The impact of aging on the subregions of the fusiform gyrus in healthy older adults
Abstract
The fusiform gyrus is known to decrease in size with increasing age. However, reported findings are inconsistent and existing studies differ in terms of the cohorts examined and/or the methods applied. Here, we analyzed age-related links in four distinct subregions of the fusiform gyrus through integrating imaging-based intensity information with microscopically defined cytoarchitectonic probabilities. In addition to age effects we investigated sex effects as well as age-by-sex interactions in a relatively large sample of 468 healthy subjects (272 females/196 males) covering a broad age range (42–97 years). We observed significant negative correlations between age and all four subregions of the fusiform gyrus indicating volume decreases over time, albeit with subregion-specific trajectories. Additionally, we observed significant negative quadratic associations with age for some subregions, suggesting an accelerating volume loss over time. These findings may serve as a frame of reference for future cross-sectional as well as longitudinal studies, not only for normative samples but also potentially for clinical conditions that present with abnormal atrophy of the fusiform gyrus. We did not detect any significant sex differences or sex-by-age interactions, suggesting that the size of the fusiform gyrus is similar in male and female brains and that age-related atrophy follows a similar trajectory in both men and women.
Significance
This cross-sectional study shows decreasing gray matter of the fusiform gyrus with increasing age, but with varying trajectories in the different subregions of the fusiform gyrus. The lack of significant sex differences points to a similarly sized fusiform gyrus in male and female brains, and the lack of significant sex-by-age interactions indicates that age-related atrophy follows a similar trajectory in both men and women. The present findings may act as a framework for future cross-sectional as well as longitudinal studies, not only in normative samples but also in clinical populations.
1 INTRODUCTION
Visual information from the retina first reaches the primary visual (or striate) cortex and is subsequently relayed to and further processed in the extrastriate cortex, which extends into the parietal and temporal lobes (Martini, 2011; Purves, 2018). Visual processing within the extrastriate cortex can be categorized into two major processing pathways: the dorsal stream or the “where” pathway leading from the extrastriate cortex to the parietal lobe is involved in spatial localization and motion analysis; the ventral stream or the “what” pathway leading from the extrastriate cortex to the temporal lobe is involved in object, color and shape recognition and high-resolution vision (Caspers et al., 2013; Purves, 2018; Ungerleider & Haxby, 1994; Ungerleider & Mishkin, 1982).
The fusiform gyrus—an object of intense scientific scrutiny (Caspers et al., 2013, 2014; Grill-Spector & Weiner, 2014; Kanwisher, McDermott, & Chun, 1997; Lorenz et al., 2017; Weiner & Grill-Spector, 2012) and also the target structure of the current study—is an essential part of the latter ventral stream. More specifically, the fusiform gyrus is involved in higher order vision processing and probably most well-known for its involvement in visual face processing—even though it also plays an important role in the visual processing of body parts, objects, places, and word forms (Andreasen et al., 1996; Grill-Spector & Weiner, 2014; Haxby et al., 1996; Kanwisher et al., 1997; Kuskowski & Pardo, 1999; McCarthy, Puce, Gore, & Allison, 1997; Sergent, Ohta, & MacDonald, 1992; Weiner & Grill-Spector, 2012). Some of these functions, such as face recognition and the ability to identify facial expressions, are known to degrade with age (Lamont, Stewart-Williams, & Podd, 2005; Ruffman, Henry, Livingstone, & Phillips, 2008; Sze, Goodkind, Gyurak, & Levenson, 2012). Thus, one would assume that the fusiform gyrus itself is affected by age-related structural atrophy.
Indeed, there is some literature addressing this phenomenon and suggesting that the size of the fusiform gyrus decreases over time (Hogstrom, Westlye, Walhovd, & Fjell, 2013; Raz, Briggs, Marks, & Acker, 1999; Raz, Gunning-Dixon, Head, Dupuis, & Acker, 1998; Wright et al., 2008). However, studies and existing reports do not discriminate between subregions of the fusiform gyrus and thus lack a more differentiated view. For example, to date, four distinct cytoarchitectonic regions have been mapped (Caspers et al., 2013; Lorenz et al., 2017), but it has not been examined whether some of those subregions are more prone to age effects than others. Furthermore, while sex differences as well as age-by-sex interactions with respect to the fusiform gyrus have been reported (Raz et al., 1997, 2004), findings are not yet conclusive and studies addressing sex effects with respect to different subregions are entirely missing.
Thus, in the current study, we set out to investigate (a) the association between age and gray matter within the fusiform gyrus, while discriminating between its different subregions, (b) possible sex differences in fusiform gray matter, while properly adjusting for individual brain size, and (c) potential age-by-sex interactions. For this purpose, we applied a state-of-the-art brain mapping technique which combines MRI-based signal intensities and cytoarchitectonically defined probabilistic maps to quantify the gray matter volume within cortical areas of the fusiform subregions (Kurth, Cherbuin, & Luders, 2015, 2017, 2019; Kurth, Jancke, & Luders, 2017, 2018; Luders, Kurth, Toga, Narr, & Gaser, 2013). Based on the existing literature, we hypothesized that the fusiform gyrus would decline with increasing age (Grady, 2002; Hogstrom et al., 2013; Kaup, Mirzakhanian, Jeste, & Eyler, 2011; Raz et al., 1998, 1999; Wright et al., 2008), but perhaps with different trajectories for different subregions. Our hypotheses with respect to possible sex differences as well as age-by-sex interactions were undirected.
2 METHODS
2.1 Study sample and image data
Image data and individual information were derived from the Open Access Series of Imaging Studies-3 (OASIS-3) database (http://oasis-brains.org). The OASIS-3 dataset is a publicly available retrospective compilation of data from 1,098 participants collected through the Knight Alzheimer's Disease Research Center at the Washington University at St. Louis, MO, USA. To compile the dataset for this study, we first inspected the accompanying clinical data to exclude participants with any documented cognitive impairment, any acute disorder, and, in addition to acute disorders, any remote neurological disorder that may have been diagnosed previously but was not deemed current or active. Still, these current diagnoses may not necessarily reflect all history of neurological or other disorders, which may affect brain structure. Therefore, the medical history of the remaining participants was checked in a second step to exclude those with a documented history of stroke or cerebrovascular disorder, a history of neurological disorder except for minor head trauma (participants with histories of acute or recent minor head trauma were still excluded), as well as a history of psychiatric disorders. In addition, a history of cardiac arrest, congestive heart failure, or cardiac bypass procedures, was deemed an exclusion criterion to avoid possible artifacts of temporary hypoperfusion and concurrent structural alterations in the brain. For the remaining 508 participants, we processed the first T1-weighted scan to identify brains that were corrupted by image artefacts or noise, which resulted in the exclusion of another 23 brains due to insufficient image quality. Finally, as participants were scanned on four different devices, scanner parameters were checked to make sure that consistent settings were used for each scanner. As only 17 of the remaining 485 participants (3.5%) were scanned using the Siemens Sonata, those were excluded as well to keep the sample as clean and the image quality as homogeneous as possible. Our final study sample included 468 participants (272 females/196 males) aged 42–97 years (mean ± SD: 68.05 ± 9.49 years) at the time of their brain scan. The majority of these participants were self-reportedly right-handed (n = 417; 89.1%); the remaining participants were either left-handed (n = 40; 8.5%) or ambidextrous (n = 11; 2.4%). Given their relatively small number (9.7%), the non-right handers were kept as part of the study sample.
For all 468 participants high-resolution T1-weighted brain images were acquired on a 1.5 Tesla Siemens Vision scanner, a 3 Tesla Siemens Biograph scanner, or a 3 Tesla Siemens Trio scanner, with the acquisition parameters for each scanner detailed elsewhere (http://oasis-brains.org). The use of three different scanners resulted in slightly different voxel sizes, specifically 1 × 1 × 1.25 mm3 for the Vision, 1.2 × 1.05 × 1.05 mm3 for the Biograph, and 1 × 1 × 1 mm3 for the Trio. All OASIS participants gave their written and informed consent to participate as well as to the public sharing of their anonymized data (Marcus, Fotenos, Csernansky, Morris, & Buckner, 2010; Marcus et al., 2007). Additional local ethics approval for the data analysis was obtained from the University of Auckland (UoA) ethics committee (Protocol No. 022375).
2.2 Data processing
The T1-weighted images were processed via the CAT12 toolbox (version 12.6) and SPM12 (r7487), as described elsewhere (Kurth et al., 2015, 2018; Kurth, Cherbuin et al., 2017; Kurth, Jancke, et al., 2017; Luders et al., 2013). More specifically, the images were corrected for magnetic field inhomogeneities and the tissue was classified into gray matter, white matter, and cerebrospinal fluid. The segmentation process was based on a maximum a posteriori estimation (Rajapakse, Giedd, & Rapoport, 1997), using a partial volume estimation algorithm (Tohka, Zijdenbos, & Evans, 2004), a spatially adapting nonlinear denoising filter (Manjon, Coupe, Marti-Bonmati, Collins, & Robles, 2010), and a hidden Markov random field model (Cuadra, Cammoun, Butz, Cuisenaire, & Thiran, 2005). In addition, white matter hyperintensities were masked to prevent their misclassification as gray matter. The resulting gray matter segments were spatially normalized to the DARTEL template within the CAT12 toolbox using linear (12-parameter affine) transformations and nonlinear (high-dimensional) warping (Ashburner, 2007). The normalized gray matter segments were then divided by the linear and nonlinear components of the Jacobian determinant derived from the normalization matrix to preserve the original voxel-wise gray matter (Ashburner & Friston, 2000; Good et al., 2001; Kurth et al., 2018). Additionally, the total intracranial volume was calculated by adding the volumes of the whole-brain tissue segments in native space (gray matter + white matter + cerebrospinal fluid) to be later included as a covariate in the statistical model.
2.3 Combining gray matter information with cytoarchitectonic tissue probabilities
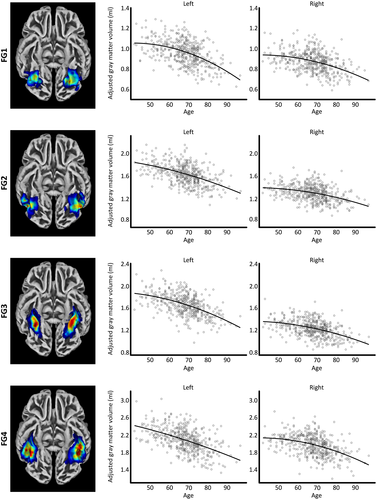
The cytoarchitectonic probability maps of the subregions of the fusiform gyrus—provided with the Anatomy Toolbox version 2.2c (Eickhoff et al., 2005)—were derived from cell body-stained histology sections of 10 postmortem brains (Caspers et al., 2013; Lorenz et al., 2017). More specifically, each brain was embedded in paraffin, sectioned into 20 µm serial slices, and stained for cell bodies. Subregions of the fusiform gyrus were then mapped under the light microscope using an observer-independent approach (Schleicher et al., 2000, 2005) based on changes in the cytoarchitecture. Subsequently, these subregions were digitized and reconstructed in 3D space, warped into MNI single-subject space, and converted into region-specific probability maps. The resulting color-coded probability maps of FG1, FG2, FG3, and FG4 (Caspers et al., 2013; Eickhoff et al., 2005; Lorenz et al., 2017) are visualized in Figure 1. To obtain the region-specific gray matter volumes from the normalized gray matter segments (see Section 2.2), the probability maps were spatially normalized to the DARTEL template to ensure an accurate correspondence with the individual gray matter segments in DARTEL space. This was followed by multiplying the respective probability maps (FG1, FG2, FG3, or FG4) with the gray matter segments. The resulting voxel-wise measures were then multiplied with the voxel volume and summed up in order to reveal the gray matter volume (in mm3) for each fusiform region, as described elsewhere (Kurth et al., 2018).
2.4 Statistical analysis
All statistical analyses were conducted in Matlab (The MathWorks, Natick, MA) using a mass-univariate general linear model. The derived volumes for the left and right FG1, FG2, FG3, and FG4 were used as dependent variables, while age, quadratic effects of age, and sex were used as the independent variables. Total intracranial volume as well as scanner were treated as variables of no interest. In addition, sex-by-age and sex-by-age2 interactions were assessed, but as neither reached significance, these terms were not included in the final statistical model. Of note, differences in variance between the sexes were found to be significant using a two-sample F-test for equal variance. Thus, a non-sphericity correction as implemented in SPM (Glaser & Friston, 2007) was applied. In addition, the residuals for left FG2 and for right FG3 were found to be not normally distributed using Lilliefors tests. Thus, significance for these two regions was established using a Monte Carlo simulation with 20,000 permutations employing the Smith procedure for covariate handling (Nichols & Holmes, 2007; Winkler, Ridgway, Webster, Smith, & Nichols, 2014). Since gray matter tissue is known to decrease with age in general, associations between regional subvolumes and age as well as age2 were assessed accordingly using one-tailed t tests. In contrast, as we did not have a directed hypothesis on the difference between male and female fusiform gyri, possible sex effects were assessed using two-tailed tests. All significance levels were Bonferroni-corrected for multiple comparisons and set at p < 0.00625 to account for the assessment of left and right FG1, FG2, FG3, and FG4 (i.e., 0.05/8). In addition, estimated annual atrophy rates were calculated using the beta estimates of the final statistical model.
3 RESULTS
In terms of linear correlations, the gray matter volumes of all four subregions (F1, F2, F3, and F4) of the fusiform gyrus were negatively associated with age. This effect was statistically significant after Bonferroni corrections. As shown in Table 1 (first column), the magnitude of these negative correlations varied among the four subregions. In contrast, negative quadratic associations (see Table 1; second column) only survived Bonferroni corrections for the left FG1 and the right FG4. In addition, a trend for significance (i.e., not surviving the correction for multiple comparisons) was evident in the right FG2 and the right FG3. The subregion-specific trajectories are depicted in Figure 1. The volume loss between age 50 and age 90 was highest within the left FG1 and lowest within the right FG2. Table 2 provides an overview of the annual volume losses at ages 50, 70, and 90 as well as the overall volume loss between age 50 and age 90.
| Side | Area | Age | Age2 | Sex | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | T (df = 461) | p | r | T (df = 461) | p | Cohen's db | T (df = 461) | p | ||
| Left | FG1 | −0.491 | 12.103 | <0.001** | −0.146 | 3.170 | <0.001** | −0.025 | 0.272 | 0.786 |
| FG2* | −0.502 | 12.450 | <0.001** | −0.064 | 1.370 | 0.149 | 0.077 | 0.821 | 0.409 | |
| FG3 | −0.529 | 13.385 | <0.001** | −0.102 | 2.208 | 0.014a | 0.143 | 1.537 | 0.125 | |
| FG4 | −0.508 | 12.667 | <0.001** | −0.038 | 0.815 | 0.208 | 0.043 | 0.458 | 0.647 | |
| Right | FG1 | −0.407 | 9.555 | <0.001** | −0.111 | 2.390 | 0.009a | 0.062 | 0.662 | 0.508 |
| FG2 | −0.378 | 8.767 | <0.001** | −0.089 | 1.926 | 0.027a | 0.186 | 1.995 | 0.047a | |
| FG3* | −0.452 | 10.873 | <0.001** | −0.100 | 2.166 | 0.034a | 0.151 | 1.623 | 0.080 | |
| FG4 | −0.432 | 10.290 | <0.001** | −0.118 | 2.544 | 0.006** | 0.186 | 2.001 | 0.046a | |
- a Trend (does not survive Bonferroni correction).
- b Positive values: males > females; negative values: females > males.
- * Significance calculated using nonparametric testing.
- ** Significant.
| Side | Area | Estimated rate of volume loss (% per year) | Total volume loss (%) | ||
|---|---|---|---|---|---|
| Age 50 | Age 70 | Age 90 | Age 50–Age 90 | ||
| Left | FG1* | −0.197 | −0.704 | −1.471 | −25.778 |
| FG2 | −0.384 | −0.614 | −0.937 | −22.247 | |
| FG3 | −0.341 | −0.691 | −1.211 | −24.982 | |
| FG4 | −0.512 | −0.709 | −0.995 | −25.141 | |
| Right | FG1 | −0.166 | −0.533 | −1.035 | −19.897 |
| FG2 | −0.178 | −0.466 | −0.844 | −17.488 | |
| FG3 | −0.263 | −0.624 | −1.144 | −22.894 | |
| FG4* | −0.185 | −0.587 | −1.155 | −21.777 | |
- * Significant quadratic effects of age.
While there was a trend for larger gray matter volumes of the right FG2 and FG4 in male brains compared to female brains, this effect did not survive Bonferroni corrections suggesting that subregions of the fusiform gyrus are similar in gray matter volume in men and women when correcting for brain size (see Table 1; third column). Similarly, there were no significant sex-by-age interactions indicating that male and female fusiform gyri tend to follow a similar trajectory with age.
4 DISCUSSION
In a sample of healthy individuals, covering a wide age range from 42 to 97 years, we investigated the magnitude and trajectories of age-related decline in subregions of the fusiform gyrus. We observed significant negative linear associations between age and all subregions of the fusiform gyrus, suggesting that increasing age is associated with decreasing gray matter volume overall. Additionally, we observed significant negative quadratic associations in some of the subregions illustrating an acceleration of volume loss over time. Overall, these findings are in accordance with prior research indicating significant age-related atrophy in the fusiform gyrus (Grady, 2002; Hogstrom et al., 2013; Raz et al., 1998, 1999, 2004), albeit there are no reports of significant quadratic associations between age and fusiform gyrus measures.
Given that the different fusiform subregions are likely to be involved in different functions (Caspers et al., 2013, 2014; Grill-Spector & Weiner, 2014; Lorenz et al., 2017), it seems notable that we observed different aging trajectories. That is, areas FG2 and FG4 have been hypothesized to correspond to the fusiform face areas (FFA-1 and FFA-2, respectively), while left FG4 is thought to correspond to the visual word form area (Caspers et al., 2013, 2014; Grill-Spector & Weiner, 2014; Lorenz et al., 2017). Areas FG1 and FG3, in contrast, have been discussed to be involved in place recognition and processing of inanimate objects (Grill-Spector & Weiner, 2014; Lorenz et al., 2017). Interestingly, the lowest volume loss between age 50 and age 90 was observed in right FG2, possibly reflecting the importance of face recognition in everyday life and its maintenance into old age. This assumption might be further supported by the hemisphere-specific volume losses (stronger age-related decline on the left than the right) as left FG2 is closely associated with the visual language processing system, while right FG2 linked with the visual face processing system (Caspers et al., 2014).
Interestingly, we did not detect any significant differences between male and female brains (and no sex-by-age interactions) with respect to the gray matter volume of the fusiform gyrus. This agrees with the outcomes of prior analyses obtaining whole-brain voxel- or vertex-wise measures which did not detect any sex differences in the fusiform gyrus either (Goldstein et al., 2002; Smith, Chebrolu, Wekstein, Schmitt, & Markesbery, 2007). Nevertheless, the missing sex differences and sex-by-age interactions seem to be in contrast with some other reports. Those latter reports, however, partly contradict each other in terms of the direction of the effect. While some have observed a lager fusiform gyrus and a steeper trajectory of age-related atrophy in men compared to women (Lotze et al., 2019; Raz et al., 2004; Xu et al., 2000), others point to a larger fusiform gyrus in women than in men (Ritchie et al., 2018).
The lack of consistency in findings—both with respect to the sex effect as well as the aforementioned missing quadratic correlations in the literature—may be due to the variety of morphometric measures and analysis approaches applied and/or the different study samples compiled. More specifically, the fusiform gyrus is frequently investigated using a region-of-interest (ROI) analysis (Gao, Conte, Richards, Xie, & Hanayik, 2019; Kanwisher et al., 1997). As further detailed elsewhere (Kurth et al., 2018), traditional ROI analyses are usually based on automated algorithms or manual tracing, which means that the method relies on the reliable detection of visible landmarks. Macroanatomical landmarks, however, are highly variable across brain scans. The methodology used in the present does not rely on the identification of macroanatomic landmarks. Instead, it derived at ROIs by integrating imaging-based intensity information with microscopically defined cytoarchitectonic probabilities. Moreover, in contrast to retrieving one overall measure treating the fusiform gyrus as a single functional unit our approach allowed for analyzing multiple subvolumes.
In addition to the differing methodology, the heterogeneity of findings across studies may also be due to differing characteristics of the study participants. For example, the age range considered is likely to impact the findings. This study is characterized by a relatively broad age range and with a focus on the later stages of life. Studies focusing on a narrower age range and/or with younger mean ages may reveal slightly different findings, such as a lack of nonlinear associations if, for example, accelerated volume loss only occurs later in life. Moreover, in older samples, the ratio of study participants suffering from mild or severe health impairments—perhaps even from preclinical conditions—may be different than in younger samples. For example, patients with diabetes mellitus have greater age-related atrophy in the fusiform gyrus compared to controls (Wu, Lin, Zhang, & Wu, 2017). While we applied a range of exclusion criteria (see Section 2.1) and ensured that subjects were free of acute and neurological disorders, the study may have contained people affected by various clinical and preclinical conditions, which are known to affect brain anatomy and/or accelerate aging trajectories (Alemany et al., 2013; Convit et al., 2000; Hedges et al., 2008; Morey et al., 2009; Wu et al., 2017; Xia et al., 2013). Finally, atrophy rates estimated from cross-sectional studies have been shown to be lower than those estimated from longitudinal studies (Fraser, Shaw, & Cherbuin, 2015; Raz et al., 2005). This might potentially limit the comparability of the present rates to observations in other studies. Thus, a replication of the current findings, preferably using longitudinal designs, will be desirable to relate them to other reports.
5 CONCLUSION
Between the ages of 42 and 97 years, the gray matter volume of the fusiform gyrus decreases with subregion-specific trajectories. While some subregions undergo stable losses over time, others are affected by increasing atrophy. Subregions of the fusiform gyrus appear to be similar in volume in male and female brains, and age-related decreases seem to follow a similar trajectory in both men and women. To our knowledge, no study has been exploring subsections of the fusiform gyrus in relation to aging and sex effects, and our present findings enhance this field of research by discriminating between four distinct subregions in each hemisphere as guided by microstructure.
DECLARATION OF TRANSPARENCY
The authors, reviewers, and editors affirm that in accordance with the policies set by the Journal of Neuroscience Research this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
ACKNOWLEDGMENTS
The authors wish to thank all researchers and participants involved in the Open Access Series of Imaging Studies. Data were provided by OASIS-3 (http://www.oasis-brains.org); Principal Investigators: Benzinger, Marcus, and Morris (NIH P50AG00561, P30NS09857781, P01AG026276, P01AG003991, R01AG043434, UL1TR000448, R01EB009352). EL is funded by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (#R01HD081720).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, F.K. and E.L.; Resources, OASIS-3 dataset; Formal Analysis, F.K.; Data Curation, M.S., F.K., and E.L.; Writing – Original Draft, M.S., F.K., and E.L.
Open Research
DATA AVAILABILITY STATEMENT
Image data and accompanying information can be obtained from the Open Access Series of Imaging Studies-3 (OASIS-3) database (http://oasis-brains.org).




