DNA damage and repair in Parkinson's disease: Recent advances and new opportunities
Abstract
Parkinson's disease (PD) is the most common movement neurodegenerative disorder. Although our understanding of the underlying mechanisms of pathogenesis in PD has greatly expanded, this knowledge thus far has failed to translate into disease-modifying therapies. Therefore, it is of the utmost urgency to interrogate further the multifactorial etiology of PD. DNA repair defects cause many neurodegenerative diseases. An exciting new PD research avenue is the role that DNA damage and repair may play in neuronal death. The goal of this mini-review was to discuss the evidence for the types of DNA damage that accumulates in PD, which has provided clues for which DNA repair pathways, such as DNA double-strand break repair, are dysfunctional. We further highlight compelling data for activation of the DNA damage response in familial and idiopathic PD. The significance of DNA damage and repair is emerging in the PD field and linking these insights to PD pathogenesis may provide new insights into PD pathophysiology and consequently lead to new therapies.
Significance
Parkinson's disease (PD) is a progressive neurodegenerative disorder. No cure or treatment exists that can stop neuronal cell death in PD. In the search for the cause of PD, many cellular pathways have been explored, though studies involving the integrity of the nuclear and mitochondrial genome have been limited. This mini-review discusses the most recent findings that support dysfunctional DNA repair in PD and how the loss of genome homeostasis may contribute to cell death. Exciting new findings linking DNA damage and repair to PD pathogenesis has the potential to expand our knowledge of the mechanisms leading to PD.
1 INTRODUCTION
Parkinson's disease (PD) is the second most common neurodegenerative disorder, affecting 2%–3% of the population over 65 years old. PD is characterized by motor impairments due to nigrostriatal degeneration and a myriad of non-motor issues (Poewe et al., 2017). Many mechanisms of pathogenesis have been proposed and evaluated for PD, including protein aggregation, autophagy defects, mitochondrial dysfunction, and membrane and protein trafficking irregularities (Kalia & Lang, 2015). Among the suggested PD mechanisms, the accumulation of DNA damage and dysfunctional DNA repair have traditionally received less attention, even though DNA damage is a central issue for cells to resolve. Unrepaired DNA damage can lead to changes in gene expression, cellular dysfunction, mutations, and carcinogenesis or cell death (Kultz, 2005). Furthermore, a decline in DNA repair capacity and the accumulation of DNA damage have been proposed to be the main drivers of cellular aging, and aging is the greatest risk factor for developing PD (Maynard, Fang, Scheibye-Knudsen, Croteau, & Bohr, 2015; Reeve, Simcox, & Turnbull, 2014).
The goal of this mini-review was to present the most compelling evidence that support that DNA damage and repair defects occur in PD (Abugable et al., 2019). We summarize the evidence in PD models and PD patient-derived tissue for the contribution of defects in each known DNA repair pathway, activation of the DNA damage response, and lack of mitochondrial genome integrity. DNA damage and repair are a mostly unexplored source of new targets for drug and biomarker discovery, and furthering our understanding of this field has the potential to give rise to PD disease-modifying therapies.
2 DNA DAMAGE AND REPAIR IN NEURONS: HOW DOES IT DIFFER FROM CYCLING CELLS?
Terminally differentiated neurons are non-replicating, long-lived cells, with high metabolic activity; as a result, they must deal with a considerable DNA damage burden. The ability of post-mitotic cells such as neurons to cope with DNA damage differs strikingly from mitotic or cycling cells. Replication-associated pathways such as mismatch repair and homologous recombination (HR) are thought to be absent from the arsenal of neuronal DNA repair (Fishel, Vasko, & Kelley, 2007). However, recent evidence suggests that HR may be active in neurons, albeit using an RNA strand as a template (Welty et al., 2018). The type of DNA damage most likely to be relevant for neurons is oxidative, for which neurons would utilize base excision repair (BER), nucleotide excision repair (NER), and non-homologous end joining (NHEJ). The differences in neuronal DNA repair capacity are important factors to consider when studying mechanisms of neurodegenerative diseases.
Not all DNA repair processes that occur in the nuclear genome are functional in the mitochondria; NER, in particular, is not active in the mitochondria, and the extent to which DNA double-strand break repair occurs is also debated (Alexeyev, Shokolenko, Wilson, & LeDoux, 2013). Since DNA damage accumulates preferentially in the mitochondrial genome than in nuclear DNA, if the compensatory systems in mitochondria such as fission/fusion and functional complementation fail, mitochondrial DNA (mtDNA) damage accumulation can lead to cellular dysfunction and cell death (Van Houten, Hunter, & Meyer, 2016). This is particularly problematic for neurons, as they are heavily reliant on mitochondria as an energy source (Kann & Kovács, 2007). Whether mtDNA repair function is similar between replicating cells and post-mitotic neurons is unknown.
3 DNA DAMAGE RESPONSE AND DNA DOUBLE-STRAND BREAK REPAIR IN PD
The DNA damage response is a network of cellular pathways that sense, signal, and repair DNA lesions. Two members of the phosphoinositide-3-kinase (PI3K)-related kinase (PIKK) family, ataxia telangiectasia mutated (ATM), and ataxia telangiectasia and Rad3 related (ATR) orchestrate the DNA damage response signaling pathway. ATM is primarily involved in DNA double-strand break repair and ATR responds to a broader range of DNA lesions that lead to single-stranded DNA, commonly originating from replication forks or repair intermediates (Awasthi, Foiani, & Kumar, 2015; Flynn & Zou, 2011). When a DNA double-strand break occurs, ATM is recruited to the lesion site, where it proceeds to phosphorylate histone H2A.X at serine 139 (also known as γH2A.X) and indicative of activation ATM autophosphorylates at serine 1981 (Paull, 2015). Activated ATM phosphorylates many effector proteins resulting in cell cycle arrest, DNA repair, or if the damage remains unrepaired, cell death. The repair of DNA double-strand breaks proceeds via the NHEJ or HR DNA repair pathways. NHEJ does not use a homologous template to guide repair of the damage, whereas HR does; as a result, HR is considered mostly an error-free mechanism and NHEJ is more prone to causing deletions or insertions (Mao, Bozzella, Seluanov, & Gorbunova, 2008).
DNA damage activates the DNA damage response signaling pathway in toxicant models of PD. Following exposure to the neurotoxin 1-methyl-4-phenylpyridinium (MPP+), ATM and its downstream effector p53 were activated leading to the induction of apoptosis (Alvira, Yeste-Velasco et al., 2007; Li, Li, Zhang, Feng, & Zhao, 2016). Inhibition of ATM with the kinase inhibitor KU-5933 was shown to ameliorate MPP+ neurotoxicity, supporting a role for ATM signaling in MPP+-induced cell death (Camins et al., 2010). Similar to MPP+, exposure to 6-hydroxydopamine (6-OHDA) caused increased polyADP-ribosylation (PARylation) and p53 activation, leading to cell death (Bernstein, Garrison, Zambetti, & O'Malley, 2011; Nair, 2006). However, there is conflicting evidence on which PIKK is stimulated by 6-OHDA exposure. Nair (2006) reported ATM activation, while in contrast, Bernstein et al. (2011) found an ATR response. This discrepancy could be attributed to the different exposure paradigms, as Bernstein and colleagues used much lower doses of 6-OHDA comparatively. Both studies were congruent in that 6-OHDA induced an apoptotic cascade, via DNA damage and p53 (Bernstein et al., 2011; Nair, 2006). DNA double-strand break-related DNA damage response signaling by both ATM and ATR was found in an in vitro rotenone model (Greene, Greenamyre, & Dingledine, 2008). Whether the DNA damage response pathway is the main driver of toxicant-induced cell death in PD models is unknown. However, recent work showed that introducing the Y142F mutation on histone H2A.X, which facilitates the binding of DNA repair factors, protected cells from MPP+-induced cell death, suggesting that stimulating DNA repair is neuroprotective (Jiang, Huang, Yen, Zubair, & Dickson, 2016).
Several studies have also linked α-synuclein (α-syn) to DNA damage and repair. α-syn accumulates in Lewy bodies and Lewy neurites and mutations or whole locus multiplication in the SNCA gene lead to familial PD (Meade, Fairlie, & Mason, 2019; Rocha, De Miranda, & Sanders, 2018). Although the presence of α-syn in the nucleus has been controversial, recent studies have consistently found evidence of nuclear α-syn (Ma et al., 2014; Pinho et al., 2019; Siddiqui et al., 2012; Zhou, Xu, Mi, Ueda, & Chan, 2013). In mice overexpressing human A53T α-syn in a PINK1 null background, mutant α-syn was also reported to be in the nucleus (Evsyukov et al., 2017). Nuclear α-syn can bind DNA and when overexpressed can cause DNA single-strand breaks and double-strand breaks, particularly under oxidative conditions (Vasquez et al., 2017). Consistent with these findings, the work by Unni's laboratory demonstrated that α-syn modulates DNA repair of DNA double-strand breaks potentially via NHEJ (Schaser et al., 2019). Interestingly they propose a model by which α-syn in Lewy bodies is sequestered in the cytoplasm thereby preventing α-syn from facilitating nuclear DNA repair. The result of the pathological α-syn is the accumulation of DNA double-strand breaks found in both mouse cortical tissue after PFF seeding and in the human cortex from subjects with dementia with Lewy bodies. It would be of interest to investigate the effect of smaller and potentially more pathogenic α-syn fragments on DNA double-strand break formation. In overexpression and seeding α-syn mouse models, similar results were found, particularly increased γH2A.X foci and activated ATM (Milanese et al., 2018). Administration of the antioxidant, N-acetyl cysteine, ameliorated these deleterious phenotypes, suggesting that oxidative stress might be responsible for causing DNA damage (Milanese et al., 2018). Exposure to sodium butyrate is also able to rescue α-syn-mediated DNA damage and downregulation of genes related to DNA damage checkpoints (Paiva et al., 2017). Overall, α-syn pathology elicits DNA damage and activates the DNA damage response.
Mutations in leucine-rich repeat kinase 2 (LRRK2) are the most common cause of late-onset familial PD, and the most prevalent mutation, p.G2019S, results in aberrant kinase activity (Paisan-Ruiz, Lewis, & Singleton, 2013). Recently, p62/SQSTM1 has been described as a LRRK2 interacting partner and substrate (Kalogeropulou et al., 2018). p62/SQSTM1 is well known as an autophagy receptor and substrate, though was recently reported to suppress DNA double-strand break repair when dysregulated by preventing histone ubiquitination (Wang et al., 2016). Although not tested, it is intriguing to hypothesize that aberrant LRRK2 activity may lead to DNA double-strand break repair dysregulation and the accumulation of DNA damage via p62/SQSTM1 phosphorylation.
4 BER AND DNA SINGLE-STRAND DAMAGE
Base lesions that are not helix distorting (e.g., 8-oxo-guanine) are repaired via BER. Briefly, after damage to a nucleobase, DNA glycosylases such as OGG1 remove the damaged base leaving an abasic site. A nick in the phosphodiesterase backbone is made by AP endonuclease 1 (APE1) or a bifunctional glycosylase with lyase activity and gives rise to a DNA single-strand break. The single-strand break is then processed by either short-patch or long-patch BER. Poly [ADP-ribose] polymerase 1 (PARP1) is best characterized as a sensor of DNA single-strand breaks that is either induced directly or as an intermediate of processing DNA lesions during BER. The final steps of BER involve end processing, gap filling, and ligation, which are performed by a host of enzymes including DNA polymerase β and x-ray repair cross-complementing 1 (Caldecott, 2008). For an extensive review of BER, please refer to Kim and Wilson (2012).
Given the plethora of evidence to support oxidative stress in PD, several groups have investigated oxidative damage to macromolecules, such as nucleic acids, in the brains of subjects with PD (Sanders & Greenamyre, 2013). Particularly in the substantia nigra (SN) of PD patients, elevated levels of 8-oxo-guanine, abasic sites, and nuclear DNA strand breaks have been reported (Alam et al., 1997; Hegde et al., 2006; Sanders, McCoy et al., 2014; Zhang et al., 1999). Perhaps as a compensatory response to the increased oxidative DNA damage, DNA glycosylases or nucleotide sanitizing enzymes are upregulated in SN dopaminergic neurons from subjects with PD (Arai et al., 2006; Fukae et al., 2005; Shimura-Miura et al., 1999). In animal models, OGG1 DNA glycosylase function is important to maintain nigrostriatal integrity, as OGG1 knockout in mice resulted in dopaminergic neuron loss with aging and conferred sensitivity to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurodegeneration (Cardozo-Pelaez, Sanchez-Contreras, & Nevin, 2012). Another BER member, APE1, is regulated by Parkin an E3 ubiquitin ligase, which when mutated can cause autosomal recessive early onset PD (Shimura et al., 2000). Interestingly, APE1 is ubiquitinated in a Parkin-dependent manner and PD-causing mutations in Parkin abrogate this ubiquitination, resulting in APE1 accumulation (Scott et al., 2017). Genetic studies further support a role for BER variants in the development of PD. Carriers of single nucleotide polymorphisms (SNPs) in the genes encoding APE1 and/or OGG1 in combination with environmental exposures were at increased PD risk, highlighting an important gene–environment interaction (Sanders et al., 2017). Individually or in combination, the BER SNPs did not increase PD risk, consistent with genome-wide association studies, but in contrast to smaller published cohorts (Chang et al., 2017; Cornetta et al., 2013; Gencer et al., 2012; Nalls et al., 2019). Future studies may investigate the cellular basis for the synergy between PD-linked BER SNPs and environmental exposures and the resulting effect on DNA damage, repair, and cell death. In total, these data support a role for BER increasing susceptibility to the development of PD, yet to date, there is no direct mechanistic evidence of a functional BER defect in PD.
PARP1 catalyzes PARylation to multiple nuclear target proteins involved in DNA repair and is suggested to underlie the pathogenic mechanism in PD (Berger et al., 2018; Dawson & Dawson, 2017; Yu, Kim, & Kim, 2016; Yun et al., 2017). In a recent study, α-syn preformed fibrils initiated nitric oxide-mediated DNA damage, which in turn activated PARP1 (Kam et al., 2018). α-syn binds to PAR which in turn accelerates its fibrillization, promoting its spread and triggering cell death via the parthenatos pathway (Koch et al., 2011). While PARP1 participates in BER and single-strand break repair, there is more recent evidence of DNA-activated PARP1 in the cellular response to DNA double-strand breaks (Beck, Robert, Reina-San-Martin, Schreiber, & Dantzer, 2014). Further investigation is required to resolve the type of DNA lesion(s) that are leading to PARP1 activation. PARP1 represents an attractive therapeutic target since inhibitors are approved by the Food and Drug Administration to treat cancer. However, in addition to its role in DNA repair, PARP1 has been found to participate in a variety of other cellular processes. Defining the potential toxicity of PARP1 inhibition due to their other roles in distinct pathways will help clarify its utility as a therapy for PD (Olsen & Feany, 2019).
5 NUCLEOTIDE EXCISION REPAIR
NER is tasked with repairing bulky lesions including cyclobutane–pyrimidine dimers, 6-4 pyrimidine–pyrimidone photoproducts (6-4PPs), chemical adducts, intrastrand crosslinks, and ROS-generated cyclopurines and the NER pathway is described in detail by Marteijn, Lans, Vermeulen, and Hoeijmakers (2014). Importantly, in the brain, the number of endogenous sources that would produce DNA lesions that are NER substrates is limited, as they are often caused by UV radiation or exposures to chemicals that cannot cross the blood–brain barrier. However, this is not the case for all NER-repaired lesions (e.g., those caused by ROS) and the repair of oxidative DNA damage via NER may be most relevant for dopamine neurons due to their vulnerability to oxidative stress (Horowitz et al., 2011). Defects in NER give rise to diseases that predispose to both cancer and neurological abnormalities, highlighting the important role NER can play in neuronal health (Sepe, Payan-Gomez, Milanese, Hoeijmakers, & Mastroberardino, 2013).
There is little known about whether NER contributes to the pathogenesis of PD. The relevance of NER to PD was recently investigated, and it was found that in PD patient-derived fibroblasts unscheduled DNA synthesis (i.e., NER capacity) is reduced (Sepe et al., 2016). In the same study, to study NER in animal models and the impact on PD pathology, two Ercc1 mouse model systems were evaluated. Excision repair cross-complementation group 1 (ERCC1) is an endonuclease responsible for making the necessary incisions before gap filling and ligation can proceed in NER (Marteijn et al., 2014). In Ercc1 mutant mice, prototypical PD pathology was observed—reduced striatal innervation, α-syn pathology, oxidative stress including γH2A.X foci, and increased sensitivity to MPTP (Sepe et al., 2016). This work suggests that ERCC1 function is important to the integrity of the nigrostriatal system. Lastly, although ERCC1 is a crucial enzyme for NER, it is also known to facilitate double-strand break repair, highlighting promiscuous cross talk of proteins in DNA repair pathways (Friboulet et al., 2013). Reflective potentially of this cross talk between pathways, Sepe et al. (2016) observed increased γH2A.X foci in dopamine neurons in both NER-deficient mouse models and persistent γH2A.X foci following gamma irradiation in PD patient-derived cells. Deciphering whether ERCC1 dysfunction is exclusive to NER, double-strand break repair or both will be crucial to understanding the role this enzyme might play in PD.
6 MITOCHONDRIAL GENOME INTEGRITY IN PD MODELS AND HUMAN TISSUE
Mitochondrial dysfunction has been proposed to be fundamental to the pathogenesis of both idiopathic and familial PD and thus has been a major research focus in the PD field (Bose & Beal, 2016). Although there has been an emphasis on investigating various facets of mitochondrial function, including bioenergetic defects in PD (Keeney, Xie, Capaldi, & Bennett, 2006; Panov et al., 2005), there is strong evidence for the disruption of mitochondrial genome maintenance. Mitochondrial dysfunction and genome integrity are linked as mtDNA damage and mutations can drive other mitochondrial phenotypes related to dysfunction, and vice versa.
Several groups have reported increased mtDNA damage and mutations in the context of the LRRK2 G2019S mutation. Our laboratory has shown increased mtDNA damage in LRRK2 G2019S PD patient-derived cells and in vitro neuron models, which is reversed upon gene correction or inhibition of LRRK2 kinase activity (Howlett et al., 2017; Sanders, Laganière et al., 2014). Taken together, these data indicate that the mtDNA damage is directly caused by the LRRK2 G2019S mutation and requires LRRK2 kinase activity. mtDNA damage may convert to a mutation, but not always (Valente et al., 2016). Interestingly, mtDNA mutations were also observed in peripheral tissues from LRRK2 G2019S subjects; mtDNA deletions were increased in fibroblasts from LRRK2 G2019S carriers compared to healthy controls, and the number of deletions was higher in PD-manifesting carriers compared to non-manifesting carriers (Ouzren et al., 2019). Since LRRK2 does not reside within the mitochondria, the mechanism by which LRRK2 exerts its effect on mitochondrial genome integrity is unclear (Biskup et al., 2006). LRRK2 is reported to interact with proteins involved in mitochondrial dynamics, inducing fission and mitochondrial clearance, suggesting a functional relationship with mitochondria (Niu, Yu, Wang, & Xu, 2012; Stafa et al., 2014; Wang et al., 2012). Future experiments may delineate the role of mitochondrial dynamics and mitophagy in LRRK2 mutant-induced mtDNA damage and mutations. In addition, the question still remains as to whether the mitochondrial genome alterations are specific to mutant LRRK2 or are more broadly applicable to other genes associated with familial forms of PD.
Beyond familial forms of PD, increased mtDNA damage is also observed with exposure to PD-linked toxicants. Acute exposure of the widely used herbicide, paraquat, increased mtDNA damage in rat primary ventral midbrain cultures and in vivo systems (Sanders et al., 2017). Consistent with these findings, paraquat and 6-OHDA, both known for causing oxidative stress, also induce mtDNA damage in the nematode Caenorhabditis elegans (González-Hunt et al., 2014). Despite widespread complex I inhibition, exposure to rotenone or MPTP induced mtDNA damage in a brain region-specific manner, highlighting mtDNA damage as a molecular marker of vulnerable neurons with the potential to be a driver of PD pathogenesis (Gureev, Shaforostova, Starkov, & Popov, 2017; Mandavilli, Ali, & Van Houten, 2000; Sanders, McCoy et al., 2014).
Evidence of mitochondrial genome integrity defects in human postmortem brain tissue from subjects with PD echo findings in the periphery and neuronal models. Persistent abasic sites in mtDNA in dopamine neurons of the SN (but not in the cortex) are found in subjects with idiopathic PD (Sanders, McCoy et al., 2014). This is not at the exclusion of other types of mtDNA lesions, as the detection of mtDNA damage is currently hindered by the lack of appropriate or sensitive tools (Gonzalez-Hunt, Wadhwa, & Sanders, 2018). Development of new tools is crucial; if specific mtDNA lesions accumulate in PD this would suggest that specific mtDNA repair pathways are dysregulated. Likely due to increased mtDNA damage, mtDNA deletions, point mutations, and transversions are increased in dopamine neurons in the SN (and not other brain regions) derived from subjects with idiopathic PD (Bender et al., 2006; Dolle et al., 2016; Kraytsberg et al., 2006; Lin et al., 2012).
Further evidence of the importance of mitochondrial homeostasis for dopaminergic neuron health is supported by the MitoPark mouse model, a conditional knockout of TFAM in dopaminergic neurons. TFAM encodes for the mitochondrial transcription factor A or TFAM, a protein that is essential for transcription and maintenance of mtDNA. This mouse model displays progressive PD-like motor phenotypes and dopaminergic neurodegeneration (Ekstrand & Galter, 2009). Interestingly, some similarities are observed in patients with inherited mitochondrial diseases caused by mutations in the mitochondrial genome or by mutations in nuclear-encoded genes that are crucial for mtDNA replication and maintenance (such as POLG or C10orf2 Twinkle). These patients exhibit severe nigrostriatal neurodegeneration compared to healthy controls (Tzoulis, Schwarzlmüller, Biermann, Haugarvoll, & Bindoff, 2016). Despite substantial striatal denervation, mitochondrial disease patients in this cohort did not present with features of clinical parkinsonism, such as tremor or rigidity. Based on the clinical findings, one might expect classical brain PD pathology such as Lewy bodies to be absent in the mitochondrial disease subjects, and this was indeed the case in a previous study with mitochondrial disease patients with POLG mutations (Tzoulis et al., 2013). However, a study focusing on older individuals with mitochondrial diseases showed increased prevalence of Lewy body pathology in this cohort compared to controls (Erskine et al., 2020). In contrast, there have been reports of patients with POLG mutations exhibiting parkinsonism (Rahman & Copeland, 2019). LRRK2 mutation carriers and idiopathic PD patients also exhibited alterations in mtDNA transcription and replication, suggesting that dysregulated maintenance of mtDNA may be a broader and shared mechanism in PD (Podlesniy et al., 2019).
Overall, the studies evaluating mitochondrial genome integrity in PD highlight the link between mitochondrial genome dyshomeostasis and nigrostriatal integrity. While the pathogenic role of mtDNA damage and mutations in PD is unclear, mtDNA damage can directly trigger neuroinflammation and cell death and a better mechanistic understanding may provide new insights into the pathophysiology of PD.
7 CONCLUSION
The pathogenic role of DNA damage and repair in PD is unclear (Figure 1). Whether nuclear and mtDNA damage and DNA repair defects are a cause or consequence of PD-related pathology and neurodegeneration remains to be determined. DNA damage can trigger cellular dysfunction including mitochondrial impairment, genetic, RNA, and protein instability, and cell death (Kultz, 2005). Interestingly, damaged mtDNA released into the cytoplasm due to stress can induce the inflammatory response and lead to neuronal death (Wilkins, Weidling, Ji, & Swerdlow, 2017). Recent findings link mtDNA, inflammation, and PD. Circulating mutated mtDNA in mitophagy-deficient and POLG mutator mice triggers an inflammatory response by STING (a regulator of the type I interferon response), and loss of STING prevented dopaminergic neurodegeneration (Sliter et al., 2018). Further investigation is warranted into how DNA damage and dysfunctional DNA repair elicit and exacerbate PD pathogenesis.
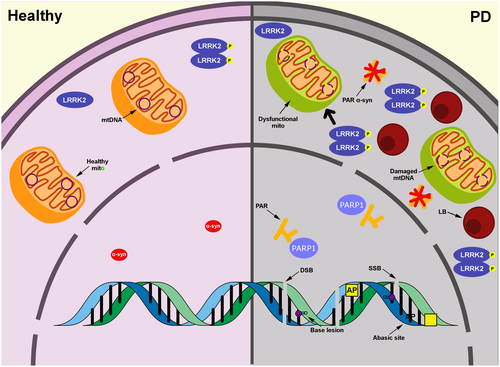
In the event of DNA damage accumulation, cycling cells initiate signaling cascades that lead to cell cycle checkpoints which halt cell division and allow for the DNA to be repaired (Lemmens & Lindqvist, 2019). Activating these same signaling cascades in post-mitotic neurons can cause cell cycle reentry, which may subsequently lead to cell death (Folch et al., 2012). Although limited, there is some evidence in the literature that cell cycle reentry occurs in PD. Increased phosphorylated retinoblastoma, a key regulator of the cell cycle, has been detected in the SN, cortex, and hippocampus tissue from idiopathic PD patients (Jordan-Sciutto, Dorsey, Chalovich, Hammond, & Achim, 2003). Additionally, duplicated DNA has been detected in SN neurons from subjects with PD (Höglinger et al., 2007). In the MPTP/MPP+ model exposure increased phosphorylation of retinoblastoma and induction of cyclin and cyclin-dependent kinases, suggesting cell cycle reentry as a possible mechanism for neuronal cell death (Alvira, Tajes et al., 2007; Camins et al., 2010; Höglinger et al., 2007). Reentry into the cell cycle has been well-documented in Alzheimer's disease, emphasizing this mechanism may represent a common pathway for neurodegeneration (Bonda et al., 2010).
To conclude, DNA damage and repair in PD is an exciting field and could elucidate new mechanisms of pathogenesis in PD, and as a result, yield new opportunities for developing biomarkers and disease-modifying therapies.
ACKNOWLEDGMENTS
The authors thank Elizabeth Thacker and Catherine Toste for their thoughtful feedback on this manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Writing – Original Draft, C.G.-H. and L.H.S.; Writing—Review & Editing, C.G.-H. and L.H.S.




