Transient neonatal shoulder paralysis causes early osteoarthritis in a mouse model
Abstract
Neonatal brachial plexus palsy (NBPP) occurs in approximately 1.5 of every 1,000 live births. The majority of children with NBPP recover function of the shoulder. However, the long-term risk of osteoarthritis (OA) in this population is unknown. The purpose of this study was to investigate the development of OA in a mouse model of transient neonatal shoulder paralysis. Neonatal mice were injected twice per week for 4 weeks with saline in the right supraspinatus muscle (Saline, control) and botulinum toxin A (BtxA, transient paralysis) in the left supraspinatus muscle, and then allowed to recover for 20 or 36 weeks. Control mice received no injections, and all mice were sacrificed at 24 or 40 weeks. BtxA mice exhibited abnormalities in gait compared to controls through 10 weeks of age, but these differences did not persist into adulthood. BtxA shoulders had decreased bone volume (−9%) and abnormal trabecular microstructure compared to controls. Histomorphometry analysis demonstrated that BtxA shoulders had higher murine shoulder arthritis scale scores (+30%), and therefore more shoulder OA compared to controls. Articular cartilage of BtxA shoulders demonstrated stiffening of the tissue. Compared with controls, articular cartilage from BtxA shoulders had 2-fold and 10-fold decreases in Dkk1 and BMP2 expression, respectively, and 3-fold and 14-fold increases in Col10A1 and BGLAP expression, respectively, consistent with established models of OA. In summary, a brief period of paralysis of the neonatal mouse shoulder was sufficient to generate early signs of OA in adult cartilage and bone.
1 INTRODUCTION
Injuries to joint-stabilizing connective tissues have long been associated with subsequent development of osteoarthritis (OA).1, 2 Substantial clinical and basic science work has demonstrated that OA develops following anterior cruciate ligament injury and concurrent mechanical destabilization of the knee.3, 4 Similarly, increased incidence of shoulder OA has been observed following massive rotator cuff injury.5 Recent work in animal models has also demonstrated that massive rotator cuff tears can lead to the development of OA.6 These findings suggest that altered biomechanical forces on a joint increase the risk of developing OA in skeletally mature humans and animals. However, it is unclear whether altered loading in developing joints leads to long-term pathologies such as OA in adult joints.
Neonatal brachial plexus palsy (NBPP) occurs in approximately 0.4–4 out of every 1,000 live births.7, 8 Most commonly, the upper trunk of the brachial plexus is injured, and patients often present with absent shoulder abduction, shoulder external rotation, elbow flexion, and forearm supination, with intact wrist extension and flexion.9 Although between 8% and 36% of children with NBPP will have permanently impaired shoulder rotation, elbow extension, and grip strength, the majority of children with NBPP only have transiently impaired upper extremity function and recover by 2 months old.10, 11 Children and adolescents with persistent brachial plexus pathology often exhibit abnormal humeral head bone morphology and altered upper extremity function, suggesting that altered soft tissue biomechanics have substantial impact on the developing joint.11-14 However, no studies have examined whether children who recover from NBPP develop persistent bone abnormalities, and the number of patients who recover from NBPP that progress to symptomatic degenerative joint disease is unknown. Given the identification of hip dysplasia (DDH) as one of the leading causes of early onset hip OA before the age of 60,15, 16 it is possible that NBPP, an injury that leads to comparable abnormalities in shoulder development,11-13 may contribute to the development of early shoulder OA.
A murine paralysis model using serial botulinum toxin A (BtxA) injections into the supraspinatus muscle of neonatal mice was used to approximate this clinical condition, as it reproduces functional deficits in mice that are comparable with the deficits observed in humans and comparable with neonatal brachial plexus neurotomy animal models.17-21 Prior work using this model found that BtxA-induced paralysis led to a substantial decrease in mineralized bone in the humeral head.19 Additionally, just 2 weeks of paralysis in neonatal mice were sufficient to cause changes in the humeral head that persisted in 16-week-old mice.18 These findings suggest that children who recover from NBPP may develop subtle yet persistent changes to the humeral head that may contribute to the development of OA.22-25 Thus, we hypothesized that transient shoulder paralysis in a mouse model would lead to the development of early OA. We used our established model of neonatal mouse shoulder BtxA-induced transient paralysis to evaluate progression of OA.
2 METHODS
2.1 Animal model
This study was approved by the Columbia IACUC, and animals were housed and bred according to standard IACUC guidelines. Pregnant female C57BL/6 J Black 6 mice were purchased from Jackson Laboratory. After birth, thirty-one neonatal BtxA mice (P0) were injected twice per week for 4 weeks with saline in the right supraspinatus muscle and BtxA (in saline) in the left supraspinatus muscle regions.17-19 Previous work showed that BtxA-induced neonatal shoulder paralysis reproduced many of the clinical outcomes in patients with NBPP, and had similar outcomes to a mouse model of neonatal brachial plexus upper trunk neurotomy.17, 19, 21 Early work establishing this model demonstrated shoulder-specific paralysis, with minimal paralysis around the elbow.19 In this study, the duration of four weeks of paralysis was chosen based on prior work, which demonstrated that this duration of paralysis leads to persistent changes in the bone around the developing enthesis, but is not associated with persistent functional deficits.18 This iteration of the model was designed to mimic the clinical scenario of transient neonatal shoulder paralysis. Following 4 weeks of injections, 23 mice were allowed to recover for 20 weeks and 8 mice were allowed to recover for 36 weeks, before being sacrificed at 24 weeks or 40 weeks old, respectively. Nineteen Control mice received no injections and were sacrificed at 24 weeks (11 mice) or 40 weeks old (8 mice). The effect of BtxA was evaluated using two comparisons: (1) BtxA shoulders were compared to the contralateral saline-injected shoulders (within-animal paired comparison) and (2) BtxA shoulders were compared to cage activity control shoulders (across animal unpaired comparison). The BtxA versus Saline comparison accounted for animal-to-animal variability and took advantage of the statistical power of paired comparison. This comparison also allowed for assessment of effects of the injection and fluid load on the joint. However, the saline-injected shoulder may be affected by animal-wide effects of unilateral shoulder paralysis. Therefore, BtxA was also compared to a separate group of cage activity controls. Gait analysis was performed on animals before sacrifice. Postmortem assays consisted of bone morphometry, atomic force microscopy, histomorphometry, and gene expression. Sample sizes for all assays were determined based on power analyses using data from our prior studies. Prior work has not shown differences in outcomes between male and female mice; nevertheless, equal numbers of male and female mice were used throughout to account for any potential effects of sex on outcomes. All assays were performed blinded to the specimen group.
2.2 Gait analysis
Gait analysis was performed on BtxA and Control mice using a high-speed camera and illuminated footprint technology (Catwalk XT gait analysis system) to detect gait metrics: stance width (forepaw (FP) and hindpaw (HP)), stance intensity, length of time on each paw (right forepaw (RF) and left forepaw (LF) stand), limb swing speed (RF and LF swing), paw print area, and stride length. Two cohorts of mice were analyzed using gait analysis. First, a group of BtxA (12 mice: 8 male, 4 female) and Control (11 mice: 5 M, 6 F) animals were evaluated at a single time point, 24 weeks old, just before sacrifice. Second, a group of BtxA (8 mice: 4M, 4F) and Control (8 mice: 4M, 4F) animals were evaluated in a longitudinal, weekly manner, from 6 weeks to 10 weeks old.
2.3 Bone morphology
To analyze humeral head bone morphometry, 24-week-old BtxA and Control mice were sacrificed and shoulders were dissected. Samples were then fixed in 4% PFA, submerged in agarose, and scanned using microCT according to previously established methods.17, 18 Samples were scanned at an energy of 70 kilovolt peaks (kVp), intensity of 142 μA, and a resolution of 5 μm (Bruker SkyScan 1272). Images were reconstructed and evaluated using a segmentation algorithm to separate cortical and trabecular bone of the humeral head proximal to the growth plate (CTAn, Bruker). Ten BtxA (7M, 3F), 10 Saline (7M, 3F), and 12 Control (6M, 6F) shoulders were scanned, and the following morphology outcomes were determined: cortical thickness, cortical bone volume, bone volume fraction (BV/TV), trabecular thickness, trabecular number, and trabecular separation. Individual trabeculae segmentation (ITS) was also used to quantitatively assess the rod-and-plate-based microstructure of these samples.26 First, the trabecular bone region was segmented from microCT scans using manual contouring. Then, digital topological analysis-based skeletonization was applied to transform the thresholded trabecular bone image into a reduced structural skeleton while accurately preserving the topology. Digital topological classification was then performed to uniquely classify each skeletal voxel as either a surface or curve. Each voxel of the recovered image was then classified to belong to either a trabecular plate (surface) or trabecular rod (curve). Based on the 3D evaluations of the subchondral trabecular bone network, morphological parameters for rods and plates including plate-to-rod ratio (PR ratio), bone volume fraction (BV/TV), axial bone volume fraction (aBV/TV), trabecular plate surface area (pTb.S), trabecular rod length (rTb.L), and respective rod and plate bone volume fraction (rBV/TV and pBV/TV), number density (rTb.N and pTb.N), and thickness (rTb.Th and pTb.Th) were assessed. The density of plate-rod (P-R Junc.D), rod-rod (R-R Junc.D), and plate-plate (P-P Junc.D) junctions were assessed. Additionally, cortical tissue mineral density (TMD) and trabecular bone mineral density (BMD) were assessed for a subset of samples (6 Btx (5M, 1F), 6 Saline (5M, 1F), and 12 Control (6M, 6F) shoulders).
2.4 Atomic force microscopy
Indentation-type atomic force microscopy (AFM) has been established as an effective means of measuring and describing early cartilage changes in the setting of OA.27-29 Twenty four-week-old mouse humeral heads from BtxA (12 shoulders: 8 M, 4 F), Saline (11 shoulders: 8 M, 3 F) and Control (7 shoulders: 4M, 3 F) groups were dissected and stored in saline at −20°C. Samples were thawed just before testing and approximately 200-micrometer-thick cuts of the humeral head were prepared using either a precision sectioning saw or an 11 blade and mounted on glass slides using cyanoacrylate adhesive. Care was taken to not damage the articular cartilage during processing, and samples were then submerged in saline containing protease inhibitors (cOmplete™ Protease Inhibitor Cocktail, Millipore Sigma). A MFP-3D-Bio Infinity AFM (Oxford Instruments) was used to perform force-indentation tests. Five to nine points were tested over a 120 × 120 μm area, centrally located within humeral head cartilage; five consecutive tests were performed at each point. Samples were interrogated using CP-FM-PS probes (sQube) with 2.8 N/m stiffness cantilevers and 3.6 μm diameter spherical tips. Each force indentation curve was fitted to the Hertz indentation model to determine sample elastic modulus, and % stiffening was calculated as the ratio between the modulus of the fifth indent and the modulus of the first indent. (indent 5/indent 1).
2.5 Histology
The OARSI Histopathology Initiative established criteria for histological assessment of OA in the mouse knee. There has been substantial work utilizing these criteria in the basic science literature.30, 31 These criteria were recently applied to the mouse shoulder using the murine shoulder arthritis scoring (MSAS) system.6 For the current study, humeral heads from 24-week-old mice were fixed in 4% PFA, decalcified in formic acid, serially dehydrated in ethanol, embedded in paraffin, and stained with toluidine blue. Sections from each sample group (9 BtxA: 7M, 2F; 9 Saline: 6M, 3F; 12 Control: 6F, 6M) were blinded, evaluated using ImageJ, and scored according to the MSAS system. Humeral head percent sphericity was calculated by first taking the mean of three measurements of humeral head circumference, measuring the length of all portions of the humeral head that fit to a straight line, and then calculating flat portions of humeral head/circumference of humeral head. Cartilage thickness was calculated by finding the mean of 10 equally spaced measurements from the surface of the humeral head cartilage to the subchondral bone. The MSAS scoring system was then used to score sphericity of the articular surface of the humeral head, cartilage cellularity, subchondral bone morphology, staining pattern, and presence of pannus.6
2.6 Gene expression
Humeral head articular cartilage gene expression was determined in 8 BtxA (4M, 4F), 8 Saline (4F, 4M), and 8 Control (4M, 4F) shoulders from 40-week old mice using established methods.32 Immediately following mouse sacrifice and dissection, tissues were frozen in liquid nitrogen and pulverized using a ball mill homogenizer (Mikro-Dismembrator U, Sartorius). RNA extraction was performed using guandinium thiocyanatephenol-chloroform (TRIzol, Thermo Fisher Scientific) and interphase separation (Phase Lock Gel, QuantaBio). RNA cleanup was performed using spin columns (RNeasy Mini Kit, Qiagen) with on-column deoxyribonuclease I treatment (Qiagen). RNA quantity and quality were determined using a spectrophotometer (NanoDrop 1000, Thermo Fisher Scientific). RNA was then reverse-transcribed into cDNA using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Cartilage-, bone- and OA-related genes (Ihh, Acan, Runx2, Dkk1, Col2A1, Col10A1, BGLAP, ALPL, and BMP2) were evaluated by SYBR Green-based quantitative reverse transcription-polymerase chain reaction on an Applied Biosystems Quant-Studio 6 flex system. Glyceraldehyde 3-phosphate dehydrogenase served as a housekeeping gene, and the relative mRNA expression level for each target gene was determined as 2-ΔΔCt.
2.7 Statistical analysis
All data are shown as mean ± standard deviation. Statistical analysis for all experiments was performed using GraphPad Prism 7 software. The threshold for statistical significance was defined at p < 0.05. Gait differences between 24-week-old BtxA and Control mice were assessed using Student t-tests. Longitudinal assessment of gait differences between BtxA and Control mice were assessed using repeated measures two-way analysis of variances (ANOVAs) followed by Sidak multiple comparison tests when appropriate. For all other outcome measures, comparisons between BtxA and Control shoulders, and Saline and Control shoulders were assessed using ANOVAs followed by Sidak multiple comparison tests when appropriate. Comparisons between BtxA and Saline shoulders were assessed using paired Student t-tests. When evaluating histology, one BtxA sample and one Saline sample did not have a contralateral shoulder for comparison, and were excluded from paired analyses. Additionally, one Saline sample was excluded from ITS analysis due to motion artifact. Thus, the corresponding BtxA sample was also excluded from paired analyses.
3 RESULTS
3.1 Gait analysis
Longitudinal gait analysis was performed on a weekly basis for a cohort of animals from 6 to 10 weeks old. When comparing gait patterns between BtxA mice and cage activity Control mice, BtxA mice had significantly shorter RF stride length (p = 0.001), shorter LF stride length (p = 0.001), longer RF stand (p = 0.0004), longer LF stand (p = 0.005), smaller RF print area (p = 0.03), and smaller LF print area (p = 0.0002) compared with Control mice (Figure 1). There were no significant differences between Control and BtxA mice in stance width FP, stance width HP, maximum intensity, RF swing, and LF swing (Figure S1). There were significant increases with time for RF stand (p = 0.02), RF print area (p < 0.0001), and LF print area (p = 0.002). Post-hoc tests showed that BtxA mice exhibited significantly smaller LF print area at weeks 6 and 7 when compared to Control mice (p < 0.05). There were no significant differences in RF print area in BtxA mice compared to Control mice at these early time points. At week 10, BtxA mice exhibited significantly shorter RF stride length (p = 0.0002) and shorter LF stride length (p = 0.0002) compared to Control mice). BtxA mice also had longer RF stand (p = 0.04) at week 10 compared to Control mice. There were no significant differences between BtxA and Control mice at other specific time points and for other examined variables.
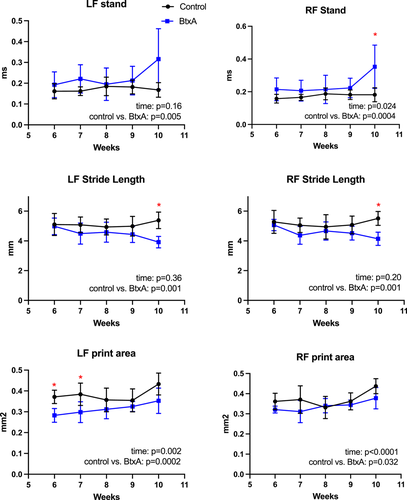
When comparing BtxA-injected (left) to Saline injected (right), at weeks 6 and 7, mean LF print was 88% (LF 0.28, RF 0.32) and 96% (LF 0.30, RF 0.31) of the size of the contralateral print, respectively. At week 10, mean LF stride was 95% of the length of the contralateral (LF stride 3.9, RF stride 4.1), and mean LF stand was 90% the length of RF stand (LF 0.3161, RF 0.3527). At all time points, mean LF print area was smaller than mean RF print area.
Gait analysis performed in a single long-term cohort of 24-week-old animals just before sacrifice showed a return to normal gait patterns (Figure 2, S2). There were no differences in FP stance width, HP stance width, maximum intensity, length of time standing on a single paw, paw print area, time length of forepaw swing and forepaw stride length between BtxA and Control mice. The left (BtxA) and right (Saline) forepaws of BtxA mice were also compared, and no statistically significant differences were observed in RF stand, LF stand, RF print area, LF print area, RF swing, LF swing, RF stride length, or LF stride length.
3.2 Bone morphometry
Based on standard microCT bone morphometry analysis, BtxA shoulders from 24-week old mice had significantly lower BV/TV when compared to contralateral Saline shoulders (p = 0.04) (Figure S3). Trabecular number and trabecular separation were significantly increased in BtxA shoulders compared to Saline shoulders (p = 0.01, p = 0.02, respectively). BtxA shoulders had significantly less cortical bone volume than both Control (p < 0.0001) and Saline shoulders (p < 0.0001) (Figure 3). No differences were observed amongst groups in cortical thickness. BtxA shoulders had significantly lower trabecular BMD than Saline shoulders (p = 0.03), however, no differences in cortical TMD were observed amongst Control, Saline and BtxA shoulders. There were also no differences in PR ratio observed amongst groups.
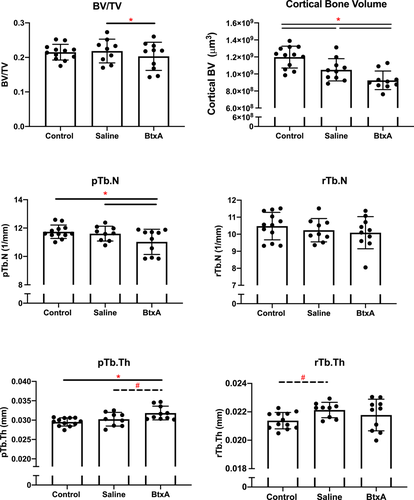
ITS analysis of microCT data showed that BtxA shoulders had significantly fewer plate-shaped trabeculae (pTb.N) than contralateral Saline shoulders (p = 0.02) and Control shoulders (p = 0.03) (Figure 3, S4). BtxA shoulders also exhibited thicker plate-shaped trabeculae (pTb.Th) compared to Saline (p = 0.09) and Control shoulders (p = 0.003). There were no significant differences between groups in PR ratio, rBV/TV, pBV/TV, BV/TV, aBV/TV, rTb.N, rTb.Th, pTb.S, rTb.L, R-R Junc.D, R-P Junc.D, and P-P Junc. D.
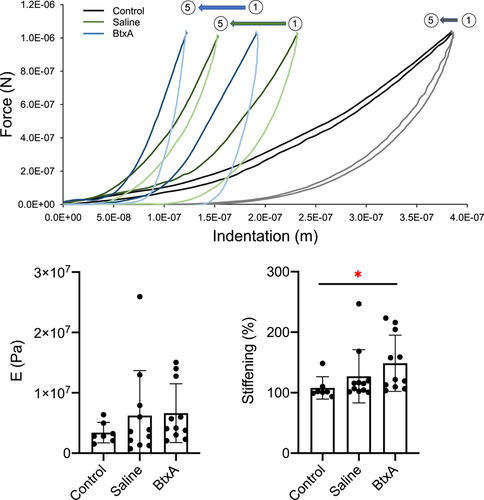
3.3 Atomic force microscopy
When comparing the moduli (E) of samples from 24-week old mice for the first indentation (indent 1), and at each subsequent indentation (indent 2 – indent 5), there were no significant differences between control, BtxA and contralateral Saline cartilage (Figure 4). Percent stiffening, defined as the ratio between indent 1 and a subsequent indent, was also evaluated. In all cases, BtxA cartilage exhibited significantly more stiffening with repeat indentation than control cartilage (indent 2/indent 1, p = 0.01; indent 3/indent 1, p = 0.01; indent 4/indent 1, p = 0.01; indent 5/indent 1, p = 0.01) (Figure 4), suggesting altered viscoelastic properties in BtxA cartilage. No statistically significant difference in stiffening was observed between Saline and Control, and BtxA and saline cartilage.
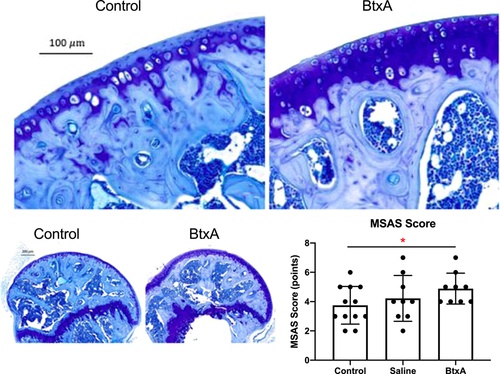
3.4 Histology
BtxA samples from 24-week old mice had significantly higher overall MSAS scores when compared to Control samples (p = 0.04), indicating that BtxA shoulders exhibited signs consistent with early OA (Figure 5). BtxA samples exhibited significantly higher cellularity scores than Control samples (p = 0.01). No differences among groups were seen in metrics that made up the MSAS score (subchondral bone quality, toluidine blue staining, presence of pannus, and sphericity amongst). No significant differences were seen in sphericity or cartilage thickness amongst BtxA, Saline, and Control groups (Figure S5).
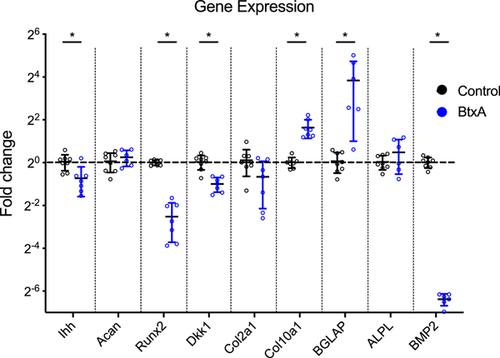
3.5 Gene expression
Gene expression changes in humeral head cartilage from 40-week old mice were measured for Ihh, Acan, Runx2, Dkk1, Col2A1, Col10A1, BGLAP, ALPL, and BMP2. There was significantly decreased gene expression in BtxA relative to Control cartilage for Runx2 (mean, 0.17-fold decrease, p < 0.0001), Dkk1 (mean, 0.49-fold decrease, p = 0.0001), and BMP2 (mean, 0.012-fold decrease, p < 0.0001) (Figure 6). There was also significantly increased gene expression between BtxA and Control cartilage for Col10A1 (mean, 3.1-fold increase, p < 0.0001) and BGLAP (mean, 13.6-fold increase, p = 0.009). There was no difference in gene expression between BtxA and Control shoulders in Ihh, Acan, Col2A1, and ALPL.
4 DISCUSSION
The current study demonstrated that transient neonatal shoulder paralysis can lead to early OA in a mouse model. Longitudinal gait analysis of juvenile mice demonstrated that young BtxA mice had significantly altered gait. However, these differences resolved in adult mice, demonstrating that BtxA mice eventually recovered full gait function after cessation of BtxA injections. Therefore, this animal model of neonatal shoulder paralysis shares features of transient NBPP, with a period of impaired function followed by functional recovery.
Using microCT analysis, we found persistent changes in the bone after recovery from transient shoulder paralysis, though the relationship between bone morphometry and OA is yet to be determined. In a study evaluating microCT changes in a DMM mouse model of knee OA, no significant difference in BMD was observed between controls and animals with early-stage OA. However, BMD was higher in animals with severe OA when compared to controls.33 In humans, there are mixed opinions regarding the relationship between BMD and OA.34, 35 ITS analysis in the current study demonstrated that BtxA shoulders exhibited fewer, thicker plate-shaped trabeculae compared to both contralateral Saline shoulders and the separate group of Control shoulders. It is possible that we observed fewer trabeculae in our adult mice due to the abnormal joint loading experienced during transient paralysis. Nevertheless, the observed thickening of trabeculae is consistent with the OA phenotype. Prior work evaluating microstructural subchondral bone changes in the setting of OA in humans showed no differences in the number of plate-shaped trabeculae beneath intact cartilage of OA samples compared to controls. However, significantly more plate-shaped trabeculae were observed in OA samples with overlying cartilage damage. Additionally, samples under both intact and damaged cartilage exhibited significantly thicker plate-shaped trabeculae when compared to controls.22
Using AFM analysis, BtxA cartilage exhibited significantly more stiffening with repeat indentation than control cartilage, suggesting altered viscoelastic properties. However, there were no statistically significant differences in stiffening when comparing Control and Saline and when comparing Saline and BtxA cartilage. There are mixed data regarding the effects of OA on the modulus of articular cartilage. In a DMM model of OA, a decrease in nanoindentation modulus relative to controls was shown to precede histological signs of degeneration.29 However, in an OA model using type IX collagen deficient mice, cartilage exhibited increased nanostiffness and unchanged microstiffness at early time points. Then, at later time points, these mice exhibited decreased nano- and micro-stiffness relative to controls. At early time points in the progression of OA, it is possible that loss of glycosaminoglycans leads to loss of water in the cartilage, particularly near the articular cartilage surface, and as a result, the AFM probe measures increased stiffness of the intact collagen network.28 However, at later stages of OA, when the cartilage surface may be fibrillated, the AFM probe may measure a decrease in stiffness due to the disrupted surface. This is consistent with the presumed loss of energy absorption in the cartilage of BtxA shoulders in the current study; these changes would be consistent with loss of viscosity and decreased permeability found in OA cartilage.
Regarding histologic assessment, BtxA shoulders exhibited significantly higher cellularity scores, consistent with a hypercellularity phenotype, when compared to Control shoulders. In a study of the progression of knee OA in a mouse model, the spatial arrangement of chondrocytes was shown to vary with disease progression. Early OA was associated with diffuse hypercellularity, with subsequent development of focal and then diffuse hypocellularity.36 Mice deficient in type IX collagen that develop OA-like changes in their joints also exhibit increased chondrocyte density at early time points.29 Thus, the hypercellularity, and likely disorganized chondrocyte proliferation observed in a majority of BtxA samples, is a finding consistent with early OA. In the current study, BtxA samples had significantly higher MSAS than Control samples, consistent with early OA. Prior work studying the relationship between subchondral bone changes and progression of OA in guinea pigs has shown that microstructural subchondral bone changes precede histological changes (as measured by OARSI scores).22 Therefore, our findings of significant microstructural changes in the subchondral bone, as seen on microCT, accompanied by relatively smaller histological changes, as seen in increased MSAS, are consistent with those observed in other established models of early OA.
Cartilage from BtxA shoulders exhibited significantly decreased expression of DKK1, BMP2, and Runx2 relative to Control shoulder cartilage. DKK1 encodes a protein that inhibits Wnt signaling and may play a role in bone formation and disease in adults. Severe OA has been associated with decreased expression of Dkk1, consistent with our findings listed above.37, 38 BMP2 encodes a secreted ligand that is a member of the TGF-beta superfamily of proteins and plays a role in osteogenesis and bone repair. BMP2 expression has been shown to be reduced in the synovial fluid of osteoarthritic human knee joints, consistent with our findings.39 Runx2 is essential for osteoblastic differentiation, as well as both intramembranous and endochondral ossification.40 Typically, OA is associated with increased expression of Runx2 in animal models and in human OA cartilage samples, thus it was unexpected to find a decrease in Runx2 expression in BtxA shoulders.41, 42 Similarly, expression of Ihh, a ligand that plays a critical role in endochondral ossification, was decreased in BtxA cartilage. In humans with OA, Ihh expression has been shown to be increased in synovial fluid and in immunohistochemical analysis of cartilage samples. However, Ihh expression was also shown to be low in patients with intact cartilage and in the early stages of osteoarthritis.43 Therefore, given that BtxA shoulder histologic samples did not exhibit substantial cartilage erosion, decreased Ihh expression in these shoulders be an indication of early OA. BtxA shoulders also had significantly increased expression of Col10A1 and BGLAP relative to controls. Col10A1 is the alpha chain of type X collagen, and is expressed by hypertrophic chondrocytes involved in endochondral ossification as well as in the mineralization zones of hyaline cartilage.44 Progression of OA is associated with increased expression of Col10A1, consistent with our findings.45BGLAP, also referred to as osteocalcin, is a bone protein secreted by osteoblasts that regulates bone remodeling and energy metabolism.46 High levels of BGLAP have been observed in osteophytic cartilage, and in the subchondral bone beneath osteophytes in osteoarthritic joints.47 Therefore, elevated levels of Col10A1 and BGLAP in our BtxA samples are consistent with the presence of early OA.
This study had a number of limitations. First, only early signs of OA were examined. Results showed early changes in gene expression, histology, bone morphometry, and mechanics consistent with the onset of OA. However, longer timepoints are needed to verify that these early changes progress into full OA, including breakdown and fibrillation of the articular cartilage. Second, the molecular mechanisms driving early OA in BtxA mice remain to be determined. The gene expression results revealed several leading candidates for bony and stiffening changes, including changes in expression of Runx2 and Col10, but further studies, including immunohistochemistry to localize protein expression patterns, are needed to determine the roles of these genes for driving OA in these mice. Third, as we have observed in previous studies, there were differences in some outcome measures between saline-injected shoulders and control shoulders of comparison animals.17-20 These differences are attributed to changes in overall animal behavior due to paralysis in one shoulder as well as local effects of the saline injection itself, and not due to systemic effects of BtxA. This conclusion is based on our previous work, which showed that outcomes after BtxA-induced paralysis matched outcomes after brachial plexus transection-induced paralysis in mice, validating the use of BtxA to model transient neonatal brachial plexus palsy.17-20 Third, due to the limited mRNA available from mouse humeral head cartilage, only a relatively small number of genes were examined. Further analysis, including earlier timepoints and genes related to mechanotransduction and matrix degradation, would help elucidate the progression of OA in this model.
A substantial portion of mouse humeral head development occurs in the first 4 weeks of life. In comparison, a relatively smaller number of developmental milestones occur in the first two months of life for humans. Prior work demonstrated persistent changes in the mouse humeral head after only 4 weeks of neonatal paralysis, despite apparently full functional recovery in the shoulder.18 Humans can have up to approximately 2 months of paralysis without experiencing permanent functional deficits whereas mice can experience at least 4 weeks of paralysis without long-term functional deficits. Therefore, despite a clear difference in the time scales for NBPP in humans compared to the BtxA mouse model, the key feature of persistent bony defects coupled with full functional recovery is similar between the two scenarios.
Recent evidence has distinguished between cartilage degeneration due to atrophy versus cartilage degeneration due to OA.48 Cartilage atrophy has a number of tissue and molecular level features that distinguish it from osteoarthritis. One of the notable features of disuse atrophy is that it is associated with relatively rapid recovery after the subject resumes normal activity. In the current study, gait analysis demonstrated functional recovery with muscle recovery from transient paralysis. However, BtxA shoulders exhibited persistent changes in the subchondral bone, abnormal viscoelastic properties in the cartilage, abnormal histologic findings, and abnormal gene expression compared to controls. The changes were consistent with early signs of OA. Although it is possible that disuse atrophy may have contributed to changes in the joint at early time points, these persistent changes are less consistent with a disuse atrophy phenotype and more consistent with OA.
Current treatment recommendations for children with NBPP include, but are not limited to, observation, regular physical exams, and daily physical therapy exercises until 3 months of age.49 However, the majority of children with NBPP recover function before 2 months old, and thus, many do not receive additional treatment or pursue long-term follow-up.10 The shortcoming of this treatment paradigm is that critical humeral head developmental milestones, including the development of preossification centers, occur by 2-months in many patients, suggesting that even transient paralysis may affect humeral head development in the long term.50 The findings in the current study have significant clinical implications, particularly given recent work identifying DDH as a source of early hip OA requiring treatment with early total hip arthroplasty (THA). A study of the Norwegian Arthroplasty Register found that DDH accounted for 26% of THAs performed before 40 years old, and the average age at diagnosis of DDH for these patients was 7.8 years.51 A comparable study of the Medical Birth Registry of Norway and the Norwegian Arthroplasty Register found that 92% of all patients who underwent THA due to an underlying dysplasia had no reported hip instability in the newborn period, as assessed on clinical exam.52 These data suggest that infants often have hip pathology that is missed or undetectable on clinical exams. The lessons learned from studying DDH, if applied to NBPP, have concerning clinical implications.
The current understanding of the natural history of transient NBPP is that children who experience recovery of their upper extremity function do not experience appreciable long-term shoulder pathology. However, in this study of an animal model of transient neonatal shoulder paralysis, we found that a brief period of paralysis in neonatal mice was sufficient to generate subchondral bone, cartilage, and gene expression changes in adult mice consistent with early OA. As with all animal models, there are limits to application of the results to clinical care. The current study demonstrated that transient neonatal paralysis can lead to early signs of OA, and this result is valuable for generating hypotheses for subsequent clinical studies. Given recent findings that DDH may contribute substantially to the development of severe, early hip OA, further clinical studies are necessary to determine what role transient NBPP may have in the development of early shoulder OA.
ACKNOWLEDGMENTS
The study was supported by NIH/NIAMS R01 AR055580 and R01 AR069668, and the OREF (with funding provided in part by Exactech Inc.). The study sponsors and sources of funding had no involvement in the study design, data collection, data analysis, data interpretation, writing of the manuscript, or decision to submit the manuscript for publication.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
All authors have made substantial contributions to this manuscript. Lynn Ann Forrester was responsible for the conception and design of the study, acquisition of data, analysis and interpretation of data, drafting and revising of the article, and final approval of the manuscript. Fei Fang, Timothy Jacobsen, Yizhong Hu, and Iden Kurtaliaj were responsible for acquisition and analysis of data, assistance in drafting the manuscript, and final approval of the manuscript. Benjamin D. Roye, X. Edward Guo, Nadeen O. Chahine, and Stavros Thomopoulos were responsible for the conception and design of the study, revising the manuscript, and final approval of the manuscript.