White matter disturbances in phenylketonuria: Possible underlying mechanisms
Abstract
White matter pathologies, as well as intellectual disability, microcephaly, and other central nervous system injuries, are clinical traits commonly ascribed to classic phenylketonuria (PKU). PKU is an inherited metabolic disease elicited by the deficiency of phenylalanine hydroxylase. Accumulation of l-phenylalanine (Phe) and its metabolites is found in tissues and body fluids in phenylketonuric patients. In order to mitigate the clinical findings, rigorous dietary Phe restriction constitutes the core of therapeutic management in PKU. Myelination is the process whereby the oligodendrocytes wrap myelin sheaths around the axons, supporting the conduction of action potentials. White matter injuries are implicated in the brain damage related to PKU, especially in untreated or poorly treated patients. The present review summarizes evidence toward putative mechanisms driving the white matter pathology in PKU patients.
Significance
Phenylketonuria (PKU) is an inherited metabolic disease that leads to the accumulation of l-phenylalanine and its metabolites in tissues and body fluids of phenylketonuric patients. Among several central nervous system alterations, white matter damage is a common finding in untreated and poorly treated patients. Myelin is important for adequate brain function and its damage is related to impaired executive functions, as observed in PKU patients. In this context, it is important to understand the mechanistic of white matter damage in PKU to mitigate cognitive impairment found in phenylketonuric patients.
1 INTRODUCTION
Classical phenylketonuria (PKU; OMIM # 261600) is an inherited metabolic disorder caused by phenylalanine 4-hydroxylase (EC # 1.14.16.1) deficiency, with an estimated worldwide prevalence of 1:10,000 newborns (Albrecht, Garbade, & Burgard, 2009). This enzyme catalyzes the hydroxylation of the essential amino acid l-phenylalanine (Phe) into l-tyrosine in the presence of tetrahydrobiopterin as the cofactor of the reaction (Flydal & Martinez, 2013). The deficiency leads to the accumulation of Phe (hyperphenylalaninemia; HPA), as well as Phe-derived metabolites (e.g., phenylpyruvate, phenyllactate. and phenylacetate), in tissues and biological fluids of phenylketonuric patients (Kaufman, 1999; Mitchell, Trakadis, & Scriver, 2011; Regier & Greene, 2017). Strict dietary Phe restriction remains the core of therapeutic management in classic PKU (Ney, Blank, & Hansen, 2014).
Intellectual disability, microcephaly, epilepsy, murine urine odor, skin and hair hypopigmentation, eczema, and psychiatric disorders are clinical features commonly attributed to PKU, especially in untreated patients (Regier & Greene, 2017). Phenylalanine 4-hydroxylase is essentially expressed in the liver and, to a lesser extent, in the kidney. However, classical PKU mainly affects central nervous system (CNS) function (Donlon, Levy, & Scriver, 2017). Decreased rates of protein synthesis and transport of large neutral amino acids into the CNS, formation of Phe oligomers and fibrils, as well as impaired energy metabolism and myelination have been shown under HPA conditions (Adler-Abramovich et al., 2012; Berti et al., 2012; Hoeksma et al., 2009; Landvogt et al., 2008). Studies evidenced that a reduction in brain protein synthesis is at least partially related to impaired transport of tyrosine to the CNS via blood–brain barrier, therefore decreasing the incorporation of this amino acid into proteins (de Groot et al., 2013; de Groot, Sijens, Reijngoud, Paans, & Spronsen, 2014). Formation of Phe amyloid-like fibrils was shown in PKU mouse models and PKU patients (Adler-Abramovich et al., 2012). These fibrils reduce cell viability and are speculated to contribute as an immunological component in the pathophysiology of the disease (Adler-Abramovich et al., 2012; Anand, Dubey, Shekhawat, & Kar, 2017). Defects in bioenergetics, in calcium homeostasis, and in the metabolism of neurotransmitters and lipids have also been associated with the accumulation of phenols and their phenylketones, with implications for the neurological manifestations associated with PKU (Schuck et al., 2015).
Impaired myelination was reported in PKU, especially in untreated patients (Anderson & Leuzzi, 2009). CNS myelination is the process by which myelin sheaths, which are produced by oligodendrocytes (OLs), surround and isolate the axons, supporting the saltatory conduction of action potentials (Barkovich & Mukherjee, 2011; Paz Soldán & Pirko, 2012). The speed of information conduction provided by myelination is vital for high-order functions, such as cognition, behavior, and emotions (Deoni et al., 2011; Nickel & Gu, 2018). Axonal integrity also depends on the support of the myelinating body, and myelin plays a role in the metabolic support to axons (Saab & Nave, 2017; Simons & Nave, 2015). Defective myelination has been associated with psychiatric conditions, such as bipolar disorder, psychosis, and schizophrenia (Lewandowski et al., 2015; Mighdoll, Tao, Kleinman, & Hyde, 2015; Nave & Ehrenreich, 2014), as well as with the pathophysiology of Alzheimer's disease (Bartzokis, Lu, & Mintz, 2007). Hypomyelination is characterized by decreased myelin production, which leads to a significant and permanent reduction of its deposition in the cerebral white matter. The loss of myelin with relative preservation of axons is called demyelination, which arises from damage to myelin sheaths or their forming cells (Bizzi et al., 2008; Love, 2006). This review gathers mechanisms affecting myelination that may contribute to the pathophysiology of PKU.
2 OLIGODENDROGENESIS AND CNS MYELINATION
OLs are highly specialized CNS cells, which form the myelin sheaths and therefore are necessary for the proper transmission of neuronal action potentials. During brain development, OLs are the last cells to be generated (El Waly, Macchi, Cayre, & Durbec, 2014). OLs are derived from tubular neuroepithelial cells, that give rise to OL precursor cells (OPCs), originally located in the subventricular zone (Skoff & Benjamins, 2014). OPCs arise from multiple restricted periventricular germinal regions, and then proliferate and differentiate into mature and functional OLs. Three sequential waves of OPCs populate the cerebral cortex (Kessaris et al., 2006).
In humans, myelination initiates in the fifth month of the fetal period (Branson, 2013). The pattern of myelination in different regions follows the distribution of OPC differentiating into myelinating OLs (Yeung et al., 2014). The process of OPCs differentiating to OLs is conserved in the adult brain, although at a much slower rate (Osso & Chan, 2017).
OPCs are produced in sequential stages. They predominantly arise from the ventral region of the neural tube in the early stages of development, and from the dorsal region of the neural tube at later stages (Richardson, Kessaris, & Pringle, 2006). Differentiation of OPCs into mature OLs is regulated by several intrinsic and extrinsic factors, which determine the extent and timing of their differentiation during CNS development (Emery, 2010; Kessaris, Pringle, & Richardson, 2008; Mitew et al., 2014; for a comprehensive review on basic mechanisms of CNS myelination see Foster, Bujalka, & Emery, 2019; Sock & Wegner, 2019; Zuchero & Barres, 2013). However, it is crucial that a small population of OPCs remain immature or quiescent during CNS development, rendering a potential source of OLs in future demyelinating conditions, as depicted in Figure 1 (Dawson, Polito, Levine, & Reynolds, 2003).
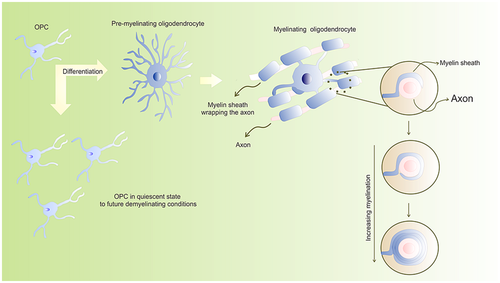
During the initial differentiation of OPCs, certain extrinsic signals act on multipotent neural progenitor cells (Figure 2), triggering a signaling cascade that converges on specific OL lineage transcriptional regulators (Naruse, Ishizaki, Ikenaka, Tanaka, & Hitoshi, 2017). Ventrally, one of the most prominent extrinsic signals is the sonic hedgehog (Shh) protein. It triggers the expression of OL lineage transcription factor 2 (Olig2) and induces the first embryonic wave of OPCs in the CNS (Orentas, Hayes, Dyer, & Miller, 1999; Pringle et al., 1996; Tekki-Kessaris et al., 2001). Therefore, exposure of the dorsal neuroepithelium to Shh triggers oligodendrogenesis, whereas the lack of Shh signaling mitigates left ventricular-derived OL appearance throughout CNS (Orentas et al., 1999; Tekki-Kessaris et al., 2001).
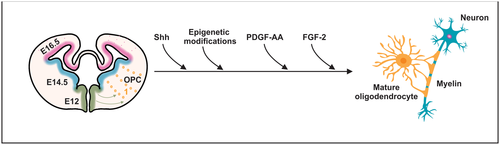
Additional extrinsic factors act independently of Shh in the differentiation of OPCs. Such clues include platelet-derived growth factor-AA (PDGF-AA) and fibroblast growth factor (FGF-2; Naruse et al., 2006). PDGF-AA binds primarily to the PDGF receptor α (PDGFRα) in immature precursor cells to promote Olig2 gene transcription (Hu et al., 2008, 2012). Several studies underscored the importance of PDGF-AA in inducing mitosis in OPCs to support survival and to regulate the rate of differentiation in OLs (Barres, Schmid, Sendnter, & Raff, 1993; Butt, Hornby, Kirvell, & Berry, 1997; Hart, Richardson, Bolsover, & Raff, 1989; Noble, Murray, Stroobant, Waterfield, & Riddle, 1988; Pringle et al., 1996; Raff, Abney, & Fok-Seang, 1985; Richardson, Pringle, Mosley, Westermark, & Dubois-Dalcg, 1988).
Axons present specific adhesion molecules, which are recognized by the OLs. Following adherence to the axon, structural changes occur in OL plasma membrane, forming the myelin sheath (Paz Soldán & Pirko, 2012). Myelinated axons have myelin segments called internodes. These structures are separated by small sections called Ranvier nodes, containing high-density sodium channels (Aggarwal, Yurlova, & Simons, 2011).
During the myelination process, the OL surrounds the axon and its cytoplasm is compressed back into the cell body, forming a multilayer wrapping (Boiko & Winckler, 2006). Myelin is mainly composed by lipids (up to 70%), such as cholesterol, phospholipids (plasmogen), and glycosphingolipids (cerebroside and sulfatide). Proteins are also important components of myelin, including myelin basic protein (MBP) and proteolipid protein (Branson, 2013).
Within the myelin membrane, glycosphingolipids are differentially located: galactocerebroside is generally present in compact myelin, whereas non-compact myelin mainly contains galactosyl sulfatide. Ceramide is the precursor for galactolipids, gangliosides, and sphingomyelin (Paz Soldán & Pirko, 2012). The free amide from galactosylceramide and galactosyl sulfatide forms hydrogen bonds with hydroxyl groups at the lipid-water interface, resulting in the formation of a tightly organized and tightly packed myelin sheath (Aggarwal et al., 2011), assembling the membrane in a stable insulating sheath (Schmitt, Cantuti Castelvetri, & Simons, 2015). It gives rise to surface appositions representing alternating extra- and intracellular spaces observed by electron microscopy (Morell & Quarles, 1999).
While immature precursor cells respond primarily to PDGF-AA, mature precursor cells respond to FGF-2. This process is mediated by different receptors, depending on the maturity stage of the OPCs (Skoff & Benjamins, 2014). FGF-2 induces mitogenesis of pro-OL (late progenitors) and inhibits the differentiation of these cells into mature OLs (Bansal & Pfeiffer, 1994; McKinnon, Matsui, Dubois-Dalcq, & Aaronsont, 1990). In addition, intraventricular microinjection of FGF-2 into mouse anterior brain increased the expression of PDGFRα and Olig2 (Naruse et al., 2006).
Epigenetic modifications also coordinate OL differentiation. To illustrate, in vitro and in vivo experiments by Zhao, Wu, Zheng, Gao, and Ju (2012) showed that overexpression of microRNA-7a induced the production of OL lineage cells in multipotent neural progenitor cells and in cerebral cortex from embryonic mice. Conversely, ablation of microRNA-7a during the differentiation of multipotent neural progenitor cells decreased the amount of mature OLs (Zhao et al., 2012). Inhibition of histone deacetylases in subventricular zone precursor cells also decreases the number of OLs by almost 50%, as well as Olig2 expression (Siebzehnrubl et al., 2007).
3 CNS DEMYELINATION IN PKU
A myriad of findings is attributed to PKU and HPA, such as behavioral problems, seizures, intellectual disability, and hypomyelination/demyelination (Dyer, 2000; Huttenlocher, 2000). Disturbances of myelination in PKU patients were originally reported in the 1950s (Alvord Jr., Stevenson, Vogel, & Engle Jr., 1950; Poser & Van Bogaert, 1959). Since then, alterations in the integrity of the cerebral white matter were described in PKU patients or in their offspring (Koch, Verma, & Gilles, 2008; Nardecchia et al., 2015; Peng, Peck, White, & Christ, 2014; Tufekcioglu et al., 2016), although alterations in the gray matter have been also detected (Bodner et al., 2012; Christ et al., 2016). The white matter pathology observed in PKU patients is diffuse throughout the brain and worsens with age, and it seems to participate in the decline of executive functions due to non-adherence to the treatment (Hawks et al., 2019). To illustrate, the microstructural development of white matter is progressively impaired in PKU children (Wesonga, Shimony, Rutlin, Grange, & White, 2016), affecting inferior executive abilities in PKU patients at different ages (Antenor-Dorsey et al., 2013). Deficiencies in the formation and maturity of myelin and axons were reported in HPA rats (Reynolds, Burri, & Herschkowitz, 1993; Reynolds, Burri, Mahal, & Herschkowitz, 1992). Behavioral changes and late cerebellar maturation were also demonstrated in rats under HPA during the first 21 days after birth, a critical period for rat brain myelination (Andersen & Guroff, 1972).
Abnormal myelination has also been associated with impaired interhemispheric connections in children affected by PKU receiving early therapy, compared to children with attention deficit/hyperactivity disorder or healthy children (Gourovitch, Craft, Dowton, Ambrose, & Sparta, 1994). Late myelination seems to occur mainly in the cerebral lobes and corpus callosum of phenylketonuric children, as shown by magnetic resonance imaging (Zhongshu, Weiming, Yukio, Cheng-(L)Ning, & Zhixing, 2001). Interestingly, Schoemans and colleagues (2010) showed that the number of OLs did not change in the corpus callosum from a 2-month-old genetic mice model of PKU. Cell viability of OL-enriched cultures was unchanged by incubation with Phe and its metabolites. Interference of these compounds on the direct quantification of myelin sheaths in myelinating cocultures was also null (Schoemans et al., 2010). However, elevated Phe levels during development of rats (from 3rd to 17th day of life) diminished OL number in corpus callosum, which can be reversed if rats are kept with physiological Phe levels for 6 weeks (Reynolds et al., 1992). It underscores that increased Phe levels can disrupt myelination, that may be partially reversed by dietary Phe restriction as shown by Zhongshu and colleagues (2001).
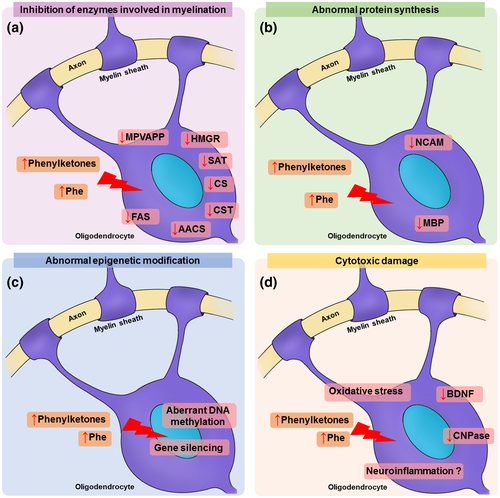
Changes in the synthesis and availability of cholesterol and other myelin constituents may contribute to hypomyelination (Saher & Stumpf, 2015). The primary role of cholesterol is to provide stability to myelin by regulating membrane fluidity and permeability. Since the availability of this lipid is rate-limiting for myelin assembly, upstream signaling systems driving myelin formation can be directly affected to cholesterol metabolism (Schmitt et al., 2015). PKU patients show low levels of membrane lipids in blood, such as docosahexaenoic acid, eicosapentaenoic acid, and cholesterol (Montoya Parra, Singh, Cetinyurek-Yavuz, Kuhn, & MacDonald, 2018; Stroup et al., 2018; Williams, Hooper, Bell, Mamotte, & Burnett, 2015). Interestingly, PKU patients have low levels of a metabolite of cholesterol metabolism in brain, 24S-(OH) cholesterol, which is inversely correlated with plasma Phe levels (Lütjohann & Von Bergmann, 2003). In fact, postmortem analysis of cerebral white matter from four PKU patients showed that cerebroside and cholesterol levels were markedly lower than in age-matched control individuals (Crome, Tymms, & Woolf, 1962). A possible mechanism for this phenomenon is the inhibition of cholesterol synthesis. Phe metabolites, in special phenylpyruvate, inhibit cholesterol synthesis from mevalonate in rat brain in vitro (Shah, Peterson, & McKean, 1969). Lower activities of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGR) and mevalonate-5-pyrophosphate decarboxylase were reported in the brain of chicks under experimental HPA (Castillo, Zafra, & Garcia-Peregrin, 1988). Similarly, Shefer et al. (2000) showed a significant decrease in the activity and protein level of HMGR in forebrain of PKU transgenic mice. In hypomyelinating tracts, a 40% reduction in MBP-positive cells expressing HMGR was also reported, implying that hypomyelination in the mouse brain may involve impaired cholesterol biosynthesis (Shefer et al., 2000). The authors also showed that Phe is a non-competitive inhibitor of HMGR (Shefer et al., 2000). Other neurodegenerative diseases with myelin injuries are caused by mutations in enzymes involved in cholesterol biosynthesis, such as 7-dehydrocholesterol reductase (e.g., Smith-Lemli-Opitz syndrome), or trafficking and storage of lipids, including cholesterol (e.g., Niemann-Pick type C disease; Saher & Stumpf, 2015).
A multitude of factors beyond cholesterol metabolism may also be implicated in PKU-related myelin pathology, including changes in other lipids and proteins (Schuck et al., 2015). Galactolipids and sulfatides play important roles in OL development and differentiation (Ranscht, Clapshaw, Price, Noble, & Seifert, 1982). In fact, these lipids are essential for myelin stabilization and maintenance. Transgenic mice lacking enzymes for the synthesis of sulfatides or galactoceramides have severe motor symptoms, thinner myelin sheaths and abnormal nodal/paranodal structures (Marcus et al., 2006; Schmitt et al., 2015). In this regard, the brain of HPA animal models shows low levels of ceramides and sulfatides and low enzyme activity of galactosylceramide sulfotransferase and adenylyltransferase sulfate (Burri, Matthieu, et al., 1990; Burri, Steffen, et al., 1990; Hommes, 1991; Matsuo, Moss, & Hommes, 1987). Impaired myelination in PKU was subsequently associated with the inhibition of fatty acid synthase and citrate synthase in the presence of phenylpyruvate in rat brain (Land & Clark, 1973). Considering that the enzymes for metabolism of ketone bodies, including acetoacetyl-CoA synthetase, are ubiquitously expressed in OLs and contribute to lipid synthesis in these cells (Pleasure et al., 1979; Poduslo, 1989), it cannot be ruled out that the inhibition by Phe and/or its metabolites on these enzymes can contribute to PKU and experimental HPA-related demyelination.
Abnormal protein composition in myelin fractions was also detected in the brain of HPA animals (Figlewicz & Druse, 1980). Decreased MBP levels, as well as changes in the levels of cyclic 2′,3′-nucleotide 3′phosphodiesterase (CNPase) and neural cell molecule adhesion (NCAM), which were shown to be critical for myelination, have been described in the brain of animals submitted to experimental HPA (Alejandre, Marco, Ramirez, Segovia, & Garcia-Peregrin, 1984; Burri, Matthieu, et al., 1990; Burri, Steffen, et al., 1990; Reynolds et al., 1992; Ushakova, Gubkina, Kachur, & Lepekhin, 1997). MBP triggers secretion of actin disassembly peptides necessary to initiate myelin wrapping around the axons (Zuchero et al., 2015). Besides, MBP concentration can directly affect the early stages of myelination (Roll-Mecak, 2019). NCAM is a protein related to cell adhesion and is expressed by neurons, astrocytes, OLs, and myelin sheaths (Bhat & Silberberg, 1988; Massaro, 2002; Palser, Norman, Saffell, & Reynolds, 2009). Ncam1−/− mice show a delay in myelination and remyelination after distress with cuprizone (Werneburg et al., 2017). In vitro data show that NCAM is associated with OPC outgrowth and survival of premyelinating OLs, probably by FGF receptors (Palser et al., 2009). In addition, CNPase is important for myelination of small axons and knockout CNPase mice show premature neurodegeneration (Edgar et al., 2009). Moreover, CNPase may protect cells from controlled cortical impact injury by increasing adenosine levels and decreasing 2′,3′-cAMP levels (Verrier et al., 2012, 2013).
Gene expression may be an alternative target for HPA effects. Aberrant pattern of DNA methylation has been identified in the postmortem brain of PKU patients (Dobrowolski et al., 2015). Altered patterns of DNA methylation in genes have also been detected in the brain of a PKU genetic mice model (Dobrowolski et al., 2016). Several abnormally expressed proteins have been reported in the brain of PKU mice, providing insightful clues to substantiate the neurological dysfunction presented by PKU patients (Imperlini, Orrù, Corbo, Daniele, & Salvatore, 2014; Oh et al., 2005). In this scenario, low levels of brain-derived neurotrophic factor (BDNF) protein and mRNA were reported in cortical neurons grown in Phe-containing medium (Li, Gu, Lu, & Liang, 2010). It was associated with decreased Erk and Akt signaling, impaired neurite development, and neuronal death (Li et al., 2010). In addition, BDNF incubated with cortical neuronal cultures decreased Phe-induced apoptosis rate by inhibiting RhoA signaling (Zhang, Zhao, Wang, & Jiao, 2010). Following a demyelinating damage with cuprizone, BDNF induces an increase in OPC proliferation (Tsiperson et al., 2015). Indeed, BDNF-induced increased proliferation and differentiation of OPC supports a way to therapeutically improve disease-related deficiencies in which demyelination is an underlying mechanism (Huang & Dreyfus, 2016). Other evidence of altered gene expression in PKU is lower transcription and translation of myocilin, a protein expressed in myelin sheaths, in a PKU mice model (Surendran et al., 2005).
OLs and myelination are closely related to neuronal activity. Neural activity evokes an intracellular calcium transient elevation which is linked with development, growth, and stabilization of myelin sheaths (Baraban, Koudelka, & Lyons, 2018; Krasnow, Ford, Valdivia, Wilson, & Attwell, 2018). OLs could also interact with neurons through PSD95 (a protein usually expressed in postsynaptic scaffolding and excitatory synapses to anchor glutamate NMDA receptors) or other scaffolds (Allison, Chervin, Gelfand, & Craig, 2000; Brasko, Hawkins, Rocha, & Butt, 2017; Hrvatin et al., 2018; Hughes & Appel, 2019; Zhang et al., 2014). Phe concentrations commonly seen in PKU patients inhibit NMDA by competing for the glycine-binding site (Glushakov et al., 2002; Glushakov, Dennis, Sumners, Seubert, & Martynyuk, 2003; Martynyuk et al., 2005). Besides glutamatergic system, aberrant myelination may decrease the synthesis of dopamine and other neurotransmitters, a phenomenon observed in HPA. Proper myelination is required to trigger signaling pathways that result in axonal maturation and upregulation of the machinery for dopamine biosynthesis (Joseph & Dyer, 2003).
The gliosis, abnormal myelination, and axonal degeneration reported in the optic nerve of rats subjected to HPA were consistent with damage to OLs (Avins, Guroff, & Kuwabara, 1975). These cells are responsible for the synthesis and maintenance of myelin, as well as for axonal support (Nave, 2010; Simons & Nave, 2015; Skoff & Benjamins, 2014). Demyelination accompanied by decreases in neuronal cell size, dendritic arborization, and synaptic spine populations were detected in the brain of untreated PKU patients (Bauman & Kemper, 1982). In fact, OL-directed lesions have been associated with hypomyelination, which may result in altered axonal integrity and neuroinflammation, subsequently leading to neurodegeneration (Desai, Guercio, Narrow, & Bowers, 2011; Ghosh et al., 2011; Kassmann et al., 2007; Kassmann & Nave, 2008; Morrison, Lee, & Rothstein, 2013; Pohl et al., 2011).
Neuroinflammation is involved in the neurodegeneration found in other demyelinating diseases, including multiple sclerosis (Peterson & Fujinami, 2007). Direct damage to gray matter may be induced by the release of inflammatory mediators and cytotoxic factors in the brain of patients with progressive multiple sclerosis, while indirect damage may be induced by activation of CNS or microglia (Calabrese et al., 2015). In the brain of a PKU mice model, upregulation of genes related to inflammation is observed, which is normalized by gene therapy (Oh et al., 2005). However, evidence of neuroinflammation occurring in the brain of PKU patients is disputed. Deon and colleagues (2015) reported increased levels of the inflammatory cytokines, interleukin-6 and interleukin-1β, in plasma and urine of PKU patients, indicating a pro-inflammatory status (Deon et al., 2015). However, Mozrzymas, Duś-Żuchowska, Kaluzny, Wenska-Chyży, and Walkowiak (2016) did not observe alterations in the levels of interleukine-6 and interleukine-8 in the blood of patients with classical PKU (Mozrzymas et al., 2016). Likewise, the levels of the biomarker for gut inflammation calprotectin are unchanged in stool samples from PKU patients (Walkowiak et al., 2013). The apparent discrepancy of these findings may be explained by the fact that PKU patients with indication of neuroinflammation were diagnosed at a late stage (Deon et al., 2015), in contrast to those from Mozrzymas and Walkowiak reports, who were diagnosed by newborn screening programs (2013, 2016).
OLs and its precursors are sensitive to alterations in redox homeostasis (Brill, Scheuer, Bührer, Endesfelder, & Schmitz, 2017; Takase et al., 2018). Several reports indicate that oxidative stress is implicated in the pathophysiology of PKU, as demonstrated in animal HPA models (Fernandes et al., 2010; Lu et al., 2011; Mazzola et al., 2011; Moraes et al., 2014; Simon et al., 2013) and in samples from PKU patients (Huemer et al., 2012; Item et al., 2017; Okano & Nagasaka, 2013; Sitta et al., 2009, 2011). The interplay between oxidative stress and bioenergetics dyshomeostasis has been associated with myelin defects. In this scenario, HPA was shown to cause energy dysfunction in the brain of rodents (Dimer et al., 2018; Schuck et al., 2015). Both mechanisms (oxidative stress and energy deficit) were associated with behavioral changes in HPA animals (Berti et al., 2012). Finally, a single PKU patient showed axonal atrophy and altered morphology of Schwann cells (Meier, Lütschg, Vassella, & Bischoff, 1975). Since peripheral nerve dysfunction is disputed in PKU patients (Cleary et al., 1994; Dyer et al., 1996; Gamstorp, Shelburne, & O'Flynn, 1966; Ludolph, Ullrich, Nedjat, Masur, & Bick, 1992), more studies are needed to investigate whether peripheral nervous system may also be a target for the pathophysiology of PKU.
4 CONCLUDING REMARKS
White matter disturbances are often found in patients with classical PKU, with devastating impact on their quality of life. However, the precise molecular mechanisms underlying these effects remain elusive. The present review hypothesizes that these white matter abnormalities may be originated from the following general mechanisms, which may take place as a combination: (a) a decrease in the activity of enzymes involved in the biosynthesis of cholesterol and other myelin precursors, (b) a decreased rate of cerebral protein synthesis, (c) epigenetic alterations, and (d) cytotoxic damage (Figure 3). More efforts are necessary to accurately identify the underlying mechanisms of the white matter pathology in PKU, allowing the development of novel and more efficient therapeutic strategies.
ACKNOWLEDGMENTS
This study was financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ). The authors thank Fernanda Malgarin, Jotele Agostini, and Jose Henrique Cararo for their contribution on data collection, and Maria Luiza Gomes for the first draft of Figures 1 and 3.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors had full access to all the data in the study and take responsibility for the integrity of the data. Conceptualization, P.F.S.; Investigation, B.K.F., M.T.R., E.L.S., G.C.F., and P.F.S.; Formal Analysis, B.K.F., M.T.R., E.L.S., G.C.F., and P.F.S.; Writing – Original Draft, B.K.F. and M.T.R.; Writing – Review & Editing, E.L.S., G.C.F., and P.F.S.; Visualization, G.C.F.; Supervision, G.C.F. and P.F.S.; Funding Acquisition, B.K.F., M.T.R., E.L.S., G.C.F., and P.F.S.




