Investigation of distal femur microarchitecture and factors influencing its deterioration: An ex vivo high-resolution peripheral quantitative computed tomography study
Tim Rolvien and Jan Hubert contributed equally to this study.
Abstract
While fractures of the distal femur are often considered as fragility fractures, detailed knowledge of the bone microarchitecture at this skeletal site is largely unavailable. Initial evaluation of a patient cohort with distal femur fractures showed a markedly increased occurrence in elderly women. The purpose of this study was to determine the extent to which demographic characteristics of distal femur fractures are reflected by general age- and sex-specific variations in local microarchitectural parameters. Fifty cadaveric femora were collected from 25 subjects (12 females, 13 males, age 25–97 years). A volume of interest within 3 cm proximal to the condyles was analyzed using high-resolution peripheral quantitative computed tomography (HR-pQCT), which revealed impaired trabecular and cortical bone microarchitecture in women compared to men as well as in osteoporotic compared to normal or osteopenic subjects, as classified by dual-energy X-ray absorptiometry (DXA) T-score. Linear regression analyzes showed negative associations between age and HR-pQCT parameters in women (e.g., cortical thickness −14 µm/year, 95% CI: −21 to −7 µm/year), but not in men (e.g., cortical thickness 1 µm/year, 95% CI: −12 to 14 µm/year). HR-pQCT parameters showed strong positive associations with areal bone mineral density (aBMD) determined by DXA at the hip in both sexes. Taken together, our findings suggest that female sex, advanced age, and low aBMD represent major risk factors for impaired microarchitecture at the distal femur. Both the diagnostic value of DXA for predicting distal femur fractures and the efficacy of bone-specific agents on fracture risk reduction should be investigated in the future.
1 INTRODUCTION
Distal femur fractures represent a rare but surgically challenging fracture site, accounting for 3%–6% of all femur fractures.1 The annual incidence ranges between 4.5 and 8.7/100.000 in the general population.2-4 Injuries involving the distal femur follow a bimodal age distribution with a peak in young men (high-energy trauma, e.g., traffic accidents) and elderly women (low-energy trauma, e.g., falls).3 Elderly women represent the patient population with the highest prevalence for extraarticular supracondylar fracture (AO 33A1).2, 4, 5 Influenced by the high prevalence of medical comorbidities in this patient population, distal femur fractures represent a relevant clinical entity with high mortality rates around 21%–38% after 1 year 6, 7 which might even exceed those of proximal femur fractures.8, 9 Given the demographic changes toward an aging population and concomitant increase in the incidence of distal femur fractures (including periprosthetic distal femur fractures), understanding the underlying causes is of major clinical interest to introduce effective prevention and treatment options.10
Based on the epidemiological findings, it appears reasonable to assume that osteoporosis is one important risk factor for distal femur fractures in the elderly.2, 5 Osteoporosis represents a skeletal disorder primarily affecting postmenopausal women, leading to an increased risk for fractures secondary to a loss of bone mass and microarchitecture.11 The diagnostic gold standard to diagnose osteoporosis is dual-energy X-ray absorptiometry (DXA), which is a two-dimensional technique measuring the areal bone mineral density (aBMD) at the proximal femur and lumbar spine. However, it has not been investigated if age-, sex-, or aBMD-dependent alterations in the bone microarchitecture of the distal femur exist, and how a potential microarchitectural deterioration might be linked to epidemiologic fracture data. High-resolution peripheral quantitative computed tomography (HR-pQCT) allows the investigation of the three-dimensional bone microarchitecture as well as volumetric bone mineral density (vBMD). The objective of this study was to review demographic characteristics of distal femur fractures and to analyze the bone microarchitecture at the distal femur by HR-pQCT with special regard to age, sex, and aBMD by DXA to improve the clinical understanding of distal femur fractures.
2 METHODS
2.1 Clinical data
A total of 75 consecutive patients diagnosed with a distal femur fracture were retrospectively identified at our department. All fractures were classified as 33 A–C according to the AO classification based on X-ray and/or computed tomography.12 Age, sex, trauma mechanism (low-energy vs. high-energy trauma) and periprosthetic fracture were evaluated. In addition, the presence of neurological, and rheumatic diseases, chronic kidney disease, and tumors with skeletal involvement were extracted from patient records.
2.2 Cadaveric specimens
Fifty femora were collected from 25 age- and BMI-matched women and men during autopsy (n = 12) women (69.6 ± 19.7 years) and (n = 13) men (63.5 ± 18.8 years). Hospital and whole-body autopsy reports were reviewed to exclude individuals with diseases that affect bone health (e.g., cancer, diabetes, glucocorticoid medication, or periods of longer immobilization). This study was approved by the local ethics committee (WF-174/20) and complied with the Declaration of Helsinki.
2.3 Dual-energy X-ray absorptiometry (DXA)
DXA (Lunar iDXA, GE Healthcare) measurements were performed at the hip (i.e., proximal femur) to assess the aBMD. A polyethylene container with a water column of 20 cm was used as a soft tissue phantom according to the recommendations of the device manufacturer and as described previously.13 The container was placed over the femur, and the femur was positioned and measured according to the routine clinical protocol. The examined regions included the femoral neck and the total hip. The lower value of both regions was used for further analysis and corresponding T-scores were generated by the manufacturer's software. T-scores represent the standard deviations of the aBMD compared to a healthy cohort of 20 to 29-years-old subjects. In accordance with the guidelines of the world health organization (WHO), the diagnosis of osteoporosis and osteopenia was based on the T-score (i.e., osteoporosis T-score ≤ −2.5, osteopenia T-score > −2.5 and ≤ −1.0).14
2.4 High-resolution peripheral quantitative computed tomography
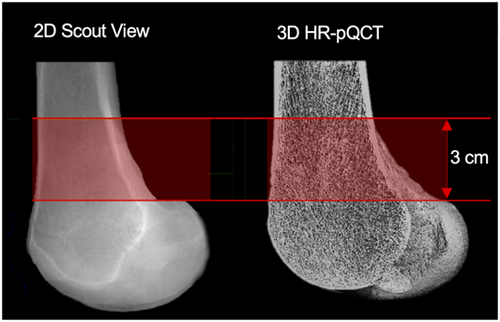
HR-pQCT measurements (XtremeCT II, Scanco Medical AG) were performed in a 3.0 cm volume of interest (VOI) at the distal femur (Figure 1) using a distinct in vivo protocol (60 kVp, 900 µA, 100 ms integration time, and a voxel size of 61 µm).15 This VOI was chosen because it corresponds to the most commonly observed site for distal femoral fractures (i.e., AO 33 A).16 It was defined using 2D scout view scans, on which the reference line was placed manually at the inflection point of the condylar-shaft-transition. All scans were acquired by one-trained technician to allow standardized measurements. Specimens were fixed within the cast to prevent motion artifacts. A standard evaluation protocol offered by the manufacturer was used to generate 3D microarchitectural datasets of the cortical and trabecular compartment. Consistent quality of the scans was controlled daily by using a manufacturer's calibration phantom. We assessed vBMD including total BMD (Tt.BMD, mg HA/cm3), trabecular BMD (Tb.BMD, mg HA/cm3), and cortical BMD (Ct.BMD, mg HA/cm3) as well as bone microarchitecture parameters including bone volume to total volume (BV/TV), trabecular number (Tb.N, 1/mm), thickness (Tb.Th, mm), and separation (Tb.Sp, mm), cortical thickness (Ct.Th, mm) and porosity (Ct.Po). Those values are identical to those used in HR-pQCT17 parameters obtained during clinical routine.
2.5 Statistical analysis
GraphPad Prism® (version 7.0, GraphPad Software) was used for statistical analyzes. We used both femora for all statistical analyzes except for the linear regression with age, for which we always used the side with the lower Ct.Th. Quantitative characteristics are presented as mean ± SD or the number. Normal distribution of the data was tested by using the Shapiro–Wilk normality test. When comparing two groups, we used the unpaired t-test for normally distributed data and the Wilcoxon–Mann–Whitney-test for nonparametric data. When comparing three groups, we used ordinary one-way ANOVA with Holm-Sidak's multiple comparison test for normally distributed data and Kruskal–Wallis test with Dunn's multiple comparison test for nonparametric data. We performed linear regression models to determine the association between age or aBMD and bone microarchitecture (Tt.BMD, BV/TV, and Ct.Th). p-values < 0.05 were considered as statistically significant.
3 RESULTS
3.1 Demographic and fracture characteristics of the clinical cohort
To gain insight into the demographic characteristics of distal femur fractures, we assessed clinical-demographic data from 75 consecutive patients who presented at our department with a distal femur fracture. Figure 2A compares a multi-fragmentary distal femur fracture with an additional proximal fibula fracture (AO 33-C3) of a 41-year-old woman after a high-energy trauma to a supracondylar fracture (AO 33-A1) of a 90-year-old woman after a low-energy trauma, illustrating the differences in fracture morphology (Figure 2A). Within the fracture cohort, most of the 75 patients were women (76%, n = 57, Figure 2B), who were on average significantly older than men (80.3 ± 16.9 vs. 56.8 ± 19.2 years, p < 0.001). Overall, the number of cases increased with age, with n = 3 fractures in the 20–29 years cohort and n = 24 fractures in the 90–99 years old (Figure 2B). This increase was primarily attributable to females, who represented the majority in the age groups ≥60 years (men n = 7 vs. women n = 50, Figure 2B).
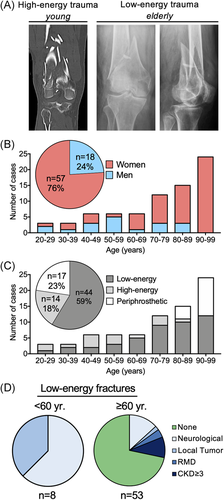
We determined whether age-specific patterns are reflected within the distribution of low-energy, high-energy trauma as well as low-energy periprosthetic fractures (Figure 2C). Patients with a low-energy fracture were significantly older than patients with a fracture that occurred in the context of high-energy trauma (75.8 ± 17.5 vs. 52.4 ± 19.3 years, p < 0.001). Specifically, while high-energy trauma caused most fractures between the ages of 20 and 59 years, the number of low-energy fractures exceeded that of high-energy fractures in patients ≥60 years old (n = 36 vs. n = 4). The proportion of periprosthetic fractures increased above 60 years, and 50% of total fractures in 90–99-year-old patients were classified as periprosthetic (Figure 2C). Of note, only one periprosthetic fracture occurred after high-energy trauma and all others occurred after low-energy trauma.
Finally, we reviewed all patients with low-energy fracture mechanisms and differentiated between patients <60 years (n = 8) and ≥60 years of age (n = 53). Interestingly, all patients <60 years either suffered from a neurological disease with relevant symptoms of paralysis (n = 5), or a local bone tumor (n = 3) (Figure 2D). In patients ≥60 years, a concomitant disease with a major systemic or local influence on bone status was present in only about one-third of the cases (Figure 2D).
3.2 Volumetric bone mineral density and bone microarchitecture of the distal femur
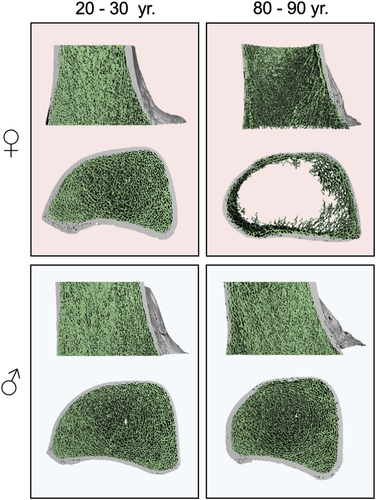
To determine the extent to which our demographic findings are reflected by general age- and sex-specific changes in bone microarchitecture parameters, we next performed an examination of distal femur microarchitecture in a group of 50 cadaveric specimens. Three-dimensional reconstructions of distal femora scanned by HR-pQCT depict the trabecular and cortical microarchitecture. These images provided initial evidence that older women may be affected by significantly deteriorated bone microarchitecture (Figure 3). Group comparisons revealed that age (69.6 ± 19.7 vs. 63.5 ± 18.8 years) and BMI (23.9 ± 8.0 vs. 23.2 ± 5.9 kg/m2) were similar between women and men (Table S1). No differences in microarchitecture parameters were observed between sides (left vs. right, p > 0.05 for all parameters) (Figure S1). Volumetric bone mineral density parameters (Tt.BMD, Tb.BMD, and Ct.BMD) were significantly lower in women compared to men (Figure 4A, Table S1). Likewise, trabecular parameters were significantly lower in women, indicated by reduced BV/TV (0.15 ± 0.07 vs. 0.21 ± 0.06, p = 0.007) and Tb.N (0.8 ± 0.4 vs. 1.1 ± 0.3 1/mm, p = 0.026) as well as a higher Tb.Sp (1.7 ± 1.3 vs. 1.1 ± 0.9 mm, p = 0.002) (Figure 4A, Table S1). No significant differences in Tb.Th (0.25 ± 0.03 vs. 0.26 ± 0.01 mm, p = 0.475) were observed. Furthermore, Ct.Th was significantly reduced in women compared to men (1.3 ± 0.3 vs 1.7 ± 0.3 mm, p < 0.0001). Interestingly, no significant differences for Ct.Po were detected (0.03 ± 0.01 vs. 0.04 ± 0.01, p = 0.740).
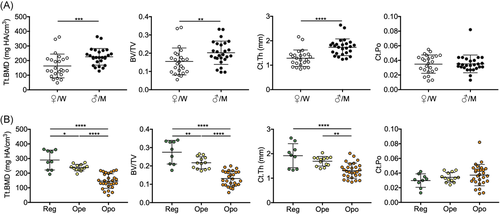
Additionally, we compared HR-pQCT data of femora with regular aBMD (Reg) values measured by DXA at the hip with those classified as osteopenia (Ope) and osteoporosis (Opo). Significantly reduced vBMD values (Tt.BMD, Tb.BMD, and Ct.BMD) as well as a decreased trabecular and cortical bone microarchitecture were detected in osteoporotic femora compared to those with regular aBMD and osteopenia (Figure 4B, Table S2). Compared to Reg and Ope, BV/TV was reduced in Opo (0.28 ± 0.06 vs. 0.22 ± 0.03 vs. 0.13 ± 0.03, both p < 0.0001) as were Tb.N (1.3 ± 0.1 vs. 1.2 ± 0.1 vs. 0.7 ± 0.3 1/mm, both p < 0.0001 ) and Ct.Th (1.9 ± 0.5 vs. 1.7 ± 0.2 vs. 1.3 ± 0.3 mm, p < 0.0001 and p = 0.002). While Tb.Th was significantly higher in Reg compared to Opo (0.28 ± 0.03 vs 0.25 ± 0.01 mm, p = 0.002), no significant differences were observed between Ope and Opo. Ct.Po did not differ significantly between Reg, Ope, and Opo (Figure 4B, Table S2).
3.3 Variables associated with microarchitectural impairment in women compared to men
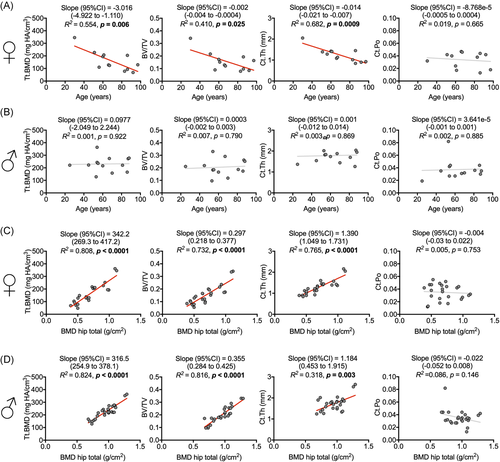
To further investigate the influence of age and aBMD on distal femur microarchitecture, linear regression models were performed in women and men. Tt.BMD, BV/TV, Ct.Th, and Ct.Po served as dependent variables while age and aBMD were used as independent variables. In contrast to men, there was a significant negative association between age and bone microarchitecture in women (Figure 5A). Specifically, Tt.BMD, BV/TV, and Ct.Th were strongly negatively associated with age in women (Tt.BMD, slope [95% CI]: −3.016 [−4.922 to −1.110] mg HA/cm3/year; p = 0.006; BV/TV, slope [95% CI]: −0.002 ± 0.001 [−0.004 to −0.0004]/year; p = 0.025 and Ct.Th, slope [95% CI]: −0.014 ± 0.003 [−0.021 to −0.007] mm/year; p < 0.001, respectively). In contrast, no age-related changes were detected in men (Figure 5B). Ct.Po was not associated with age in neither women nor men. We also analyzed the association of aBMD measured by DXA at the hip and the bone microarchitecture of the distal femur, revealing high associations in both women and men (Figure 5C,D). Of note, aBMD at the hip also decreased significantly with age in women, whereas no significant association between age and aBMD was found in men (Figure S2).
4 DISCUSSION
While the general importance of bone microarchitecture assessment for an accurate prediction of fracture risk has been outlined by our group and others in the recent years,18-20 a detailed analysis of the local distal femur microarchitecture by HR-pQCT had not previously been performed. In this study, we demonstrated severe age-related microarchitectural deterioration of the distal femur by HR-pQCT in women but not in men, which was mirrored by clinical fracture data. In both sexes, distal femur microarchitecture was strongly influenced by aBMD at the hip assessed by DXA, which represents the clinical gold standard to diagnose osteoporosis. The results of this study may not only be important for fracture prediction but also for surgical treatment, as reduced bone microarchitecture (e.g., osteoporotic bone) can challenge successful surgery and influence the choice and positioning of implant material.21-23
Reviewing bone microarchitecture in humans, it is important to note that its characteristics as well as age- and sex-related changes vary throughout the skeleton.24-26 For example, BV/TV values ranging from 8.3% in vertebrae to 15.8% in the femoral neck 27 have been reported, highlighting the limited transferability of known characteristics in-between different anatomical regions. Comparable patterns of microarchitectural deterioration with age were observed in other anatomical regions of the skeleton.28-31 A 10-years-decrease of −7% for BV/TV and of −5% for Ct. Th was described for the microarchitecture of the proximal femur in women.32 Extrapolation of the presented age-related development within this study corresponds to a BV/TV reduction of around −8% and Ct.Th of around −6% per age decade, indicating a comparable degree of cortical and trabecular reduction of the proximal and distal femur in women. Men also showed an age-related loss of the trabecular and cortical bone microarchitecture in the proximal femur in the respective previous study,32 suggesting a possible difference in the age-related development of bone microarchitecture at the proximal and distal femur in men.
Interestingly, although an increase of Ct.Po up to 87% with age has been described in other anatomic regions such as the surgical neck of the humeral head,33 a similar age-related change was not observed in the distal femur. One reason for the lack of increase could be the so-called phenomenon of trabecularization of cortical bone, which results in cortical bone showing morphological similarities to trabecular bone during aging.34-38 In fact, recently described results for cortical porosity measurements showed a high degree of resolution dependence and although the second-generation of in vivo HR-pQCT provides a higher resolution compared to the first-generation device, it is still lower compared to ex vivo micro-CT (e.g., synchrotron radiation micro-CT)—the gold standard for measuring cortical porosity.39, 40 Therefore, synchrotron radiation micro-CT may be needed to investigate whether the lack of increases in Ct.Po was due to insufficient spatial resolution or represented a distinct characteristic of the distal femur.
Due to its high predictive potential for risk assessment of other fragility fractures such as hip and vertebral fractures, the WHO has established the measurement of aBMD via DXA as the basis for the diagnosis of osteoporosis. DXA measurements have become the gold standard for fracture risk assessment worldwide.41 In this study, we found strong positive associations between aBMD measured by DXA at the proximal femur and microarchitecture parameters at the distal femur. Therefore, it is reasonable to assume that DXA measurement at the proximal femur is also strongly related to the risk of distal femur fractures. Nevertheless, the predictive value of DXA on distal femur fractures is likely to be lower than for other fragility fractures, which is mainly related to the rarity of this fracture entity.
Overall, our clinical data underscore that distal femur fractures in patients <60 years of age occurred only when relevant concomitant diseases (mainly involving disuse conditions) were present, whereas this was not the case in old age. This suggests that a large proportion of distal femur fractures in the elderly are attributable to age-related alterations in bone health (i.e., primary osteoporosis).
Despite the high consistency of the clinical data with the findings on microarchitecture, some limitations of this study need to be addressed. First, we did not perform biomechanical testing to evaluate bone strength. Therefore, we cannot determine the influence of individual bone microarchitecture parameters on fracture resistance. Second, the microarchitecture analysis was limited to 50 femora from 25 cadavers, potentially limiting generalizability of our results. Third, we were not able to investigate potential regional differences in bone microarchitecture (e.g., medial vs. lateral). Although a recent study investigating local differences revealed that the most distal region in the condylar region had the highest local BMD, no structural parameters were analyzed and the effect of age and sex was could not be determined due to the small number of specimens.42 Therefore, it remains questionable which region is structurally most suitable for screw placement. Third, the clinical analysis was limited to key demographic parameters, which did not allow for detailed analysis of individual factors that may have contributed to fracture development. This includes possible loosening and wear of knee prostheses in the cases with periprosthetic fracture.
In conclusion, we here present the first assessment of bone microarchitecture at the distal femur showing an inverse association with age in women but not in men. Consistent with the presented clinical data, our ex vivo analysis provides a microarchitectural explanation for the categorization of distal femur fractures as fragility fractures. With knowledge of the risk factors for impaired distal femur microarchitecture, such as poor aBMD by DXA (i.e., the gold standard of osteoporosis assessment), female sex, and advanced age, patients at high risk for distal femur fractures can be identified. Nonetheless, the diagnostic value of DXA in predicting distal femur fractures and the efficacy of bone-specific treatments on fracture risk reduction need to be further investigated in the future.
CONFLICT OF INTERESTS
The authors declare that there are no conflicts of interests.
AUTHOR CONTRIBUTIONS
Constantin Schmidt, Christoph Riedel, Tim Rolvien, and Jan Hubert wrote the manuscript. Constantin Schmidt, Christoph Riedel, Julian Stürznickel, Herbert Mushumba, Maximilian M. Delsmann, Christian Ries, Sebastian Kleiss, and Peter Bannas collected data and performed data analysis. Peter Bannas, Frank Timo Beil, Michael Amling, Klaus Püschel, Tim Rolvien, and Jan Hubert supervised the study. Frank Timo Beil, Michael Amling, Klaus Püschel, Tim Rolvien, and Jan Hubert conceived and designed the study. All authors reviewed, read, and approved the final manuscript.