins in single analysis, affording large lists of candidate drug targets in preclinical studies and candidate biomarkers in clinical studies. However, for rational and efficient drug discovery and development, these candidates must be validated, and so it is important to understand the roles of these proteins, for example, how these proteins influence ADMET/efficacy of drugs and how these proteins are in turn affected by drugs.
In the validation step, quantitative analysis of protein expression is a useful strategy for comparison between control and target conditions. Antibody-based quantification, such as immunoblotting and various immunoassays including enzyme-linked immunosorbent assay (ELISA), with specific antibodies have been used for this purpose, but the preparation of suitable antibodies can be difficult, especially when the amino acid sequences of candidate proteins are very similar. Even if antibodies are available, cross-reactivity remains an issue. For example, whole proteome analysis of yeast proteins demonstrated that one polyclonal antibody reacted with 1700 different yeast proteins.3 Furthermore, the results of quantification of soluble epidermal growth factor receptor varied by over 1000-fold among three different ELISA assays.4 Such problems have led to a focus on pharmacogenomics, although gene expression levels do not always correlate to the protein expression levels.5
Recently, new methodology for protein quantification has been developed on the basis of liquid chromatography–linked tandem MS (LC–MS/MS) with multiple reaction monitoring (MRM). The standard proteomics approach has been to identify proteins in biological samples as comprehensively as possible from mass spectral information, this is called global proteomics. The new method is called quantitative targeted absolute proteomics (QTAP) because the proteins to be quantified are first selected as target proteins, and only these target proteins are quantified to measure their absolute expression levels. QTAP overcomes many of the difficulties associated with protein quantification with antibodies (Table 1), and is expected to open up the new research field of pharmacoproteomics (PPx). In this review, we introduce the technical features of QTAP as a protein quantification methodology and summarize its advantages with reference to recently reported results, and discuss some possible applications of QTAP in drug discovery and development.
| Antibody-Based Analysis | LC–MS/MS-Based Analysis | ||
|---|---|---|---|
| Quantification | Binding assay using antibody | Direct quantification of peptide | |
| Method availability | Antibody needed to be available | Target peptide need to be available | |
| Method development | Takes long time for development, including antigen preparation, immunization, and specificity validation | ≪ | About 1 month for development, including target peptide selection, peptide synthesis, and MS optimization |
| Antigen protein or peptide is necessary | ≪ | Target peptide is selected from sequence information | |
| Specificity | Depending on antibody, and difficult to validate specificity | ≪ | High specificity by mass filter, and specificity is confirmed by database |
| Difficult to prepare specific antibodies for modification and mutation in proteins | < | Easy to distinguish modification and mutation by mass | |
| Sensitivity | High sensitivity (pg/mL–ng/mL) | > | Medium sensitivity (ng/mL) |
| Multiprotein assay | 1 protein/1 assay | ≪ | 37 proteins/1 assay |
| Multisample assay | 96 or more samples/1 assay | ≫ | 1 sample/1 assay |
| Instrument | Simple instrumentation | > | Expensive and high-tech instrumentation |
- “<” or “≪” indicates advantages of LC–MS/MS-based analysis, and “>” or “≫” indicates advantages of antibody-based analysis. The sensitivity, multiprotein assay and multisample assay refer to ELISA as an antibody-based analysis.
- LC–MS/MS, liquid chromatography-linked tandem mass spectrometry.
PRINCIPLES AND METHODOLOGY
Protein Quantification by MRM Using LC–MS/MS
Quantification by LC–MS/MS has been performed for small molecular compounds, such as drugs, and it was considered that a similar quantification strategy might be available for proteins. However, proteins are too large to separate by reversed-phase high-performance liquid chromatography (HPLC) and their mass lies above the range of the mass filter in MS for quantification [mass-to-charge ratio (m/z) <2800 in 4000QTRAP, <1250 in API5000 (ABSCIEX, Foster City, CA)]. Therefore, proteins must first be digested with a protease, such as trypsin, and then peptide(s) specific for the target protein can be quantified by LC–MS/MS. Trypsin digestion is also an important process for quantification of membrane proteins, which generally exhibit low solubility and high aggregability due to the presence of hydrophobic regions. Trypsin digestion produces peptides from various regions of target membrane proteins, and a peptide with appropriate hydrophobicity can be selected for LC–MS/MS-based quantification.
To achieve highly selective quantification, the chosen peptide is quantified by MRM using triple quadrupole (Triple Q) MS. Triple Q MS employs three chambers, of which the first and third (Q1 and Q3) are mass filters that pass the peptide ion having the target mass. In the second chamber, the peptide ion is fragmented by collision with N2 gas (Fig. 1). The use of two mass filters provides high selectivity and a high signal-to-noise ratio. The combination of Q1 and Q3 mass filters is called the transition, which can be changed every 10 ms, and up to 300 different peptide ions can be quantified simultaneously in a single analysis.
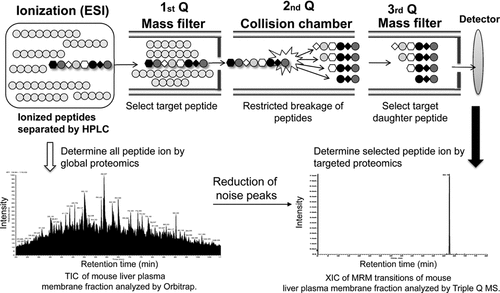
Detection of target peptide by multiple reaction monitoring (MRM) analysis using a triple quadrupole tandem mass spectrometer (Triple Q MS). The total ion chromatogram (TIC) presents the ion intensity of all peptides determined by nanoLC-Orbitrap. The extracted ion chromatogram (XIC) presents ion intensity determined by MRM transition of the target peptide. In MRM analysis, the target peptide is selected by two mass filters (Q1 and Q3), resulted in a highly selective chromatogram, as shown in the right XIC, by reducing noise from the complex peptide sample, shown in the left TIC.
An internal standard is essential to accurately quantify the absolute amounts (Fig. 2). For peptide quantification, a stable isotope-labeled peptide having the same amino acid sequence as the target peptide is used as the internal standard. The labeled internal standard peptide is eluted at exactly the same retention time as the target peptide, but it can be distinguished from the target peptide by MS due to the difference in mass. Furthermore, the absolute amount of the target peptide can be calculated from the target peptide to internal standard peak ratio in chromatograms. Each target peptide in a protein sample is quantified by measuring four different MRM transitions, which consist of the same Q1 and four different Q3, and the internal standard peptide is quantified by measuring the four corresponding MRM transitions (multiplexed-MRM analysis) to improve the accuracy.6 Therefore, eight MRM transitions in total are required for one protein, and 37 different proteins can be simultaneously quantified by using the maximum of 300 MRM transitions in a single analysis.
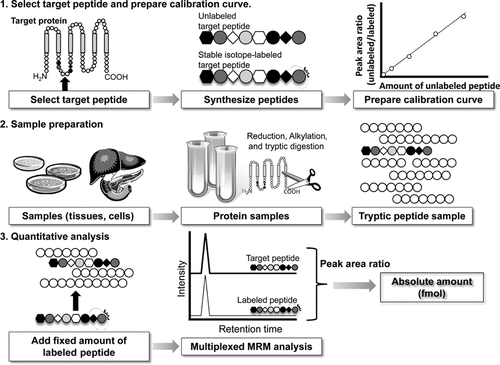
Procedure of sample preparation and quantification by MRM analysis.
Because at least one stable isotope-labeled peptide is necessary for quantifying each protein, the cost of synthesizing labeled peptides (about $1000–$1500 each for 5 nmol quantities) could be an issue for multiplexed-MRM analysis. However, only 20–500 fmol of each labeled peptide is spiked in each protein sample, and therefore the cost of labeled peptide for a single sample is very small compared with the cost of other reagents such as trypsin and LC solvents. One approach to reduce cost would be to use a peptide set from an established peptide library. The AQUA Peptide Library (Sigma–Aldrich, St. Louis, MO) provides amounts of 100 pmol at about $300 per peptide for registered proteins. Another solution is to synthesize stable isotope-labeled protein containing target peptides by using an Escherichia coli or in vitro cell-free protein synthesis system.7 The sensitivity of the method also depends on the available equipment, such as LC and MS, and the cost of high-sensitivity instruments can be significant.
We have used this approach to quantify proteins in whole lysate of brain capillaries; plasma membrane fraction of liver, kidney, and platelets; and microsomal fraction of liver.6,8-12 Other groups have employed it for protein quantification in plasma, serum, and yeast.5,13-16 LC–MS/MS-based quantification is applicable to many kinds of protein samples such as ELISA and immunoblotting. The proteins in the sample are first reduced and alkylated under solubilizing conditions in the presence of guanidine chloride or urea (Fig. 2). Then, the reduced and alkylated proteins are digested with trypsin to produce tryptic peptides. A fixed amount of stable-isotope-labeled internal standard peptides is added and the mixture is analyzed by multiplexed MRM. The absolute amount of each target peptide is determined from the target peptide to internal standard peak ratio.
PRINCIPLES AND ADVANTAGES
Sequence-based Target Peptide Selection for Multiplexed-MRM Analysis
The most important issue for high sensitivity and reliability in protein quantification by MRM analysis is the selection of the target peptide. The target peptide should have a unique amino acid sequence and give a high intensity in MS analysis. One selection strategy has been to identify the target peptide by global proteomics. Picotti et al.5 have developed a database of peptides based on previous proteomics experiments, called the MRM Atlas, and they reported an analysis of 1500 yeast proteins. Table 2 shows our results in an analysis of membrane transporters in plasma membrane fraction of mouse liver, quantified with HPLC-4000 QTRAP and identified with nanoLC-Orbitrap. Global proteomics could identify relatively low-expressed transporters, but only a few peptides were identified per protein in many cases. For accurate quantification, trypsin digestion efficiency, peptide specificity, posttranslational modification, and polymorphisms should be considered, and a small number of peptides identified by global proteomics is unlikely to be sufficient to select appropriate target peptides for MRM analysis.9
| Transporter | Expression Level (fmol/µg Protein) | Number of Identified Peptides |
|---|---|---|
| Oatp1 | 42.9 | 11 |
| Na/K ATPase | 33.5 | 25 |
| Mct1 | 18.8 | 3 |
| Ntcp | 17.1 | 3 |
| Nat | 10.3 | 3 |
| Bcrp | 8.51 | 4 |
| Mrp2 | 7.05 | 12 |
| Bsep | 6.65 | 22 |
| Mrp6 | 5.11 | 9 |
| Mrp3 | 3.64 | 12 |
| Abcg8 | 3.54 | 2 |
| Abcg5 | 2.82 | 3 |
| Gat2 | 2.79 | 2 |
| 4F2hc | 2.06 | 4 |
| Glut1 | 1.87 | 0 |
| Oatp2 | 1.65 | 3 |
- For quantitative analysis, plasma membrane fraction of mouse liver prepared by sucrose density gradient centrifugation (23 µg protein) was analyzed by conventional HPLC and Triple Q MS (4000QTRAP).6 For peptide identification, plasma membrane fraction of mouse liver (0.5 µg protein) was analyzed by nanoLC and Fourier-transform ion cyclotron resonance MS (LTQ Orbitrap) and peptides were identified with protein discoverer.
We have developed selection criteria based on amino acid sequence, including peptide length, amino acid content, and database annotation information.6 Kamiie et al.6 reported that among 85 peptides from human serum albumin only 14 (16%) were detected at above 500 counts/fmol, whereas 23 of 31 peptides (74%) selected using our criteria were detected at above 500 counts/fmol. Our criteria are still being improved, and all 27 peptides selected for human transporters were detected at above 500 counts/fmol.
Developing detection systems using antibodies usually takes about a year, and sometimes a suitable specific antibody cannot be obtained at all. In contrast, with multiplexed MRM, 1 month is sufficient to develop the quantification method, including 3 weeks for peptide synthesis, and it is not necessary to prepare target proteins (i.e., antigens in the case of antibody preparation). It is also possible to make a decision as to whether or not the method is feasible within the first 10 min. The procedure for sequence-based peptide selection is similar to that in the case of quantitative reverse transcription-polymerase chain reaction (RT-PCR) (Fig. 3). Sequence-based target peptide selection makes it possible to have “on-demand” protein quantification.
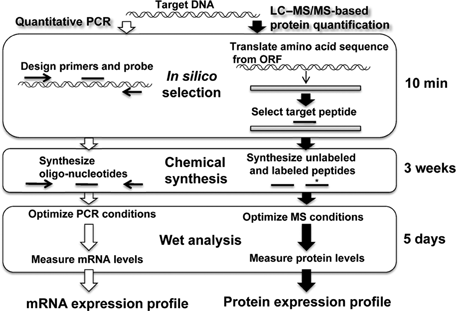
Method development procedures for LC–MS/MS-based protein quantification and quantitative real time RT-PCR.
ADVANTAGES AND LIMITATIONS
Comparison of LC–MS/MS-Based Quantification with Antibody-based Quantification
The principle of protein quantification using LC–MS/MS is to quantify specific peptides generated from target proteins by trypsin digestion. As MS selects the peptide based on molecular weight (m/z) with a mass filter, a major advantage of this quantification method is high selectivity, in contrast to the issue of cross-reactivity in antibody-based methods. A single amino acid difference in a peptide changes the m/z, and is able to be distinguished by MS (Table 1). An example of the superiority of LC–MS/MS is provided by the cytochrome P450 (CYP) enzyme isoforms, for which the preparation of specific antibodies is difficult due to the high sequence similarity among the isoforms. Wang et al.17 quantified the CYP3A family, including CYP3A4 and CYP3A5, in pooled human liver microsomes. Langenfeld et al.18 quantified CYP2D6 in 30 liver specimens, and Seibert et al.19 quantified CYP1A2 and 2E1 in eight liver specimens. In contrast, by applying sequence-based target peptide selection, we recently developed a method for simultaneous quantification of 11 CYP isoforms, CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4, 3A5, and 3A43, and used it to quantify their absolute protein expression amounts in human liver microsomes in the range of 1–80 fmol/µg protein.9 Furthermore, posttranslational modification, such as phosphorylation, results in a change of m/z, so that LC–MS/MS can separately quantify both modified and nonmodified protein. Quantification of other phosphorylated proteins, such as focal adhesion kinase, estradiol receptor α, and HER2, by LC–MS/MS has also been reported.20,21
Simultaneous quantification of multiple proteins is another advantage of LC–MS/MS-based quantification (Table 1). Thirty-seven proteins can currently be quantified simultaneously, and we have reported the simultaneous quantification of 34 transporters and two membrane proteins in mouse brain capillaries, liver, and kidney.6 It is also possible to increase the number of target proteins for quantification by repeating the LC–MS/MS analysis. We recently reported the quantification of 114 membrane proteins in human brain capillaries.12 Therefore, LC–MS/MS is an excellent tool to quantify a large number of target proteins such as transporters, enzymes, receptors, channels, and biomarker candidates.
On the contrary, a limitation of this method is the longer analysis time of LC–MS/MS compared with ELISA and immunoblotting (Table 1). LC–MS/MS analyzes samples one by one, and a single analysis takes about 2 h using regular HPLC, so a maximum of 12 samples can be analyzed in 1 day. The use of ultra-performance liquid chromatography can shorten the analysis time, but the number of proteins that can be quantified simultaneously is reduced. ELISA and immunoblot measure single proteins, but it is possible to analyze 96 or more samples in parallel by ELISA, and more than 10 samples by immunoblotting. Therefore, antibody-based quantification, especially ELISA, is useful methodology to determine the expression levels of one or several proteins in large number of samples, for example, for clinical diagnosis.
Another advantage of LC–MS/MS is the procedure of method development (Table 1). For antibody preparation, antigen preparation, involving antigen protein purification or antigen peptide synthesis, is necessary. Even after immunization, antibody purification and validation of the specificity take a long time. In contrast, quantification using LC–MS/MS does not require the target protein to be purified. Instead, the target peptide for quantification can be selected by computer on the basis of amino acid sequence information in about 10 min.6,9 The specificity of the target peptide can also be confirmed by database search. This feature, that is, the ability to efficiently develop a quantification method for candidate proteins from only sequence information, is important for rapid progress in protein research.
Sensitivity is an important factor for quantification, as well as accuracy and reliability (Table 1). The sensitivity of ELISA is in the range from pg/mL to ng/mL, and that of LC–MS/MS-based quantification is of ng/mL order (0.1–1 pmol/mL).22 Thus, LC–MS/MS-based quantification is currently less sensitive than ELISA, but the continuing improvement of MS instruments is expected to narrow or eliminate this difference in the near future.
Recent improvement in LC–MS/MS-based quantification is high-resolution MRM (HR-MRM) using quadrupole time-of-flight (QTOF) MS. One of the factors affecting the accuracy of quantification values is noise derived from peptides other than the target peptide in the sample. The noise peaks sometimes overlap with the target peptide peak, and/or raise the baseline of the target peptide peak, leading to inaccuracy of the quantification values. Triple Q MS allows the peptide to be passed through mass filters within a small filter range around the m/z set for the target peptide [0.7 unit for QTRAP 5500 (ABSCIEX, Foster City, CA) and 0.1 unit for TSQ Vantage (Thermo Fisher Scientific, Waltham, MA)]. Narrower filter range (or higher resolution) of the mass filters results in less noise in the chromatogram. Triple Q MS gives high accuracy and a wide dynamic range of quantification, but the mass resolution is low. In contrast, QTOF MS gives high mass resolution, but the dynamic range is narrow and the scan time is longer. Recently, MS instrument development has improved the dynamic range and scan speed of QTOF MS, and the latest QTOF is able to perform HR-MRM [0.02 unit for TripleTOF 5600 (ABSCIEX, Foster City, CA)]. By HR-MRM analysis with TripleTOF 5600, reduction in noise levels was observed, and accuracy of quantification data was improved compared with standard MRM analysis when protein samples of high complexity, such as whole tissue lysate, were analyzed. The calibration curve was also linear from 0.25 fmol to at least 100 fmol (greater amounts were not examined). Further MS instrument development to afford higher resolution and greater sensitivity is expected to extend the applicability of LC–MS/MS-based protein quantification.
Antibodies are used for various purposes, not only ELISA and immunoblotting assay but also localization analysis. Expression of proteins is inhomogeneous in cells and tissues, and localization information provided by immunohistochemical analysis is important to clarify the protein function. As 1 µg protein can be analyzed by nano-LC–MS/MS, the combination of MRM analysis and laser microdissection is useful methodology to determine protein expression in specific tissue regions. Furthermore, MS is becoming available for localization analysis, in the form of imaging MS.23,24 Although the present methodology does not have sufficient sensitivity to determine protein localization, this is also a promising future direction in proteomics.
APPLICATION
Quantification of Membrane Transporter Protein
Membrane proteins are difficult to analyze quantitatively by western blotting or ELISA due to their poor solubility and high aggregability, although membrane proteins include important drug targets and play important roles in ADMET. By applying multiplexed-MRM analysis, we have quantified 43 transporters and Na+/K+ ATPase in mouse; 47 transporters, three junction proteins, two receptors, and Na+/K+ ATPase in monkey; and 106 transporters, 14 membrane proteins, and Na+/K+ ATPase in human (Supplemental Tables 1–3).6,8
This simultaneous quantification enabled us to screen expressed membrane proteins in biological samples, and we have applied it to identify ATP-binding cassette (ABC) transporters expressed in human platelets.11 In initial screening, mRNA expression levels of 49 ABC transporters were examined by quantitative RT-PCR. Then, the protein expression levels of 13 mRNA-positive ABC transporters were determined by multiplexed-MRM analysis. Finally, ABCA7, B4, C1, C3, and C4 were confirmed to be expressed in plasma membrane of human platelets. This report showed that the combination of extensive mRNA screening by PCR or DNA chip analysis, coupled with accurate protein quantification by multiplexed MRM, is an effective strategy to identify membrane proteins expressed in target cells and tissues. Furthermore, the absolute protein expression levels provide a basis to decide the order of analysis among numbers of candidate proteins. This report also demonstrated that mRNA expression levels and protein expression levels of these five ABC transporters in plasma membrane were not correlated.
Some transporters form functional multiprotein complexes in the plasma membrane. Determination of the absolute expression amounts of the proteins composing such complexes is important for understanding the stoichiometry of the proteins. For example, amino acid transport system L consists of two membrane proteins, LAT1 and 4F2hc, and plays an important role in blood-to-brain amino acid supply at the plasma membrane of brain capillary endothelial cells.25,26 Multiplexed-MRM analysis showed that the absolute protein expression levels of LAT1 and 4F2hc were 2.19 fmol/µg protein and 16.4 fmol/µg protein in mouse brain capillaries.6 The lower expression of LAT1 suggests that changes in LAT1, but not in 4F2hc, protein expression are involved in regulation of the transport activity of system L in mouse brain capillaries.
Interspecies Comparison of Blood–Brain Barrier Transporters
Species differences, especially between human and animals, are of critical importance in preclinical and clinical studies during drug development. To date, comparisons have been performed at the functional level, such as by positron emission tomography (PET) studies, and at the mRNA level by PCR and DNA chip analyses. QTAP-based ADME research using the combination of in silico peptide selection and multiplexed-MRM LC–MS/MS analysis enables the direct comparison of protein expression levels among animals and human, and this should reflect function better than mRNA analysis, as well as being more comprehensive than functional analysis. Recently, we have clarified the absolute protein expression levels of transporters in human, monkey, and mouse brain capillaries, providing basic information about species differences in transport function at the blood–brain barrier (BBB) (Fig. 4).6,8,12
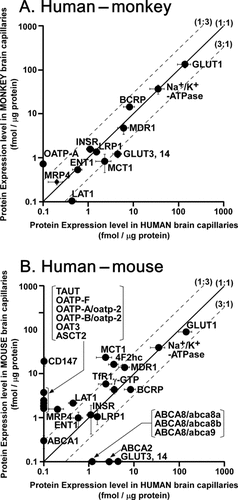
Comparison of absolute protein expression levels of transporters in brain capillaries between (A) human and monkey and (B) human and mouse. The protein expression levels are taken from our previous reports: human,12 monkey,8 and mouse.6 In panel (B), mouse abca8a, abca8b, and abca9 were compared as possible homologues of human ABCA8. Human OATP-A and OATP-B were compared as possible homologues of mouse oatp-2 at the BBB. The solid line passing through the origin represents the line of identity, and the broken lines represent threefold differences. Each point represents mean ± SD. The molecules on the horizontal (mouse and monkey) or vertical (human) axis are under the limits of quantification.
MDR1/mdr1a/ABCB1 and BCRP/ABCG2 are major gatekeepers for multiple hydrophobic drugs, and function to pump out the drugs from brain capillary endothelial cells to the circulating blood and to attenuate drug distribution to the brain. Compared with human brain capillaries, MDR1 protein expression was not significantly different in monkey brain capillaries, whereas it was 2.33-fold greater in mouse brain capillaries. In contrast, BCRP protein expression was 1.74-fold greater in monkey, but it was 1.85-fold lower in mouse. This result indicated that, in addition to differences in substrate specificity and affinity, differences in the expression levels of transporters contribute to species differences in transporter function at the BBB, as well as drug distribution to the brain. The lower expression of MDR1 in human and monkey brain capillaries would lead us to predict higher distribution of MDR1 substrates into the brain of human and monkey compared with that of mice. Indeed, a PET study demonstrated that human brain penetration of [18F]altanserin and [11C]GR205171, substrates of MDR1, was 4.5- and 8.6-fold greater than in rodents, respectively.27 Monkey brain penetration of [11C]verapamil and [11C]GR205171 was 4.1- and 2.8-fold greater than in rodents, respectively.
Organic anion transporters serve to transport water-soluble anionic drugs and brain metabolites across the BBB. Mouse brain capillaries contain following four organic anion transporters: MRP4/ABCC4, OAT3, oatp1a4/oatp2, and oatp1c1/oatp14 (1.59, 1.97, 2.11, and 2.41 fmol/µg protein, respectively).6 In contrast, OAT3, OATP1C1, and OATP1A2 (human ortholog of oatp1a4) were under the limits of quantification in human (<0.348, <0.208, and <0.695 fmol/µg protein, respectively).12 MRP4 was detected, but the expression amount was 8.2-fold smaller than that in mouse. Monkey showed a similar expression pattern to human, although OATP1A2 was detected, whereas OAT3 and OATP1C1 were under the limits of quantification.8 The difference in protein amount of MRP4 was 1.47-fold between monkey and human (0.286 and 0.195 fmol/µg protein, respectively). Ro64-0802, an active form of the anti-influenza virus agent oseltamivir, undergoes active efflux mediated by OAT3 and MRP4 at the BBB.28 Recently, abnormal behavior has been reported in teenagers or younger people prescribed oseltamivir, although a rodent study showed no specific central nervous system or behavioral effects after administration of doses corresponding to at least 100 times the clinical dose.29 Therefore, a possible hypothesis to explain oseltamivir toxicity in humans is that low expression of MRP4 and OAT3 in the human BBB results in reduced efflux of Ro64-0802 from the brain, leading to greater accumulation in the brain, which in turn induces adverse effects on the central nervous system. Furthermore, oseltamivir is converted to Ro 64-0802 by carboxylesterase 1A1 (CES1A1) in the liver, and CES1A1 was reported to be predominantly expressed in human brain capillaries.30,31 Therefore, the species differences in expression of converting enzymes are also necessary to be clarified by the further studies.
Brain energy sources such as glucose, lactate, and ketone bodies are supplied to the brain via glucose transporter 1 (GLUT1) and monocarboxylate transporter 1 (MCT1). In human and monkey, the GLUT1 level is much greater than the MCT1 level (61-fold and 155-fold greater, respectively), which is a reasonable expression pattern to promote efficient production of ATP from glucose, rather than lactate and ketone bodies, and to avoid brain accumulation of lactate, which could cause acidosis in the brain. Unlike human and monkey, however, mouse brain capillaries highly express MCT1 (23.7 fmol/µg protein) as well as GLUT1 (90.0 fmol/µg protein), suggesting that not only glucose but also lactate and ketone bodies are efficiently supplied to the mouse brain. The energy metabolic rate in mouse brain is greater than that in human and monkey brain.32 Hence, a possible explanation for high expression of MCT1 protein is to support the fast energy metabolism in mouse brain.
Supply of amino acids across the BBB is important for the synthesis of proteins and neurotransmitters in brain. System L, corresponding to LAT1 and 4F2hc, plays an important role in supplying large neutral amino acids such as leucine, tryptophan, tyrosine, and phenylalanine to the brain. The protein levels of LAT1 and 4F2hc in human were 19.7% and 21.2% of those in mouse, whereas LAT1 was under the detection limit in monkey (<0.326 fmol/µg protein). The cerebral protein synthesis rate in human brain [0.345–0.614 nmol/(min·g)] has been estimated to be lower than that in rodent brain [3.38 nmol/(min·g)] by PET using l-[1-11C]leucine and a three-compartment model,33 and the brain concentration of serotonin generated from tryptophan has been shown to be lower in humans than that in mice (20 versus 679 ng/g brain in the frontal cortex).34,35 Cerebral protein and serotonin synthesis are likely affected by amino acid availability in the brain and tryptophan transport into the brain, respectively.36 Therefore, the supply of amino acids via system L at the human BBB could be smaller than that at the mouse BBB, in accordance with the protein expression amounts of LAT1 and 4F2hc.
It is also critical to clarify developmental changes in the protein levels of transporters at the BBB to understand the risk of adverse drug reaction in brain and the effect of changes in brain metabolism of endogenous substances. Significant developmental changes in expression levels of mdr1a, glut1, and mct1 at rodent BBB have been reported; the protein expressions of mdr1a and glut1 were significantly smaller at birth than in adult rat,37,38 and the expression of mct1 dramatically increased during the suckling period and then decreased in adults in mice.39 In contrast, in situ hybridization showed that bcrp expression did not significantly change from E13 stage to adult.40 However, most of the above findings were based on rodent data. As shown in our studies, there is a significant species difference in the protein levels of transporters between mouse and human, so it is questionable whether or not the reported developmental changes in rodents are applicable to human. So, we analyzed developmental changes in monkey, in which the protein levels of transporters are similar to those in human.8 Unlike rodents, no significant difference was observed in MDR1 and GLUT1 levels between neonates (1 day after birth) and adults. The MCT1 level was the highest in neonates (at 1 day after birth), followed by that in children and adults. In contrast to MCT1, the BCRP level significantly increased with postnatal development and was 2.3-fold greater in adults than that in neonates (at 1 day after birth). These findings in monkey should be helpful to improve our understanding of the brain distribution of drugs and endogenous compounds in the developmental phase in human.
ADVANCED APPLICATION
Reconstruction of in Vivo Transporter Protein Function
Variability, such as species difference, in vitro/in vivo difference, age difference, and normal/disease difference, in physiological function must be taken into account in drug discovery and development, as well as clinical application. Drug concentrations in the tissue and plasma, pharmacological and/or toxicological responses, and the pathophysiological state of the drug target organ depend upon the activities of functional proteins such as transporters, enzymes, channels, receptors, and so on. Because their activities are likely to correlate with their protein levels, QTAP of the functional proteins is expected to be an effective approach to understand such differences in physiological function and to overcome them in the clinical context.
An immortalized mouse brain capillary endothelial cell line, TM-BBB4, has been characterized as in vitro mouse BBB model that retains the in vivo permeability of various compounds across the BBB.41 The results suggest that TM-BBB4 preserves the in vivo functions of several transporters. However, the transport rate of 3-o-methyl-d-glucose (3-OMG), a substrate of GLUT1, was significantly different between TM-BBB4 and in vivo [30.0 and 234 µL/(min·g brain), respectively]. Therefore, we investigated whether the in vitro/in vivo difference in transport activity of GLUT1 can be understood by means of QTAP (Fig. 5). The protein level of GLUT1 was found to be 2.94 fmol/µg protein in TM-BBB4. Therefore, the intrinsic transport activity (transport rate per protein) of GLUT1 was estimated to be 10.2 [µL/(min·g brain)]/(fmol GLUT1/µg protein) in TM-BBB4, by dividing the transport rate of 3-OMG by the protein level of GLUT1.
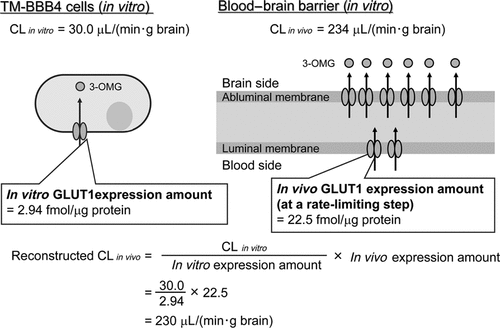
Reconstruction of GLUT1 transport activity at in vivo brain capillaries from absolute expression amount and in vitro transport activity.
Immunohistochemical analysis has shown that GLUT1 protein is localized both at luminal and abluminal membranes of in vivo brain capillary endothelial cells with a luminal–abluminal expression ratio of approximately 1:3.42 This indicates that transport via GLUT1 at the luminal membrane is a rate-limiting step in 3-OMG penetration into the brain. Hence, one-fourth of GLUT1 protein (90 fmol/µg protein × 1/4 = 22.5 fmol/µg protein) was estimated to contribute to the in vivo penetration of 3-OMG. By integrating the intrinsic transport activity and the expression level of contributing GLUT1 protein, expected in vivo penetration of 3-OMG via GLUT1 could be calculated to be 230 µL/(min·g brain). This reconstructed value agreed extremely well with the observed value [234 µL/(min·g brain)]. This result illustrates well the value of QTAP to understand in vitro/in vivo differences in physiological activities of functional proteins.
STRATEGY AND PROSPECTS
QTAP-based Drug Discovery and Development
Great efforts have been directed to drug discovery and development in both the pharmaceutical industry and academia in the past decades. There have been remarkable developments in the methodology of life science, including whole-genome sequencing, genome-wide association study, and global proteomics, which have facilitated the discovery of disease-related proteins, but we are still far away from a “gold-rush era” of new drugs for these target proteins. The processes of drug ADMET/efficacy are all governed by protein function, and the lack of quantitative information at the protein level generally makes it necessary to treat the human body as a “black box.” A basic workflow for QTAP-based study is proposed in this review and is shown in Figure 6. QTAP-based study could underpin rational strategies for PPx-based stratification of preclinical research, for example, “target identification,” “lead optimization,” “toxicity profiling,” and “animal model”, as well as clinical research. Tables 3 and 4 summarize possible applications of QTAP-based studies in preclinical research (Table 3) and clinical research (Table 4), focusing on critical problems in drug discovery and development, for example, lack of efficacy in human, toxicity in human, and poor clinical safety.43 A quantitative atlas of functional proteins in animal models and humans in normal and diseased states would provide the basis for enormous advances in drug discovery and development by allowing reconstruction and/or prediction of the ADMET/efficacy of candidate small molecular or protein drugs. We believe QTAP-based studies will have a revolutionary effect on drug discovery and development research.
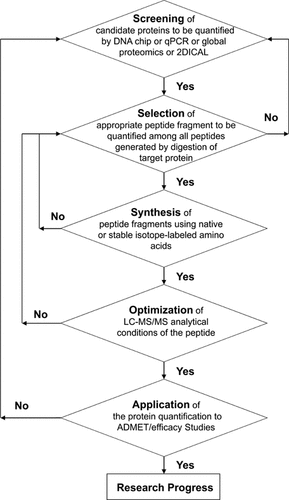
A basic workflow of QTAP-based study. two-dimensional image converted analysis of liquid chromatography and mass spectrometry (2DICAL) is the differential protein expression analysis by label-free quantitative global proteomics developed by Ono et al.46
| Research Stage | Applications |
|---|---|
| Target identification | Comparison of expression amounts of candidate drug target proteins. |
| Comparison of drug target protein expression amounts in the target organ of animal model and human. | |
| Analysis of phosphorylation of target proteins involved in signal transduction. | |
| Analysis of drug target protein turnover in the target organ. | |
| Lead optimization | Validation and quality control of in vitro screening system by protein quantification of drug target. |
| Validation and quality control of in vitro evaluation system by quantification of proteins involved in ADME. | |
| Rational optimization by extrapolation of in vivo activities based on the in vitro intrinsic activities and expression amounts of proteins relevant to ADME and efficacy. | |
| Quality control of protein drugs (antibody, growth factor, and cytokine). | |
| Toxicity profiling | Validation and quality control of in vitro toxicity study system by protein quantification. |
| Validation and quality control of animal models, such as chimeric (uPA/SCID) mouse with humanized liver,44 in toxicity studies. | |
| Discovery and analysis of marker proteins involved in hepatic and/or extrahepatic toxicity in animal models. | |
| Analysis of protein modification involved in hepatic and extrahepatic toxicity. | |
| Animal model | Analysis of interspecies differences in expression of proteins involved in ADME and efficacy. |
| Validation and quality control of animal models, including knockout, transgenic, and human artificial chromosome transfer mice,45 by quantification of proteins related to the genetic modification. | |
| Rational extrapolation of pharmacokinetics, pharmacodynamics, and toxicokinetics in human based on protein expression amounts and activities in animal models. | |
| Analysis of protein–drug concentration in plasma. |
- QTAP, quantitative targeted absolute proteomics; ADMET, absorption, distribution, metabolism, elimination, and toxicity.
| Research stage | Applications |
|---|---|
| Phase I | Quality control of protein drugs. |
| PK studies of protein drugs by measuring plasma concentration time profile. | |
| Analysis of hepatic and/or extrahepatic toxicity marker proteins in plasma and/or urine. | |
| Phase II | Quality control of protein drugs. |
| PK studies of protein drugs by measuring plasma concentration–time profile. | |
| Analysis of hepatic and/or extrahepatic toxicity marker proteins in plasma and/or urine. | |
| Analysis of surrogate marker proteins involved in drug efficacy. | |
| Analysis of drug target protein expression in target tissue and/or plasma of patients. | |
| Phase III | Quality control of protein drugs. |
| PK studies of protein drugs by measuring plasma concentration–time profile. | |
| Analysis of hepatic and/or extrahepatic toxicity marker proteins in plasma and/or urine. | |
| Analysis of surrogate marker proteins involved in drug efficacy. | |
| Analysis of drug target protein expression in target tissue specimen and/or plasma of patients. |
- QTAP, quantitative targeted absolute proteomics; PK, pharmacokinetics.
CONFLICT OF INTEREST
Tetsuya Terasaki and Sumio Ohtsuki are a full professor and an associate professor of Tohoku University (Sendai, Japan), respectively, and are also directors of Proteomedix Frontiers. This research was not supported by Proteomedix Frontiers and their positions at Proteomedix Frontiers do not present any financial conflicts. The other authors declared no conflict of interest.
Acknowledgements
The studies mentioned in this review were supported in part by a Grant for Development of Creative Technology Seeds Supporting Program for Creating University Ventures from Japan Science and Technology Agency (JST), and the Industrial Technology Research Grant Program from New Energy and the Industrial Technology Development Organization of Japan, as well as a Grant-in-Aid for Scientific Research (S) 18109002 from the JSPS, a Grant-in-Aid for Scientific Research on Priority Area 17081002 from The Ministry of Education, Culture, Sports, Science, and Technology Japan, and a grant for Creation of Strategic Innovation Project from JST.




